Expression of INPP5D Isoforms in Human Brain: Impact of Alzheimer’s Disease Neuropathology and Genetics
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA and RNA Extraction from Human Brain Tissue
2.2. PCR Amplification and Quantitation
2.3. Allelic Expression Imbalance
2.4. INPP5D Isoform Stability
2.5. Statistics
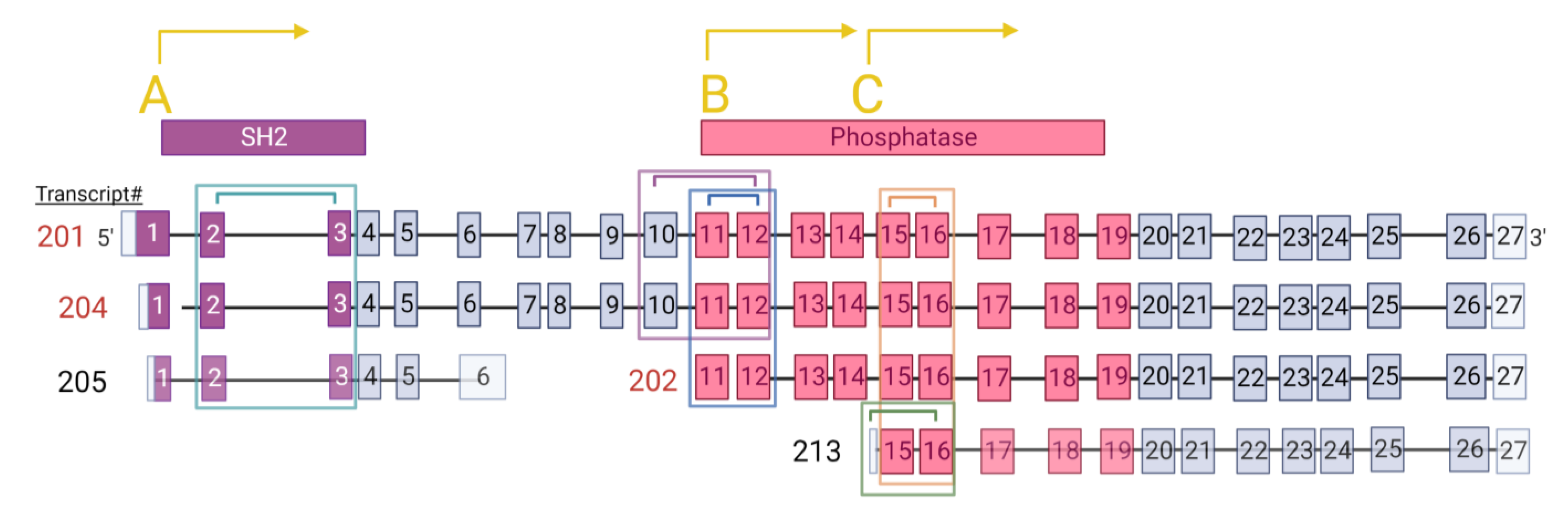
3. Results
3.1. Quantitation of INPP5D Isoforms as a Function of AD Neuropathology and Genetics
3.2. Identification of Novel INPP5D Isoforms and Quantitation with AD Neuropathology
3.3. Cycloheximide Assay to Quantify Variants Undergoing Nonsense-Mediated Decay
3.4. Allelic Expression Imbalance for SNP-Associated Effects on Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Data Accreditation Statement
References
- Gatz, M.; Reynolds, C.A.; Fratiglioni, L.; Johansson, B.; Mortimer, J.A.; Berg, S.; Fiske, A.; Pedersen, N.L. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 2006, 63, 168–174. [Google Scholar] [CrossRef]
- Karch, C.M.; Jeng, A.T.; Nowotny, P.; Cady, J.; Cruchaga, C.; Goate, A.M. Expression of novel Alzheimer’s disease risk genes in control and Alzheimer’s disease brains. PLoS ONE 2012, 7, e50976. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Yamazaki, K.; Ozaki, Y.; Sao, T.; Yoshida, T.; Mori, T.; Mori, Y.; Ochi, S.; Iga, J.I.; Ueno, S.I. INPP5D mRNA Expression and Cognitive Decline in Japanese Alzheimer’s Disease Subjects. J. Alzheimers Dis. 2017, 58, 687–694. [Google Scholar] [CrossRef]
- Wightman, D.P.; Jansen, I.E.; Savage, J.E.; Shadrin, A.A.; Bahrami, S.; Holland, D.; Rongve, A.; Borte, S.; Winsvold, B.S.; Drange, O.K.; et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 2021, 53, 1276–1282. [Google Scholar] [CrossRef]
- Lin, P.B.; Tsai, A.P.; Soni, D.; Lee-Gosselin, A.; Moutinho, M.; Puntambekar, S.S.; Landreth, G.E.; Lamb, B.T.; Oblak, A.L. INPP5D deficiency attenuates amyloid pathology in a mouse model of Alzheimer’s disease. Alzheimers Dement. 2022, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; An, X.; Han, Y.; Ma, R.; Yang, K.; Zhang, L.; Chi, J.; Li, W.; Llobet -Navas, D.; Xu, Y.; et al. Overexpression of JARID1B promotes differentiation via SHIP1/AKT signaling in human hypopharyngeal squamous cell carcinoma. Cell Death Dis. 2016, 7, e2358. [Google Scholar] [CrossRef]
- Malik, M.; Parikh, I.; Vasquez, J.B.; Smith, C.; Tai, L.; Bu, G.; La Du, M.J.; Fardo, D.W.; Rebeck, G.W.; Estus, S. Genetics ignite focus on microglial inflammation in Alzheimer’s disease. Mol. Neurodegener. 2015, 10, 52. [Google Scholar] [CrossRef]
- Efthymiou, A.G.; Goate, A.M. Late onset Alzheimer’s disease genetics im plicates microglial pathways in disease risk. Mol. Neurodegener. 2017, 12, 43. [Google Scholar] [CrossRef]
- Song, W.M.; Colonna, M. The identity and function of microglia in neurodegeneration. Nat. Immunol. 2018, 19, 1048–1058. [Google Scholar] [CrossRef]
- Song, W.M.; Colonna, M. The Microglial Response to Neurodegenerative Disease. Adv. Immunol. 2018, 139, 1–50. [Google Scholar] [CrossRef]
- Sayed, F.A.; Kodama, L.; Fan, L.; Carling, G.K.; Udeochu, J.C.; Le, D.; Li, Q.; Zhou, L.; Wong, M.Y.; Horowitz, R.; et al. AD—linked R47H-TREM2 mutation induces disease-enhancing microglial states via AKT hyperactivation. Sci. Transl. Med. 2021, 13, eabe3947. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.B.; Janelidze, S.; Strandberg, O.; Whelan, C.D.; Zetterberg, H.; Blennow, K.; Palmqvist, S.; Stomrud, E.; Mattsson-Carlgren, N.; Hansson, O. Microglial activation protects against accumulation of tau aggregates in nondemented individuals with underlying Alzheimer’s disease pathology. Nat. Aging 2022, 2, 1138–1144. [Google Scholar] [CrossRef]
- Jain, N.; Lewis, C.A.; Ulrich, J.D.; Holtzman, D.M. Chronic TREM2 activation exacerbates Abeta-associated tau seeding and spreading. J. Exp. Med. 2023, 220. [Google Scholar] [CrossRef]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hagg, S.; Athanasiu, L.; et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019, 51, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; DeStafano, A.L.; Bis, J.C.; Beecham, G.W.; Grenier-Boley, B.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef]
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef]
- Peng, Q.; Malhotra, S.; Torchia, J.A.; Kerr, W.G.; Coggeshall, K.M.; Humphrey, M.B. TREM2—and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci. Signal 2010, 3, ra38. [Google Scholar] [CrossRef]
- Li, C.; Zhao, B.; Lin, C.; Gong, Z.; An, X. TREM2 inhibits inflammatory responses in mouse microglia by suppressing the PI3K/NF-kappaB signaling. Cell Biol. Int. 2019, 43, 360–372. [Google Scholar] [CrossRef]
- Mondal, S.; Subramanian, K.K.; Sakai, J.; Bajrami, B.; Luo, H.R. Phosphoinositide lipid phosphatase SHIP1 and PTEN coordinate to regulate cell migration and adhesion. Mol. Biol. Cell 2012, 23, 1219–1230. [Google Scholar] [CrossRef]
- Wang, S.; Sudan, R.; Peng, V.; Zhou, Y.; Du, S.; Yuede, C.M.; Lei, T.; Hou, J.; Cai, Z.; Cella, M.; et al. TREM2 drives microglia response to amyloid-beta via SYK-dependent and -independent pathways. Cell 2022, 185, 4153–4169. [Google Scholar] [CrossRef]
- Iguchi, A.; Takatori, S.; Kimura, S.; Hori, Y.; Sasaki, J.; Saito, T.; Saido, T.C.; Ikezu, T.; Takai, T.; Sasaki, T.; et al. INPP5D Modulates TREM2 Loss-of-Function Phenotypes in a Mouse Model of Alzheimer Disease. Neuron 2021, 61, 3844721. [Google Scholar] [CrossRef]
- Castranio, E.L.; Hasel, P.; Haure-Mirande, J.V.; Ramirez Jimenez, A.V.; Hamilton, B.W.; Kim, R.D.; Glabe, C.G.; Wang, M.; Zhang, B.; Gandy, S.; et al. Microgli.al INPP5D limits plaque formation and glial reactivity in the PSAPP mouse model of Alzheimer’s disease. Alzheimers Dement. 2022, 1–14. [Google Scholar] [CrossRef]
- Machiela, M.J.; Chanock, S.J. LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015, 31, 3555–3557. [Google Scholar] [CrossRef] [PubMed]
- Grear, K.E.; Ling, I.F.; Simpson, J.F.; Furman, J.L.; Simmons, C.R.; Peterson, S.L.; Schmitt, F.A.; Markesbery, W.R.; Liu, Q.; Crook, J.E.; et al. Expression of SORL1 and a novel SORL1 splice variant in normal and Alzheimers disease brain. Mol. Neurodegener. 2009, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Chiles, J., 3rd; Xi, H.S.; Medway, C.; Simpson, J.; Potluri, S.; Howard, D.; Liang, Y.; Paumi, C.M.; Mukherjee, S.; et al. Genetics of CD33 in Alzheimer’s disease and acute myeloid leukemia. Hum. Mol. Genet. 2015, 24, 3557–3570. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.C.; Katsumata, Y.; Simpson, J.F.; Fardo, D.W.; Estus, S. Analysis of Genetic Variants Associated with Levels of Immune Modulating Proteins for Impact on Alzheimer’s Disease Risk Reveal a Potential Role for SIGLEC14. Genes 2021, 12, 1008. [Google Scholar] [CrossRef]
- Shaw, B.C.; Snider, H.C.; Turner, A.K.; Zajac, D.J.; Simpson, J.F.; Estus, S. An Alternatively Spliced TREM2 Isoform Lacking the Ligand Binding Domain is Expressed in Human Brain. J. Alzheimers Dis. 2022, 87, 1647–1657. [Google Scholar] [CrossRef]
- Pievani, M.; Rasser, P.E.; Galluzzi, S.; Benussi, L.; Ghidoni, R.; Sabattoli, F.; Bonetti, M.; Binetti, G.; Thompson, P.M.; Frisoni, G.B. Mapping the effect of APOE epsilon4 on gray matter loss in Alzheimer’s disease In Vivo. Neuroimage 2009, 45, 1090–1098. [Google Scholar] [CrossRef]
- Tan, R.H.; Pok, K.; Wong, S.; Brooks, D.; Halliday, G.M.; Kril, J.J. The pathogenesis of cingulate atrophy in behavioral vari ant frontotemporal dementia and Alzheimer’s disease. Acta Neuropathol. Commun. 2013, 1, 30. [Google Scholar] [CrossRef]
- Nelson, P.T.; Braak, H.; Markesbery, W.R. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009, 68, 1–14. [Google Scholar] [CrossRef]
- Malik, M.; Simpson, J.F.; Parikh, I.; Wilfred, B.R.; Fardo, D.W.; Nelson, P.T.; Estus, S. CD33 Alzheimer’s risk -altering polymorphism, CD33 expression, and exon 2 splicing. J. Neurosci. 2013, 33, 13320–13325. [Google Scholar] [CrossRef] [PubMed]
- Parikh, I.; Fardo, D.W.; Estus, S. Genetics of PICALM expression and Alzheimer’s disease. PLoS ONE 2014, 9, e91242. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.K.; Shaw, B.C.; Simpson, J.F.; Estus, S. Identification and Quantitation of Novel ABI3 Isoforms Relative to Alzheimer’s Disease Genetics and Neuropathology. Genes 2022, 13, 1607. [Google Scholar] [CrossRef]
- Parikh, I.; Medway, C.; Younkin, S.; Fardo, D.W.; Estus, S. An intronic PICALM polymorphism, rs588076, is associated with allelic expression of a PICALM isoform. Mol. Neurodegener. 2014, 9, 32. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Isolation of DNA fragments from polyacrylamide gels by the crush and soak method. CSH Protoc. 2006, 2006, 1–6. [Google Scholar] [CrossRef]
- Tsai, A.P.; Lin, P.B.; Dong, C.; Moutinho, M.; Casali, B.T.; Liu, Y.; Lamb, B.T.; Landreth, G.E.; Oblak, A.L.; Nho, K. INPP5D expression is associated with risk for Alzheimer’s disease and induced by plaque-associated microglia. Neurobiol. Dis. 2021, 153, 105303. [Google Scholar] [CrossRef]
- Consortium, G.T. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290. [Google Scholar] [CrossRef]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas -Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subs et associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef]
- Huang, K.L.; Marcora, E.; Pimenova, A.A.; Di Narzo, A.F.; Kapoor, M.; Jin, S.C.; Harari, O.; Bertelsen, S.; Fairfax, B.P.; Czajkowski, J.; et al. A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat. Neurosci. 2017, 20, 1052–1061. [Google Scholar] [CrossRef]
- He, L.; Loika, Y.; Kulminski, A.M. Allele-specific analysis reveals exon- and cell-type-specific regulatory effects of Alzheimer’s disease-associated genetic variants. Transl. Psychiatry 2022, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Meilandt, W.J.; Xie, L.; Gandham, V.D.; Ngu, H.; Barck, K.H.; Rezzonico, M.G.; Imperio, J.; Lalehzadeh, G.; Huntley, M.A.; et al. Trem2 restrains the enhancement of tau accumulation and neurodegeneration by beta -amyloid pathology. Neuron 2021, 109, 1283–1301. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Song, W.M.; Huang, S.C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A.; et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017, 170, 649–663. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Y.; Grajales-Reyes, G.; Colonna, M. TREM2 dependent and independent functions of microglia in Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 84. [Google Scholar] [CrossRef]
- Leyns, C.E.G.; Ulrich, J.D.; Finn, M.B.; Stewart, F.R.; Koscal, L.J.; Remolina Serrano, J.; Robinson, G.O.; Anderson, E.; Colonna, M.; Holtzman, D.M. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. USA 2017, 114, 11524–11529. [Google Scholar] [CrossRef] [PubMed]
- Gratuze, M.; Leyns, C.E.; Sauerbeck, A.D.; St -Pierre, M.K.; Xiong, M.; Kim, N.; Serrano, J.R.; Tremblay, M.E.; Kummer, T.T.; Colonna, M.; et al. Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J. Clin. Invest 2020, 130, 4954–4968. [Google Scholar] [CrossRef]








| rs35349669 | |||
|---|---|---|---|
| rs10933431 | TT | TC | CC |
| GG | 0 | 2 | 2 |
| GC | 1 | 15 | 7 |
| CC | 10 | 19 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajac, D.J.; Simpson, J.; Zhang, E.; Parikh, I.; Estus, S. Expression of INPP5D Isoforms in Human Brain: Impact of Alzheimer’s Disease Neuropathology and Genetics. Genes 2023, 14, 763. https://doi.org/10.3390/genes14030763
Zajac DJ, Simpson J, Zhang E, Parikh I, Estus S. Expression of INPP5D Isoforms in Human Brain: Impact of Alzheimer’s Disease Neuropathology and Genetics. Genes. 2023; 14(3):763. https://doi.org/10.3390/genes14030763
Chicago/Turabian StyleZajac, Diana J., James Simpson, Eric Zhang, Ishita Parikh, and Steven Estus. 2023. "Expression of INPP5D Isoforms in Human Brain: Impact of Alzheimer’s Disease Neuropathology and Genetics" Genes 14, no. 3: 763. https://doi.org/10.3390/genes14030763
APA StyleZajac, D. J., Simpson, J., Zhang, E., Parikh, I., & Estus, S. (2023). Expression of INPP5D Isoforms in Human Brain: Impact of Alzheimer’s Disease Neuropathology and Genetics. Genes, 14(3), 763. https://doi.org/10.3390/genes14030763





