Meta-Analysis of 49 SNPs Covering 25,446 Cases and 41,106 Controls Identifies Polymorphisms in Hormone Regulation and DNA Repair Genes Associated with Increased Endometrial Cancer Risk
Abstract
:1. Introduction
2. Materials and Methods
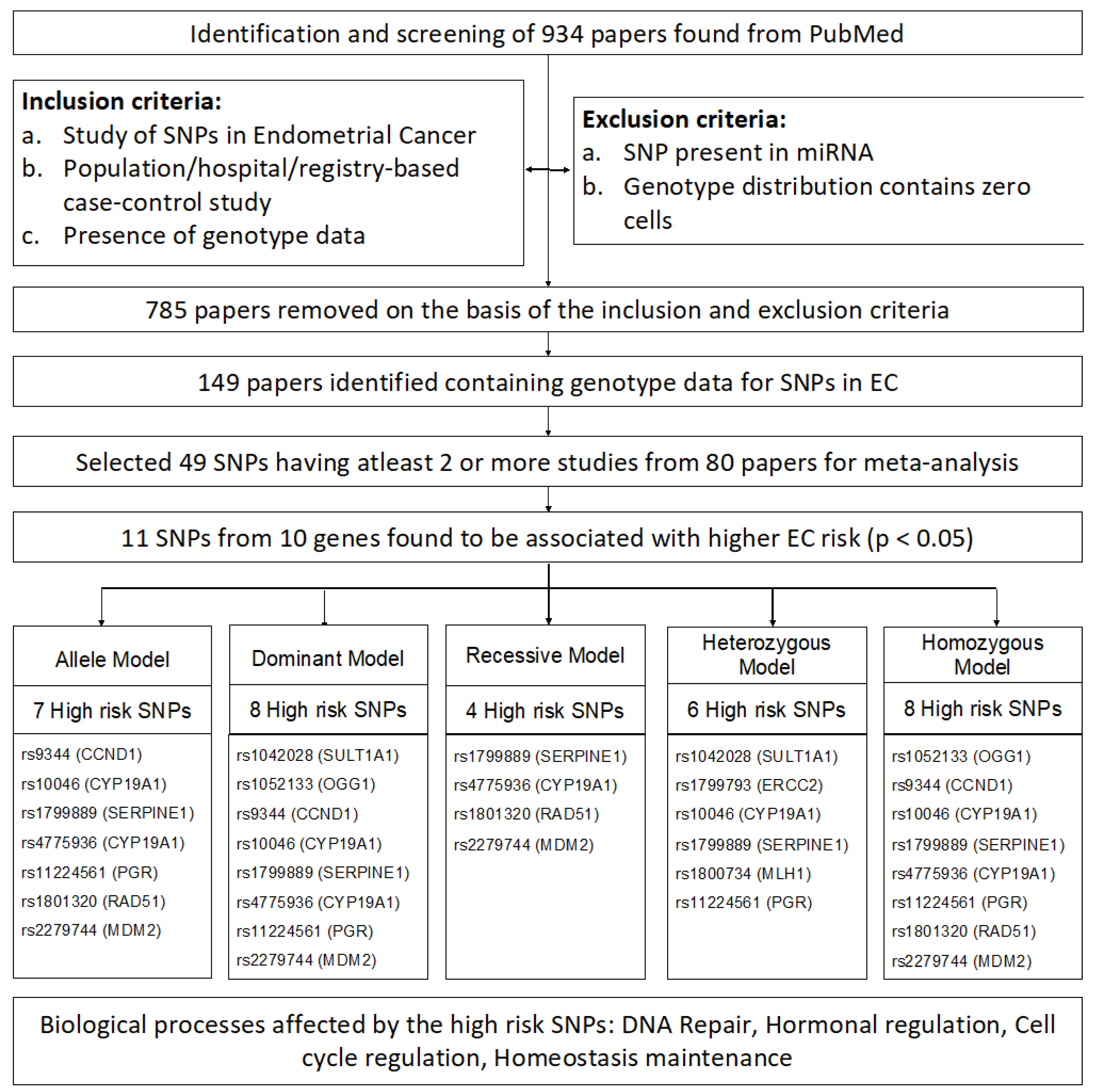
2.1. Screening and Selection
2.2. Data Collection
2.3. Meta-Analysis
3. Results
4. Discussion
4.1. Hormone-Related Genes
4.2. DNA Repair-Related Genes
4.3. Other Genes
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Olson, S.H.; Bandera, E.V.; Orlow, I. Variants in Estrogen Biosynthesis Genes, Sex Steroid Hormone Levels, and Endometrial Cancer: A HuGE Review. Am. J. Epidemiol. 2006, 165, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Niederacher, D.; An, H.-X.; Cho, Y.-J.; Hantschmann, P.; Bender, H.G.; Beckmann, M.W. Mutations and Amplification of Oncogenes in Endometrial Cancer. Oncology 1999, 56, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Das, A.P.; Saini, S.; Agarwal, S.M. A comprehensive meta-analysis of non-coding polymorphisms associated with precancerous lesions and cervical cancer. Genomics 2022, 114, 110323. [Google Scholar] [CrossRef]
- Das, A.P.; Chopra, M.; Agarwal, S.M. Prioritization and Meta-analysis of regulatory SNPs identified IL6, TGFB1, TLR9 and MMP7 as significantly associated with cervical cancer. Cytokine 2022, 157, 155954. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing. R Found. Stat. Comput. Vienna Au. 2020. Available online: https://www.r-project.org/ (accessed on 19 December 2022).
- Kovalchik, S. RISmed: Download Content from NCBI Databases. 2021. [Google Scholar]
- Mantel, N.; Haenszel, W. Statistical Aspects of the Analysis of Data From Retrospective Studies of Disease. Gynecol. Oncol. 1959, 22, 719–748. [Google Scholar] [CrossRef] [Green Version]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Évid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Yutani, H. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef] [Green Version]
- Cornel, K.M.C.; Bongers, M.Y.; Kruitwagen, R.P.F.M.; Romano, A. Local estrogen metabolism (intracrinology) in endometrial cancer: A systematic review. Mol. Cell. Endocrinol. 2018, 489, 45–65. [Google Scholar] [CrossRef]
- O’Mara, T.A.; Fahey, P.; Ferguson, K.; Marquart, L.; Lambrechts, D.; Despierre, E.; Vergote, I.; Amant, F.; Hall, P.; Liu, J.; et al. Progesterone receptor gene variants and risk of endometrial cancer. Carcinogenesis 2010, 32, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Hevir, N.; Šinkovec, J.; Rižner, T.L. Disturbed expression of phase I and phase II estrogen-metabolizing enzymes in endometrial cancer: Lower levels of CYP1B1 and increased expression of S-COMT. Mol. Cell. Endocrinol. 2011, 331, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, J.F.; Fitz Gerald, G.A. Chapter 4-Steroid Hormones and Other Lipid Molecules Involved in Human Reproduction. In Yen and Jaffe’s Reproductive Endocrinology; Strauss, J.F., Barbieri, R.L., Eighth, E., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 75–114.e7. ISBN 978-0-323-47912-7. [Google Scholar]
- Nakajima, M.; Yokoi, T. Chapter 19-MicroRNA: Regulation of P450 and Pharmacogenetics. In Handbook of Pharmacogenomics and Stratified Medicine; Padmanabhan, S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 385–401. ISBN 978-0-12-386882-4. [Google Scholar]
- Allen, N.E.; Key, T.J.; Dossus, L.; Rinaldi, S.; Cust, A.; Lukanova, A.; Peeters, P.H.; Onland-Moret, N.C.; Lahmann, P.H.; Berrino, F.; et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 2008, 15, 485–497. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, T.A.; Glubb, D.M.; Amant, F.; Annibali, D.; Ashton, K.; Attia, J.; Auer, P.L.; Beckmann, M.W.; Black, A.; Bolla, M.K.; et al. Identification of nine new susceptibility loci for endometrial cancer. Nat. Commun. 2018, 9, 3166. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.H.; Thompson, D.J.; O’Mara, T.A.; Painter, J.N.; Glubb, D.M.; Flach, S.; Lewis, A.; French, J.D.; Freeman-Mills, L.; Church, D.; et al. Five Endometrial Cancer Risk Loci Identified through Genome-Wide Association Analysis. Nat. Genet. 2016, 48, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.N.; O’Mara, T.A.; Morris, A.P.; Cheng, T.H.T.; Gorman, M.; Martin, L.; Hodson, S.; Jones, A.; Martin, N.G.; Gordon, S.; et al. Genetic overlap between endometriosis and endometrial cancer: Evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 2018, 7, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-Lorence, S.; Amarneh, B.; Ito, Y.; Fisher, C.R.; Michael, M.D.; et al. Aromatase Cytochrome P450, The Enzyme Responsible for Estrogen Biosynthesis. Endocr. Rev. 1994, 15, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.E.; Feigelson, H.S. Hormonal carcinogenesis. Carcinogenesis 2000, 21, 427–433. [Google Scholar] [CrossRef]
- Paynter, R.A.; Hankinson, S.E.; Colditz, G.A.; Kraft, P.; Hunter, D.J.; De Vivo, I. CYP19(aromatase) haplotypes and endometrial cancer risk. Int. J. Cancer 2005, 116, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lundin, E.; Wirgin, I.; Lukanova, A.; Afanasyeva, Y.; Krogh, V.; Axelsson, T.; Hemminki, K.; Clendenen, T.V.; Arslan, A.A.; Ohlson, N.; et al. Selected polymorphisms in sex hormone-related genes, circulating sex hormones and risk of endometrial cancer. Cancer Epidemiol. 2012, 36, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Key, T.J.; Pike, M.C. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: Its central role in explaining and predicting endometrial cancer risk. Br. J. Cancer 1988, 57, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vivo, I.; Huggins, G.S.; Hankinson, S.E.; Lescault, P.J.; Boezen, M.; Colditz, G.A.; Hunter, D.J. A functional polymorphism in the promoter of the progesterone receptor gene associated with endometrial cancer risk. Proc. Natl. Acad. Sci. USA 2002, 99, 12263–12268. [Google Scholar] [CrossRef] [Green Version]
- Nebert, D.W.; Dalton, T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960. [Google Scholar] [CrossRef]
- Hirata, H.; Hinoda, Y.; Okayama, N.; Suehiro, Y.; Kawamoto, K.; Kikuno, N.; Rabban, J.T.; Chen, L.M.; Dahiya, R. CYP1A1, SULT1A1, andSULT1E1 polymorphisms are risk factors for endometrial cancer susceptibility. Cancer 2008, 112, 1964–1973. [Google Scholar] [CrossRef]
- Gulyaeva, L.F.; Mikhailova, O.N.; PustyInyak, V.O.; Kim, I.V.; Gerasimov, A.V.; Krasilnikov, S.E.; Filipenko, M.L.; Pechkovsky, E.V. Comparative Analysis of SNP in Estrogen-metabolizing Enzymes for Ovarian, Endometrial, and Breast Cancers in Novosibirsk, Russia. Adv. Exp. Med. Biol. 2008, 617, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahayri, Z.N.; Patrinos, G.P.; Wattanapokayakit, S.; Iemwimangsa, N.; Fukunaga, K.; Mushiroda, T.; Chantratita, W.; Ali, B.R. Variation in 100 relevant pharmacogenes among emiratis with insights from understudied populations. Sci. Rep. 2020, 10, 21310. [Google Scholar] [CrossRef]
- Das, A.P.; Saini, S.; Tyagi, S.; Chaudhary, N.; Agarwal, S.M. Elucidation of Increased Cervical Cancer Risk Due to Polymorphisms in XRCC1 (R399Q and R194W), ERCC5 (D1104H), and NQO1 (P187S). Reprod. Sci. 2022, 1–15. [Google Scholar] [CrossRef]
- Michalska, M.M.; Samulak, D.; Romanowicz, H.; Smolarz, B. Association of polymorphisms in the 5′ untranslated region of RAD51 gene with risk of endometrial cancer in the Polish population. Arch. Gynecol. Obstet. 2014, 290, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Samulak, D.; Michalska, M.; Góralczyk, B.; Szyłło, K.; Lewy, J.; Sporny, S.; Kokołaszwili, G.; Burzyński, M.; Romanowicz-Makowska, H. 135G>C and 172G>T polymorphism in the 5′ untranslated region of RAD51 and sporadic endometrial cancer risk in Polish women. Pol. J. Pathol. 2011, 62, 157–162. [Google Scholar]
- Aka, P.; Mateuca, R.; Buchet, J.-P.; Thierens, H.; Kirsch-Volders, M. Are genetic polymorphisms in OGG1, XRCC1 and XRCC3 genes predictive for the DNA strand break repair phenotype and genotoxicity in workers exposed to low dose ionising radiations? Mutat. Res. 2004, 556, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Helland, A.; Børresen-Dale, A.-L.; Peltomäki, P.; Hektoen, M.; Kristensen, G.B.; Nesland, J.M.; de la Chapelle, A.; Lothe, R.A. Microsatellite instability in cervical and endometrial carcinomas. Int. J. Cancer 1997, 70, 499–501. [Google Scholar] [CrossRef]
- Ryan, N.A.J.; Glaire, M.A.; Blake, D.; Cabrera-Dandy, M.; Evans, D.G.; Crosbie, E.J. The proportion of endometrial cancers associated with Lynch syndrome: A systematic review of the literature and meta-analysis. Genet. Med. J. Am. Coll. Med. Genet. 2019, 21, 2167–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugasawa, K. Chapter Four-Mechanism and Regulation of DNA Damage Recognition in Mammalian Nucleotide Excision Repair. In DNA Repair; Zhao, L., Kaguni, L.S., Eds.; Academic Press: San Diego, CA, USA, 2019; Volume 45, pp. 99–138. ISBN 1874-6047. [Google Scholar]
- Lunn, R.M.; Helzlsouer, K.J.; Parshad, R.; Umbach, D.M.; Harris, E.L.; Sanford, K.K.; Bell, D.A. XPD polymorphisms: Effects on DNA repair proficiency. Carcinogenesis 2000, 21, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkson, S.G.; Wood, R.D. Polymorphisms in the human XPD (ERCC2) gene, DNA repair capacity and cancer susceptibility: An appraisal. DNA Repair 2005, 4, 1068–1074. [Google Scholar] [CrossRef]
- Gilabert-Estellés, J.; Ramón, L.A.; Braza-Boïls, A.; Gilabert, J.; Chirivella, M.; España, F.; Estellés, A. Plasminogen activator inhibitor-1 (PAI-1) 4 G/5 G polymorphism and endometrial cancer. Influence of PAI-1 polymorphism on tissue PAI-1 antigen and mRNA expression and tumor severity. Thromb. Res. 2011, 130, 242–247. [Google Scholar] [CrossRef]
- Köhler, U.; Hiller, K.; Martin, R.; Langanke, D.; Naumann, G.; Bilek, K.; Jänicke, F.; Schmitt, M. Tumor-Associated Proteolytic Factors uPA and PAI-1 in Endometrial Carcinoma. Gynecol. Oncol. 1997, 66, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.K.; Bødker, J.S.; Christensen, A.; Dupont, D.M.; Hansen, M.; Jensen, J.K.; Kjelgaard, S.; Mathiasen, L.; Pedersen, K.E.; Skeldal, S.; et al. Plasminogen activator inhibitor-1 and tumour growth, invasion, and metastasis. Thromb. Haemost. 2004, 92, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Ueda, M.; Yamamoto, M.; Nunobiki, O.; Toji, E.; Sato, N.; Izuma, S.; Okamoto, Y.; Torii, K.; Noda, S. Murine double-minute 2 homolog single nucleotide polymorphism 309 and the risk of gynecologic cancer. Hum. Cell 2009, 22, 49–54. [Google Scholar] [CrossRef]
- Kubbutat, M.H.G.; Jones, S.N.; Vousden, K.H. Regulation of p53 stability by Mdm2. Nature 1997, 387, 299–303. [Google Scholar] [CrossRef]
- Michael, D.; Oren, M. The p53 and Mdm2 families in cancer. Curr. Opin. Genet. Dev. 2002, 12, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.L.; Hu, W.; Bond, E.E.; Robins, H.; Lutzker, S.G.; Arva, N.C.; Bargonetti, J.; Bartel, F.; Taubert, H.; Wuerl, P.; et al. A Single Nucleotide Polymorphism in the MDM2 Promoter Attenuates the p53 Tumor Suppressor Pathway and Accelerates Tumor Formation in Humans. Cell 2004, 119, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; He, G.; Hou, M.; Chen, L.; Chen, S.; Xu, A.; Fu, Y. Cell Cycle Regulation by Alternative Polyadenylation of CCND1. Sci. Rep. 2018, 8, 6824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kang, S.B.; Lee, H.P. Cyclin D1 polymorphism and the risk of endometrial cancer. Gynecol. Oncol. 2005, 97, 431–435. [Google Scholar] [CrossRef]
- Ashton, K.A.; Proietto, A.; Otton, G.; Symonds, I.; McEvoy, M.; Attia, J.; Gilbert, M.; Hamann, U.; Scott, R.J. The influence of the Cyclin D1 870 G>A polymorphism as an endometrial cancer risk factor. BMC Cancer 2008, 8, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghamdi, J.; Padmanabhan, S. Chapter 12-Fundamentals of Complex Trait Genetics and Association Studies. In Handbook of Pharmacogenomics and Stratified Medicine; Padmanabhan, S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 235–257. ISBN 978-0-12-386882-4. [Google Scholar]
- Wang, X.; Glubb, D.M.; O’Mara, T.A. 10 Years of GWAS discovery in endometrial cancer: Aetiology, function and translation. Ebiomedicine 2022, 77, 103895. [Google Scholar] [CrossRef] [PubMed]
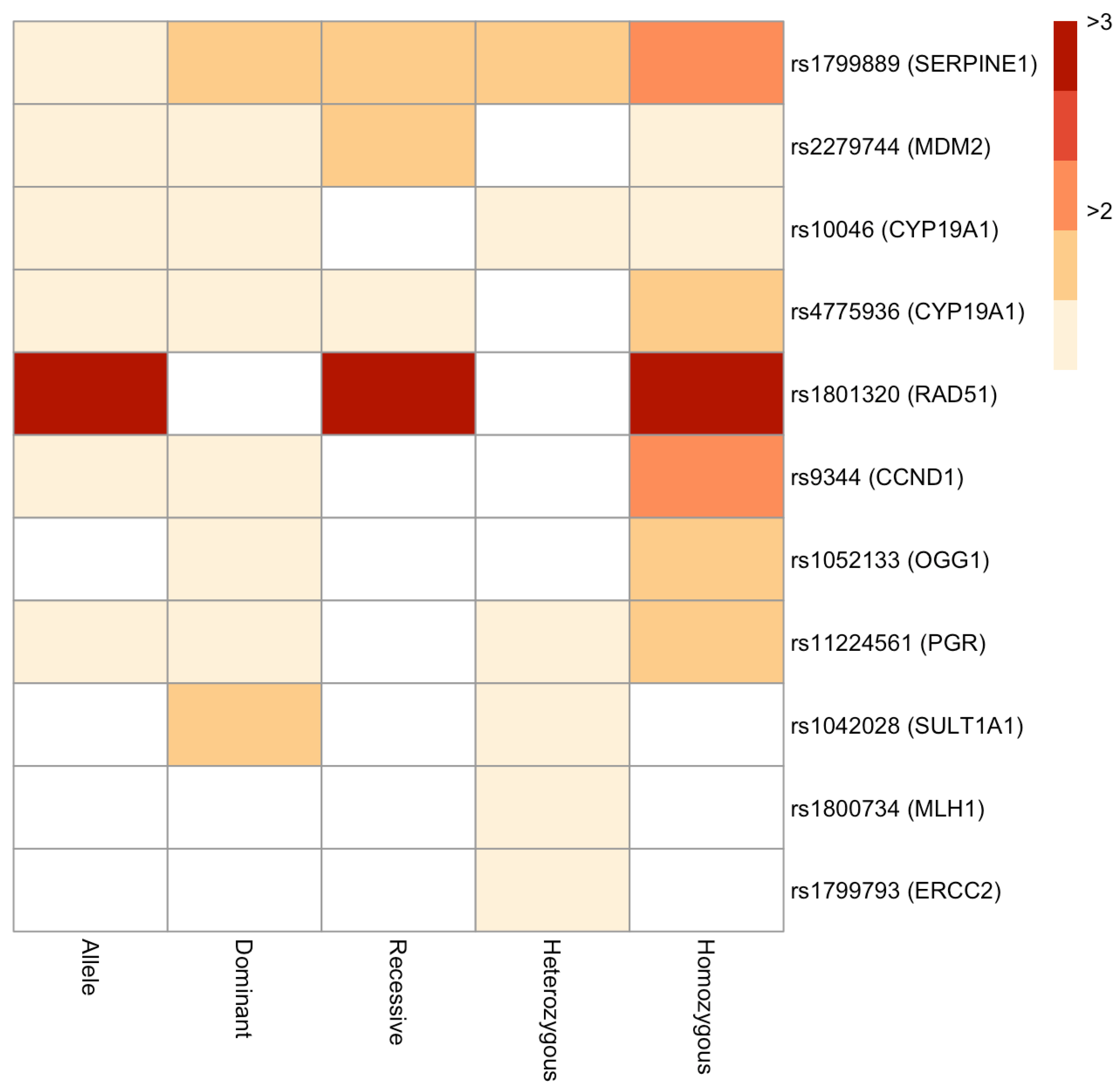
| RSID | Model | Study | OR (95% CI) | Weight (%) | Cochran’s Q | I2 | T2 | Pooled OR (95% CI) | p-Value | Egger’s p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| rs9344 | Fixed | Kang et al. (2005) | 1.69 (1.14–2.5) | 27.83 | 0.26 | 21.45 | 0.01 | 1.4 (1.13–1.74) | 0.002280 | NA |
| Ashton et al. (2008) | 1.29 (0.99–1.67) | 72.17 | ||||||||
| rs10046 | Fixed | Paynter et al. (2005) | 1.22 (0.98–1.51) | 39.43 | 0.94 | 0 | 0 | 1.21 (1.06–1.39) | 0.005794 | NA |
| Lundin et al. (2012) | 1.21 (1.01–1.44) | 60.57 | ||||||||
| rs1799889 | Fixed | Gilabert-Estellés et al. (2012) | 1.44 (1.1–1.88) | 53.98 | 0.91 | 0 | 0 | 1.45 (1.19–1.77) | 0.000244 | NA |
| Su et al. (2011) | 1.47 (1.1–1.97) | 46.02 | ||||||||
| rs4775936 | Fixed | Paynter et al. (2005) | 1.28 (1.03–1.59) | 39.47 | 0.79 | 0 | 0 | 1.25 (1.09–1.44) | 0.001213 | NA |
| Lundin et al. (2012) | 1.23 (1.03–1.47) | 60.53 | ||||||||
| rs11224561 | Fixed | Xu_SECS et al. (2009) | 1.15 (1–1.31) | 55.99 | 0.71 | 0 | 0 | 1.19 (1.08–1.31) | 0.000572 | 0.74 |
| O’Mara_ANECS et al. (2011) | 1.26 (1.06–1.49) | 33.81 | ||||||||
| O’Mara_LES et al. (2011) | 1.2 (0.89–1.63) | 10.2 | ||||||||
| rs1801320 | Random | Krupa et al. (2011) | 7.23 (3.2–16.35) | 15.69 | 0 | 90.72 | 0.18 | 4.29 (2.02–9.11) | 0.008642 | 0.24 |
| Michalska et al. (2014) | 2.55 (2.16–3) | 29.58 | ||||||||
| Romanowicz-Makowska et al. (2012) | 3.81 (2.87–5.05) | 27.54 | ||||||||
| Smolarz et al. (2011) | 6.32 (4.68–8.53) | 27.19 | ||||||||
| rs2279744 | Random | Walsh et al. (2007) | 1.38 (0.87–2.19) | 7.2 | 0.01 | 58.26 | 0.05 | 1.26 (1.05–1.52) | 0.019979 | 0.29 |
| Terry_NHS et al. (2008) | 1.21 (1.04–1.41) | 13.47 | ||||||||
| Ashton et al. (2009) | 1.13 (0.86–1.48) | 10.98 | ||||||||
| Ueda et al. (2009) | 1.2 (0.83–1.74) | 8.86 | ||||||||
| Nunobiki et al. (2009) | 1.17 (0.79–1.73) | 8.35 | ||||||||
| Knappskog_Haukeland et al. (2012) | 1.06 (0.91–1.23) | 13.47 | ||||||||
| Knappskog_MoMaTEC et al. (2012) | 1.18 (1.02–1.36) | 13.59 | ||||||||
| Zajac et al. (2012) | 2.67 (1.83–3.9) | 8.69 | ||||||||
| Yoneda et al. (2013) | 1.33 (0.97–1.83) | 9.94 | ||||||||
| Okamoto et al. (2015) | 0.96 (0.53–1.72) | 5.46 |
| RSID | Model | Study | OR (95% CI) | Weight (%) | Cochran’s Q | I2 | T2 | Pooled OR (95% CI) | p-Value | Egger’s p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| rs1042028 | Fixed | Gulyaeva et al. (2008) | 1.28 (0.81–2.02) | 55.09 | 0.17 | 47.01 | 0.05 | 1.6 (1.17–2.21) | 0.003737 | NA |
| Hirata et al. (2008) | 2 (1.28–3.14) | 44.91 | ||||||||
| rs1052133 | Fixed | Krupa et al. (2011) | 0.84 (0.26–2.7) | 2.87 | 0.34 | 11.7 | 0.05 | 1.31 (1.09–1.56) | 0.003153 | 0.64 |
| Cincin et al. (2012) | 1.95 (1.16–3.26) | 9.56 | ||||||||
| Smolarz et al. (2018) | 1.33 (1.04–1.71) | 50.79 | ||||||||
| Sobczuk et al. (2012) | 1.26 (0.69–2.28) | 8.92 | ||||||||
| Romanowicz-Makowska et al. (2011) | 1.39 (0.86–2.25) | 13.19 | ||||||||
| Hosono et al. (2013) | 0.85 (0.51–1.42) | 14.67 | ||||||||
| rs9344 | Fixed | Kang et al. (2005) | 1.73 (0.86–3.47) | 24.22 | 0.57 | 0 | 0 | 1.46 (1.02–2.07) | 0.036328 | NA |
| Ashton et al. (2008) | 1.37 (0.91–2.06) | 75.78 | ||||||||
| rs10046 | Fixed | Paynter et al. (2005) | 1.58 (1.08–2.33) | 33.22 | 0.35 | 0 | 0.01 | 1.37 (1.09–1.72) | 0.007032 | NA |
| Lundin et al. (2012) | 1.26 (0.95–1.67) | 66.78 | ||||||||
| rs1799889 | Fixed | Gilabert-Estellés et al. (2012) | 1.75 (1.12–2.73) | 60.17 | 0.98 | 0 | 0 | 1.74 (1.23–2.47) | 0.001878 | NA |
| Su et al. (2011) | 1.73 (0.98–3.06) | 39.83 | ||||||||
| rs4775936 | Fixed | Paynter et al. (2005) | 1.53 (1.07–2.18) | 34.08 | 0.26 | 21.76 | 0.01 | 1.3 (1.05–1.61) | 0.015277 | NA |
| Lundin et al. (2012) | 1.18 (0.91–1.55) | 65.92 | ||||||||
| rs11224561 | Fixed | Xu_SECS et al. (2009) | 1.45 (1.08–1.95) | 22.72 | 0.57 | 0 | 0 | 1.29 (1.11–1.49) | 0.000710 | 0.96 |
| O’Mara_ANECS et al. (2011) | 1.27 (1.05–1.53) | 58.98 | ||||||||
| O’Mara_LES et al. (2011) | 1.14 (0.8–1.61) | 18.31 | ||||||||
| rs2279744 | Fixed | Walsh et al. (2007) | 1.09 (0.57–2.1) | 2.57 | 0.71 | 0 | 0.03 | 1.15 (1.04–1.28) | 0.007404 | 0.41 |
| Terry_NHS et al. (2008) | 1.15 (0.94–1.41) | 25.2 | ||||||||
| Ashton et al. (2009) | 1.14 (0.79–1.65) | 7.83 | ||||||||
| Ueda et al. (2009) | 0.81 (0.42–1.56) | 2.99 | ||||||||
| Nunobiki et al. (2009) | 0.71 (0.35–1.42) | 2.82 | ||||||||
| Knappskog_Haukeland et al. (2012) | 1.1 (0.9–1.36) | 25.64 | ||||||||
| Knappskog_MoMaTEC et al. (2012) | 1.24 (1.01–1.51) | 25.49 | ||||||||
| Zajac et al. (2012) | 1.68 (0.89–3.17) | 2.15 | ||||||||
| Yoneda et al. (2013) | 1.42 (0.86–2.36) | 3.78 | ||||||||
| Okamoto et al. (2015) | 0.81 (0.32–2.01) | 1.52 |
| RSID | Model | Study | OR (95% CI) | Weight (%) | Cochran’s Q | I2 | T2 | Pooled OR (95% CI) | p-Value | Egger’s p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| rs1799889 | Fixed | Gilabert-Estellés et al. (2012) | 1.59 (0.99–2.58) | 47.02 | 0.87 | 0 | 0 | 1.64 (1.19–2.27) | 0.002664 | NA |
| Su et al. (2011) | 1.68 (1.09–2.61) | 52.98 | ||||||||
| rs4775936 | Fixed | Paynter et al. (2005) | 1.25 (0.88–1.77) | 46.43 | 0.38 | 0 | 0.01 | 1.4 (1.11–1.77) | 0.004165 | NA |
| Lundin et al. (2012) | 1.54 (1.13–2.1) | 53.57 | ||||||||
| rs1801320 | Random | Krupa et al. (2011) | 16 (3.22–79.56) | 10.63 | 0 | 90.68 | 0.31 | 10.03 (3.57–28.19) | 0.005741 | 0.33 |
| Michalska et al. (2014) | 4.68 (3.67–5.97) | 31.94 | ||||||||
| Romanowicz-Makowska et al. (2012) | 10.77 (6.98–16.62) | 28.98 | ||||||||
| Smolarz et al. (2011) | 18.45 (11.63–29.29) | 28.45 | ||||||||
| rs2279744 | Random | Walsh et al. (2007) | 2.55 (1.06–6.1) | 6.45 | 0 | 67.82 | 0.14 | 1.64 (1.18–2.26) | 0.007311 | 0.18 |
| Terry_NHS et al. (2008) | 1.57 (1.17–2.1) | 13.3 | ||||||||
| Ashton et al. (2009) | 1.23 (0.73–2.08) | 10.25 | ||||||||
| Ueda et al. (2009) | 1.91 (1.04–3.49) | 9.24 | ||||||||
| Nunobiki et al. (2009) | 2 (1.04–3.83) | 8.71 | ||||||||
| Knappskog_Haukeland et al. (2012) | 1.01 (0.74–1.38) | 13.07 | ||||||||
| Knappskog_MoMaTEC et al. (2012) | 1.24 (0.93–1.64) | 13.4 | ||||||||
| Zajac et al. (2012) | 4.67 (2.7–8.08) | 9.94 | ||||||||
| Yoneda et al. (2013) | 1.49 (0.88–2.53) | 10.25 | ||||||||
| Okamoto et al. (2015) | 1.14 (0.41–3.15) | 5.38 |
| RSID | Model | Study | OR (95% CI) | Weight (%) | Cochran’s Q | I2 | T2 | Pooled OR (95% CI) | p-Value | Egger’s p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| rs1042028 | Fixed | Gulyaeva et al. (2008) | 1.29 (0.77–2.17) | 50.16 | 0.43 | 0 | 0.01 | 1.5 (1.06–2.14) | 0.023277 | NA |
| Hirata et al. (2008) | 1.72 (1.06–2.78) | 49.84 | ||||||||
| rs1799793 | Fixed | Weiss_CARE et al. (2005) | 1.2 (0.89–1.62) | 35.23 | 0.93 | 0 | 0 | 1.22 (1.02–1.45) | 0.031413 | NA |
| Doherty_SEER et al. (2011) | 1.22 (0.98–1.52) | 64.77 | ||||||||
| rs10046 | Fixed | Paynter et al. (2005) | 1.6 (1.07–2.41) | 32.24 | 0.23 | 31.39 | 0.02 | 1.31 (1.03–1.67) | 0.025399 | NA |
| Lundin et al. (2012) | 1.18 (0.87–1.58) | 67.76 | ||||||||
| rs1799889 | Fixed | Gilabert-Estellés et al. (2012) | 1.61 (1.01–2.56) | 60.24 | 0.83 | 0 | 0 | 1.56 (1.08–2.25) | 0.018038 | NA |
| Su et al. (2011) | 1.48 (0.82–2.68) | 39.76 | ||||||||
| rs1800734 | Fixed | Beiner et al. (2006) | 1.51 (1.2–1.91) | 91 | 0.18 | 45.51 | 0.09 | 1.45 (1.16–1.81) | 0.001132 | NA |
| Poplawski et al. (2015) | 0.81 (0.34–1.94) | 9 | ||||||||
| rs11224561 | Fixed | Xu_SECS et al. (2009) | 1.42 (1.04–1.94) | 22.11 | 0.47 | 0 | 0.01 | 1.24 (1.07–1.45) | 0.004969 | 0.88 |
| O’Mara_ANECS et al. (2011) | 1.24 (1.02–1.51) | 59.13 | ||||||||
| O’Mara_LES et al. (2011) | 1.05 (0.73–1.51) | 18.76 |
| RSID | Model | Study | OR (95% CI) | Weight (%) | Cochran’s Q | I2 | T2 | Pooled OR (95% CI) | p-Value | Egger’s p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| rs1052133 | Fixed | Krupa et al. (2011) | 0.96 (0.06–16.25) | 0.93 | 0.1 | 45.58 | 0.17 | 1.65 (1.3–2.1) | 0.000035 | 0.45 |
| Cincin et al. (2012) | 0.65 (0.13–3.32) | 3.70 | ||||||||
| Smolarz et al. (2018) | 1.93 (1.46–2.55) | 67.50 | ||||||||
| Sobczuk et al. (2012) | 3.03 (0.75–12.16) | 2.33 | ||||||||
| Romanowicz-Makowska et al. (2011) | 1.86 (0.65–5.32) | 4.99 | ||||||||
| Hosono et al. (2013) | 0.75 (0.39–1.42) | 20.55 | ||||||||
| rs9344 | Fixed | Kang et al. (2005) | 3.08 (1.34–7.05) | 22.64 | 0.21 | 35.07 | 0.08 | 1.98 (1.28–3.06) | 0.002235 | NA |
| Ashton et al. (2008) | 1.66 (0.99–2.78) | 77.36 | ||||||||
| rs10046 | Fixed | Paynter et al. (2005) | 1.55 (0.99–2.43) | 38.47 | 0.87 | 0 | 0 | 1.5 (1.14–1.99) | 0.004126 | NA |
| Lundin et al. (2012) | 1.48 (1.03–2.11) | 61.53 | ||||||||
| rs1799889 | Fixed | Gilabert-Estellés et al. (2012) | 2.2 (1.24–3.92) | 53.79 | 0.95 | 0 | 0 | 2.23 (1.46–3.42) | 0.000231 | NA |
| Su et al. (2011) | 2.26 (1.2–4.26) | 46.21 | ||||||||
| rs4775936 | Fixed | Paynter et al. (2005) | 1.61 (1.05–2.49) | 40.12 | 0.95 | 0 | 0 | 1.6 (1.22– 2.1) | 0.000806 | NA |
| Lundin et al. (2012) | 1.59 (1.11–2.26) | 59.88 | ||||||||
| rs11224561 | Fixed | Xu_SECS et al. (2009) | 1.48 (1.08–2.01) | 70.92 | 0.63 | 0 | 0.02 | 1.55 (1.2–2.01) | 0.000828 | 0.2 |
| O’Mara_ANECS et al. (2011) | 1.59 (0.93–2.7) | 23.77 | ||||||||
| O’Mara_LES et al. (2011) | 2.38 (0.94–6.04) | 5.31 | ||||||||
| rs1801320 | Random | Krupa et al. (2011) | 25.33 (4.48–143.32) | 12.49 | 0 | 83.01 | 0.48 | 7.44 (2.16–25.61) | 0.014058 | 0.19 |
| Michalska et al. (2014) | 3.72 (2.77–5) | 31.26 | ||||||||
| Romanowicz-Makowska et al. (2012) | 5.41 (3.22–9.09) | 28.57 | ||||||||
| Smolarz et al. (2011) | 13 (7.27–23.24) | 27.69 | ||||||||
| rs2279744 | Fixed | Walsh et al. (2007) | 2.29 (0.89–5.89) | 2.15 | 0.23 | 23.41 | 0.07 | 1.43 (1.23–1.66) | 0.000004 | 0.41 |
| Terry_NHS et al. (2008) | 1.6 (1.17–2.18) | 22.32 | ||||||||
| Ashton et al. (2009) | 1.29 (0.73–2.26) | 7.87 | ||||||||
| Ueda et al. (2009) | 1.36 (0.62–2.98) | 3.97 | ||||||||
| Nunobiki et al. (2009) | 1.27 (0.55–2.93) | 3.6 | ||||||||
| Knappskog_Haukeland et al. (2012) | 1.07 (0.77–1.49) | 24.95 | ||||||||
| Knappskog_MoMaTEC et al. (2012) | 1.36 (1–1.85) | 24.84 | ||||||||
| Zajac et al. (2012) | 3.5 (1.73–7.08) | 2.87 | ||||||||
| Yoneda et al. (2013) | 1.76 (0.93–3.3) | 5.36 | ||||||||
| Okamoto et al. (2015) | 0.95 (0.29–3.12) | 2.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.P.; Chaudhary, N.; Tyagi, S.; Agarwal, S.M. Meta-Analysis of 49 SNPs Covering 25,446 Cases and 41,106 Controls Identifies Polymorphisms in Hormone Regulation and DNA Repair Genes Associated with Increased Endometrial Cancer Risk. Genes 2023, 14, 741. https://doi.org/10.3390/genes14030741
Das AP, Chaudhary N, Tyagi S, Agarwal SM. Meta-Analysis of 49 SNPs Covering 25,446 Cases and 41,106 Controls Identifies Polymorphisms in Hormone Regulation and DNA Repair Genes Associated with Increased Endometrial Cancer Risk. Genes. 2023; 14(3):741. https://doi.org/10.3390/genes14030741
Chicago/Turabian StyleDas, Agneesh Pratim, Nisha Chaudhary, Shrishty Tyagi, and Subhash M. Agarwal. 2023. "Meta-Analysis of 49 SNPs Covering 25,446 Cases and 41,106 Controls Identifies Polymorphisms in Hormone Regulation and DNA Repair Genes Associated with Increased Endometrial Cancer Risk" Genes 14, no. 3: 741. https://doi.org/10.3390/genes14030741




