Abstract
Telomere shortening or loss of shelterin components activates DNA damage response (DDR) pathways, leading to a replicative senescence that is usually coupled with a senescence-associated secretory phenotype (SASP). Recent studies suggested that telomere aberration that activates DDR may occur, irrespective of telomere length or loss of shelterin complex. The blind mole-rat (Spalax) is a subterranean rodent with exceptional longevity, and its cells demonstrate an uncoupling of senescence and SASP inflammatory components. Herein, we evaluated Spalax relative telomere length, telomerase activity, and shelterin expression, along with telomere-associated DNA damage foci (TAFs) levels with cell passage. We show that telomeres shorten in Spalax fibroblasts similar to the process in rats, and that the telomerase activity is lower. Moreover, we found lower DNA damage foci at the telomeres and a decline in the mRNA expression of two shelterin proteins, known as ATM/ATR repressors. Although additional studies are required for understanding the underling mechanism, our present results imply that Spalax genome protection strategies include effective telomere maintenance, preventing early cellular senescence induced by persistent DDR, thereby contributing to its longevity and healthy aging.
1. Introduction
Significant efforts have been made towards the understanding of telomeres’ role in cellular senescence, aging, and age-associated diseases [1,2,3,4]. Telomeres are repetitive DNA sequences coated by the shelterin complex and located at the tips of eukaryotic chromosomes. Together, they shield the exposed ends and thereby, the genes located at the subtelomeric region, providing chromosomal stability by preventing their recognition as double strand breaks (DSB) and the activation of the DNA damage response (DDR) [5]. Telomeres can bypass the “end-replication” complexity caused by the incapability of the conventional polymerases to fully replicate the lagging DNA strand by recruiting a reverse transcriptase, known as telomerase, or by alternative mechanisms [6,7,8,9].
Since telomerase expression is restricted in most somatic cells, telomeric DNA progressively shortens with cell division [10]. As telomeres become shorter, they lose the ability to recruit sufficient shelterin components, which results in the exposure to DDR machinery, eventually leading to cellular senescence [11]. Recent studies have suggested that telomere aberrations triggering DDR can occur, irrespective of telomere length or the loss of the shelterin components (reviewed in [12]), indicating additional mechanisms inducing early senescence. Indeed, we have recently demonstrated shortening of telomeres in the blind mole-rat, of the genus Nannospalax (hereafter, Spalax), despite its long lifespan [13] and healthy aging processes [14], indicating a crucial role of telomeres’ integrity maintenance, rather than its elongation.
The senescent phenotype is usually accompanied by the senescence-associated secretory phenotype (SASP) that entails pro-inflammatory cytokines, as well as growth factors and extracellular matrix degrading proteins [15,16,17,18]. The chronic presence of senescent cells and a persistent SASP causes local and systemic inflammation that contributes to the development of age-associated diseases, which reinforce the role of cellular senescence in both aging and cancer. Nonetheless, senescence is associated with beneficial aspects, including tissue repair and wound healing, as well as embryonic development (reviewed in [19]). Hence, cell senescence is thought to contribute to keeping organisms relatively free from cancer in early life, but to possibly promote aging and age-associated pathologies in an antagonistic-pleiotropic manner later in life.
Throughout millions of years of underground evolution, Spalax has evolved superior survival mechanisms under hypoxia, which eventually promote cancer resistance and healthy aging [20,21,22]. Strikingly, the uncoupling of senescence and the SASP inflammatory response was recently discovered in Spalax [14]. Spalax is a solitary rodent that exhibits exceptional longevity (~20 years [23]), despite its small body size. Subterranean rodents naturally face great challenges due to repeated exposure to acute hypoxia and to internally-produced DNA-damaging substances that are released during hypoxia-reoxygenation cycles [24], yet Spalax seem to avoid such deleterious effects by efficient DNA repair mechanisms that protect cells from damage and subsequent oncogenic processes [25]. Notwithstanding these findings, limited data is available about Spalax telomere maintenance and telomerase activity and their contribution to cellular senescence in this wild animal. We have recently demonstrated that telomeres shorten with age in different Spalax tissues, similar to the process observed in short lived animals [13]; however, a brain transcriptome study showed that genes involved in telomere maintenance and associated with cellular resistance to DNA damage and telomere length regulation, such as MRE11A, RNASEH, and TELO2, were upregulated [26].
Here, we hypothesize that DNA integrity maintenance, including telomere regions, rather than telomere length maintenance, is a strategy that evolved in Spalax to support its exceptional healthy aging and longevity. To test this hypothesis, we employed primary fibroblast isolated from Spalax and laboratory rats in a cell culture model and measured telomere length, telomerase activity, telomere associated damage, and the expression of the shelterin complex mRNA.
2. Materials and Methods
2.1. Cell Culture
Blind mole-rat and laboratory rat (Rattus norvegicus) primary cultured fibroblast cells were tested. Cells were used for the evaluation of telomere length, telomerase activity, shelterin complex gene expression, and immuno-fluorescence analyses. Cells were isolated from three different newborn individuals (males and females, at an estimated age of 2–3 days), as described elsewhere [27]. The cell samples are listed in Supplementary Table S1.
The primary cell isolation protocol was approved by the Institutional Ethics Committee of the University of Haifa (reference #671/19).
The fibroblasts were grown in DMEM–F12 medium (supplemented with 10% FBS, L-glutamine (2 mM), and penicillin–streptomycin (100 U/mL, 0.1 mg/mL, respectively)) in a standard CO2 incubator. Growth media and supplements were purchased from Biological Industries (Beit HaEmek, Israel). In this study, we used young (second passage, P2), or senescent (fifth passage, P5) cells. Passage was recorded when a confluent culture was sub-cultured into 3 plates of the same area. Senescence was determined when the cells acquired an enlarged, flattened morphology and showed positive staining for senescence-associated β-galactosidase (Supplementary Figure S1). Other senescence markers (e.g., p21, p53, cell population doubling) were well described in previous studies of primary fibroblasts [14,25].
2.2. DNA Extraction
DNA was extracted from Spalax and rat primary cells using a High Pure PCR Template Preparation Kit (Roche, Penzberg, Germany), following the manufacturer’s protocol. All DNA samples were tested for purity and integrity using a Nanophotometer NP80 (Implen, Munchen, Germany).
2.3. Telomere Measurement
The average relative telomere length in primary cells was measured using qPCR (quantitative polymerase chain reaction) on a LightCycler 480 II (Roche, Penzberg, Germany), as previously described [28,29], and modified for use in Spalax and rats. The relative telomere length (T/S) of the samples was calculated as the ratio of the telomere concentration (T) to the single copy gene (S), relative to the reference sample standard curve. The erythropoietin (EPO) gene was used as a single copy gene for Spalax [30] and rats. The primers are listed in Supplementary Table S2. Both reactions for telomeres and single copy genes were run on the same plate in duplicates for each sample, along with a negative control of water. All reactions used 10 ng of DNA in a final volume of 20 µL containing 10 µL SYBER green Master Mix, 2 µL primers, 6 µL water, and 2 µL DNA sample. The reaction components and the LightCycler program are detailed in the Supplementary Tables S3 and S4.
2.4. RNA Extraction and Real-Time Quantitative PCR
RNA was extracted from cultured cells by using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA), following the manufacturer’s instructions. The RNA samples were quantified on a Nanodrop® spectrophotometer, and quality was assessed on the Agilent Bioanalyzer. RNA samples were treated with DNase I (DNA-free, Ambion, Austin, TX, USA), and 1 µg was taken for first-strand cDNA synthesis (iScript, Bio-Rad, Hercules, CA, USA) in a 20 μL volume. Aliquots of 1 μL of cDNA were used for each real-time PCR reaction. Species-specific primers were designed for each shelterin component transcript and telomerase using Primer3 software (Applied BioSystems, Austin, TX, USA), based on the published sequences (Supplementary Tables S5 and S6). Relative quantification of gene transcription was performed by using Fast SYBR Green (Applied BioSystems) and 1 µL of cDNA generated from 50 ng total RNA. Serial dilutions of the cDNA with the highest expression level for each target gene were used to build a relative standard curve and to test the amplification efficiency for each experiment. Samples were tested in triplicate. The amplification parameters were as follows: 95 °C for 5 min, followed by 45 cycles of 95 °C for 10 s, 60 °C for 10 s, and 75 ° C for 10 s. To verify a single product with a fixed melting temperature, the melting curve protocol was applied. The quantification relied on equal amounts of total RNA used in each sample, and the reliability of this method was tested and confirmed by cyclophilin and actin housekeeping genes (HKG) for Spalax and rats. The data are presented as relative gene expression values normalized to total RNA.
2.5. Telomerase Activity
Telomerase activity was measured in cultured cells, according to the telomeric repeat amplification protocol (TRAP) method [31], a sensitive and specific PCR-based functional enzyme assay. The assay was performed using the TeloTAGGG telomerase PCR ELISA PLUS (Roche Diagnostics GmbH, Singapore), following the manufacturer’s instructions.
Telomerase activity was measured according to the telomeric repeat amplification protocol (TRAP).
Briefly, each pellet from Spalax and rat primary cells was homogenized in 200 μL of lysis reagent. After a 30 min incubation on ice, the lysate was centrifuged at 16,000× g for 30 min at 4 °C, and the supernatant was aliquoted. Control templates, which contain positive telomerase template DNA with the same sequence as a telomerase product with eight telomeric repeats, were included in the kit. The PCR-based analysis was carried out in a 50 μL reaction mixture containing 3 μL cell extract. An internal standard was amplified by telomeric substrate primers in order to avoid false negative results. Telomerase positive results were confirmed by repeated TRAP assay, with heat pretreatment of cellular lysates (at 85 °C for 15 min) to monitor for false positive results. Lysis buffer reagent controls were included in each reaction to monitor for the possibility of reagent contamination. Positivity was detected when the substrate turned blue and then yellow upon the addition of the stop reagent, with the color conversion maximizing the sensitivity of the readings. Sample absorbance was measured within 30 min using a spectrophotometer at 450 nm against a blank (reference wavelength at 650 nm). Samples were considered as positive when the difference between the absorbance of the sample and the absorbance of the negative control was higher than the two-fold background activity. Telomerase activity was expressed as a ratio by comparing the signal of the sample to the signal of the high positive control template containing 0.1 μmol/μL DNA.
2.6. Immunofluorescence
Fibroblasts from late passages from both Spalax and rats were seeded in 6-well plates on glass coverslips at ~40 k cells/well and incubated overnight. The cells were then fixed with cold methanol for 10 min (−20 °C), permeabilized with 0.2% Triton X-100™ in PBS for 15 min, and blocked for one hour with 5% BSA solution in PBS. Staining: coverslips were incubated with the first primary antibody (anti γ-H2A.X, Abcam, Cambridge, UK) for 2 h at room temperature (diluted 1:700 in the staining blocking solution containing 0.05% triton and 1% BSA), washed three times, and incubated with the secondary antibody (goat anti-rabbit IgG Alexa fluor® 488, Abcam, Cambridge, UK) in the dark for 1 h at room temperature (diluted 1:300 in the staining blocking solution), and washed three times with washing buffer containing 0.05% triton. The coverslips were then post-fixed with cold methanol for 10 min, air-dried, and stained with anti-TRF2 conjugated with Alexa fluor® 594 (Novus Biologicals, CO, USA) at a concentration 1:200 in staining blocking solution and incubated in the dark overnight (4 °C). After a final washing, the coverslips were inverted onto slides containing Vectashield mounting medium and DAPI. The cells’ nuclei were visualized under a fluorescent microscope (Leica DMi8, equipped with Leica DFC365FX camera) and counted using Foci Counter software.
2.7. Statistical Analysis
All statistical analyses were performed by using JMP14 (SAS Institute Inc., Cary, NC, USA) and GraphPad Prism8 (San Diego, CA, USA). The correlation between rTL and age was calculated using Pearson’s correlation test. The lines on the graphs represent simple linear regression adjusted for age. Statistical comparisons were made using the Mann–Whitney U test for two groups.
3. Results
3.1. Relative Telomere Length and Telomerase Activity in Fibroblast Cells
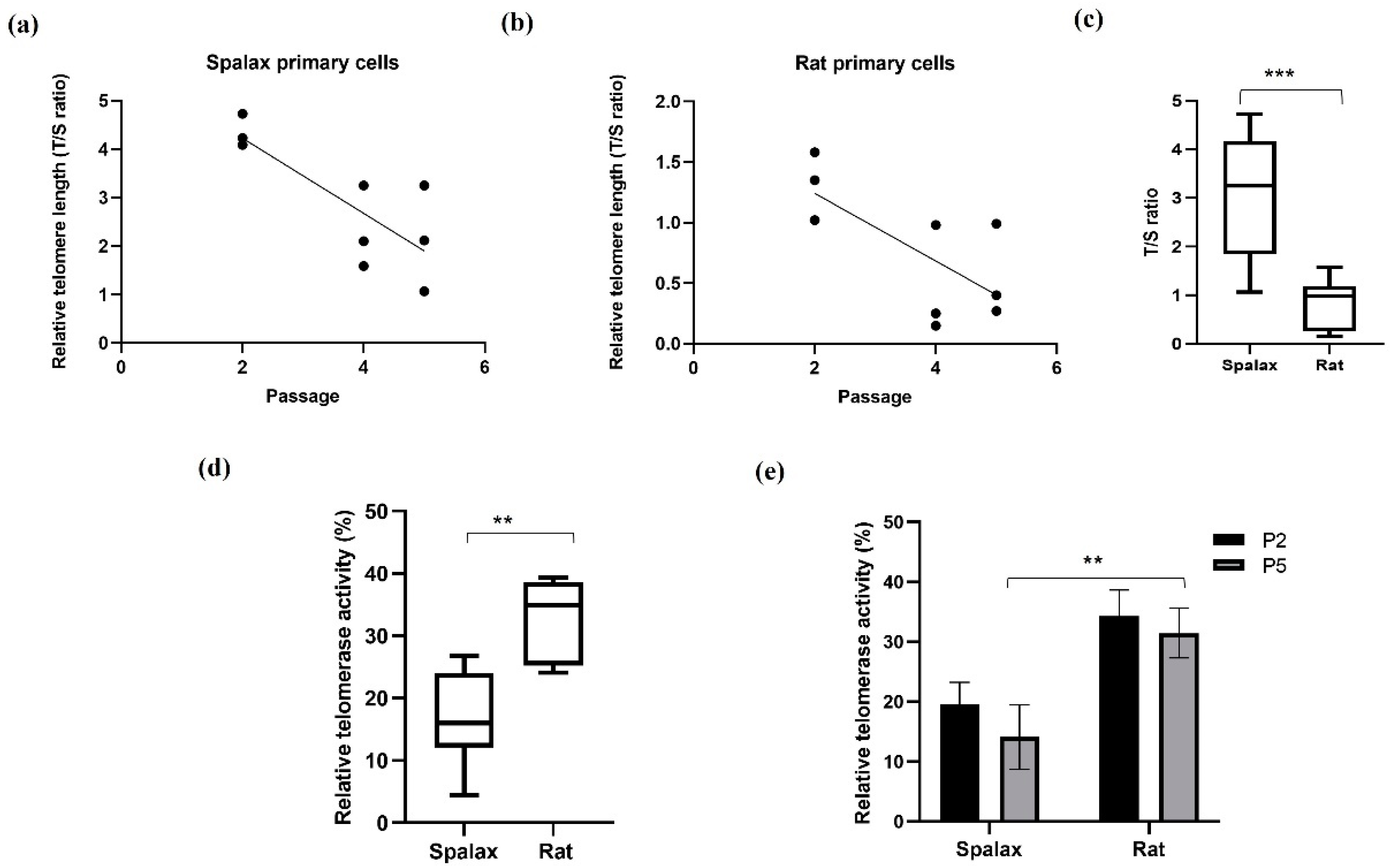
We first analyzed the relative telomere length (rTL) and relative telomerase activity (rTA) in Spalax and rat fibroblasts from different passages (where the fifth passage demonstrated the senescence phenotype in both species). rTL in Spalax fibroblasts decreased with the cell passages (slope = −0.7750, F1,7 = 12.6, p < 0.01, R2 = 0.6429) (Figure 1a), similar to the results observed in short-lived rats (slope = −0.2793, F1,7 = 6.969, p < 0.05, R2 = 0.4989) (Figure 1b), and in line with our previous in vivo study [13]. Nonetheless, rTL was significantly higher in Spalax fibroblasts compared with those of rats (p < 0.0001) (Figure 1c), and both species showed a similar rTL decrease rate. We next tested telomerase activity (%) in lysates from primary fibroblasts. Overall rTA in Spalax was lower than in rats (Figure 1d), with no significant differences in the rTA between passage 2 and passage 5 (terminal passage for the primary cells) of the same species (Figure 1e). These results indicate a Spalax-specific strategy of long telomeres combined with inhibited telomerase activity.
Figure 1.
Relative telomere length (telomere to single copy gene (T/S) ratio) and relative telomerase activity in Spalax and rats’ primary fibroblasts. (a) Relative telomere length (rTL) as a function of passages in Spalax primary fibroblasts (slope = −0.7750, F1,7 = 12.6, p < 0.01, R2 = 0.6429); (b) relative telomere length (rTL) as a function of passages in rat primary fibroblast cells (slope = −0.2793, F1,7 = 6.969, p < 0.05, R2 = 0.4989) (Pearson’s); (c) range of rTL between Spalax and rat fibroblasts (boxplots represent data from a,b); (d) range of relative telomerase activity (rTA) between Spalax and rat fibroblasts (both passages); (e) rTA in Spalax and rat primary fibroblast cells with cell passage (n = 3, cells from three different individuals of Spalax and rats). ** p < 0.01 and *** p < 0.001 (Mann–Whitney U test).
3.2. Telomere Associated DNA Damage Foci (TAFs)
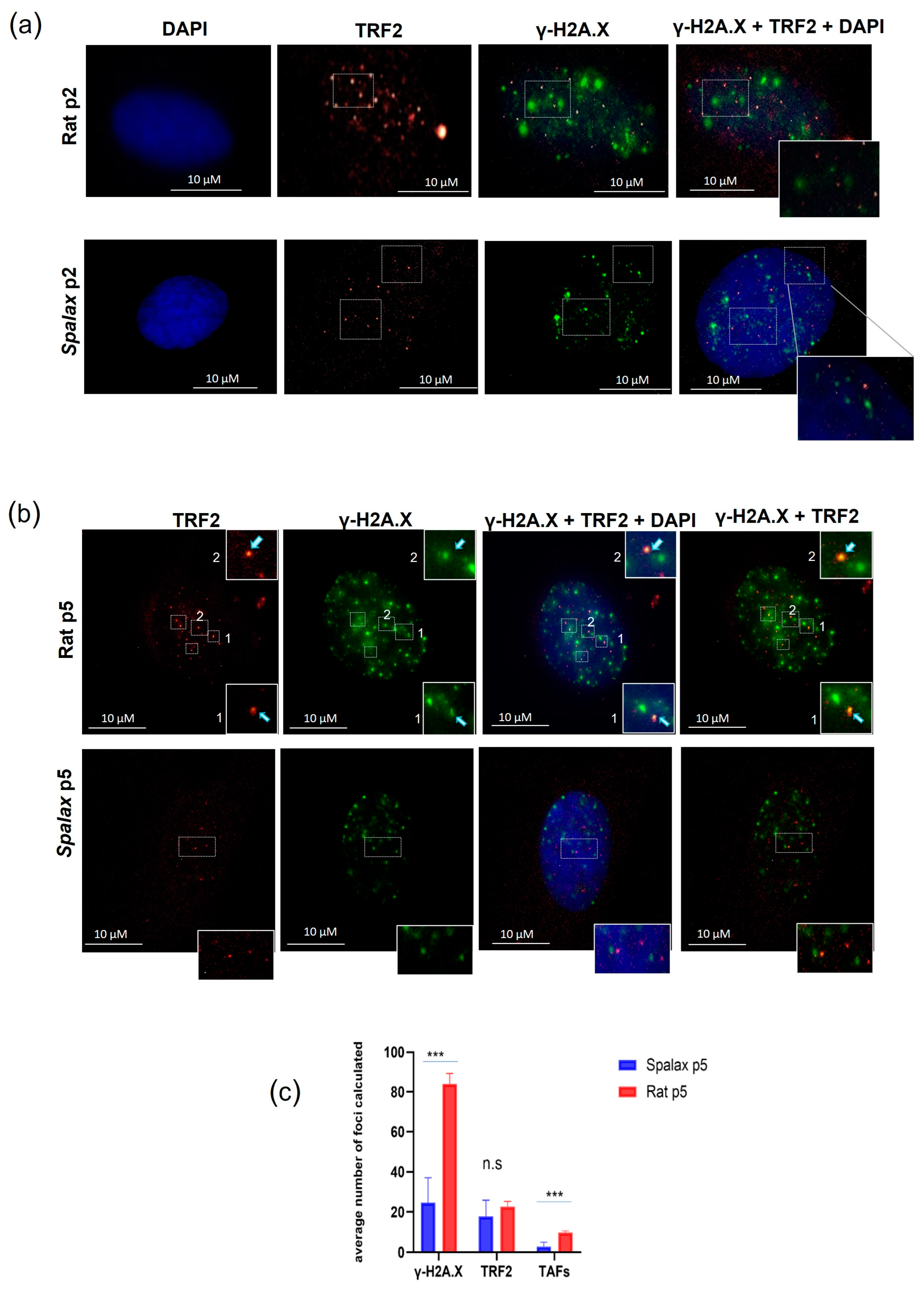
Telomere associated foci (TAFs) are DNA damaged foci occurring in telomeres and can be induced as a result of the telomere shortening during replicative senescence or by the disruption of the shelterin complex. The uncapped telomeres recruit DDR factors, such as γ-H2A.X, and initiate signaling cascade, eventually leading to replicative senescence [32]. However, new studies show that damage in telomeres can occur in a length-independent manner [33]. Here, we investigated the accumulation of TAFs in senescent Spalax and rat fibroblasts by co-immunostaining for the DNA damage marker γ-H2A.X and the telomeric protein TRF2 (Figure 2). Our results are in line with previously reported higher general DNA damage in rat cells [14]. Indeed, senescent Spalax fibroblasts exhibited significantly less DNA damage in general than did those of the rats (Supplementary Figure S2) and specifically harbored less damage at the telomeric site (Figure 2a,b). While the average number of foci counted for the telomere marker did not differ significantly between rat and Spalax senescent fibroblasts (p = 0.1706) (Figure 2c), the average number counted for the colocalized markers γ-H2A.X and TRF2 was significantly higher in rats (p < 0.0001), indicating telomere-associated DNA damage. Conversely, the colocalization of TRF2 and γ-H2A.X among the nuclei of the Spalax senescent fibroblasts was almost absent.
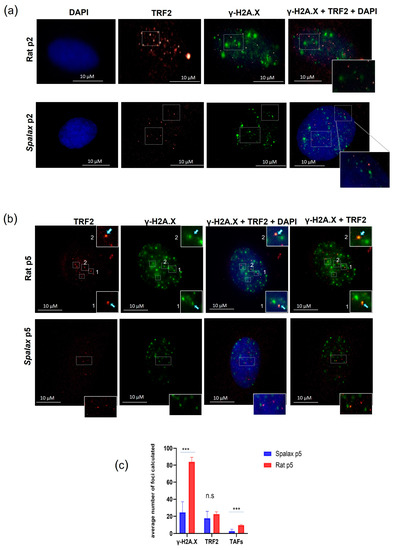
Figure 2.
TAFs formation in Spalax and rat cells. Immunostaining for TRF2 (red) and γ-H2A.X (green) cell nuclei were counterstained with DAPI (blue) for early-passage cells (a) and late-passage, senescent cells (b). Examples of double stained foci are marked with rectangles. (c) The average number of γ-H2A.X, TRF2 and TAFs (co-localized) foci counted in 20 randomly chosen nuclei with γ-H2A.X positive foci using FociCounter. *** p < 0.001 (Mann–Whitney U-test).
3.3. Shelterin Core Components’ mRNA Expression Dynamics in Primary Fibroblast Cells
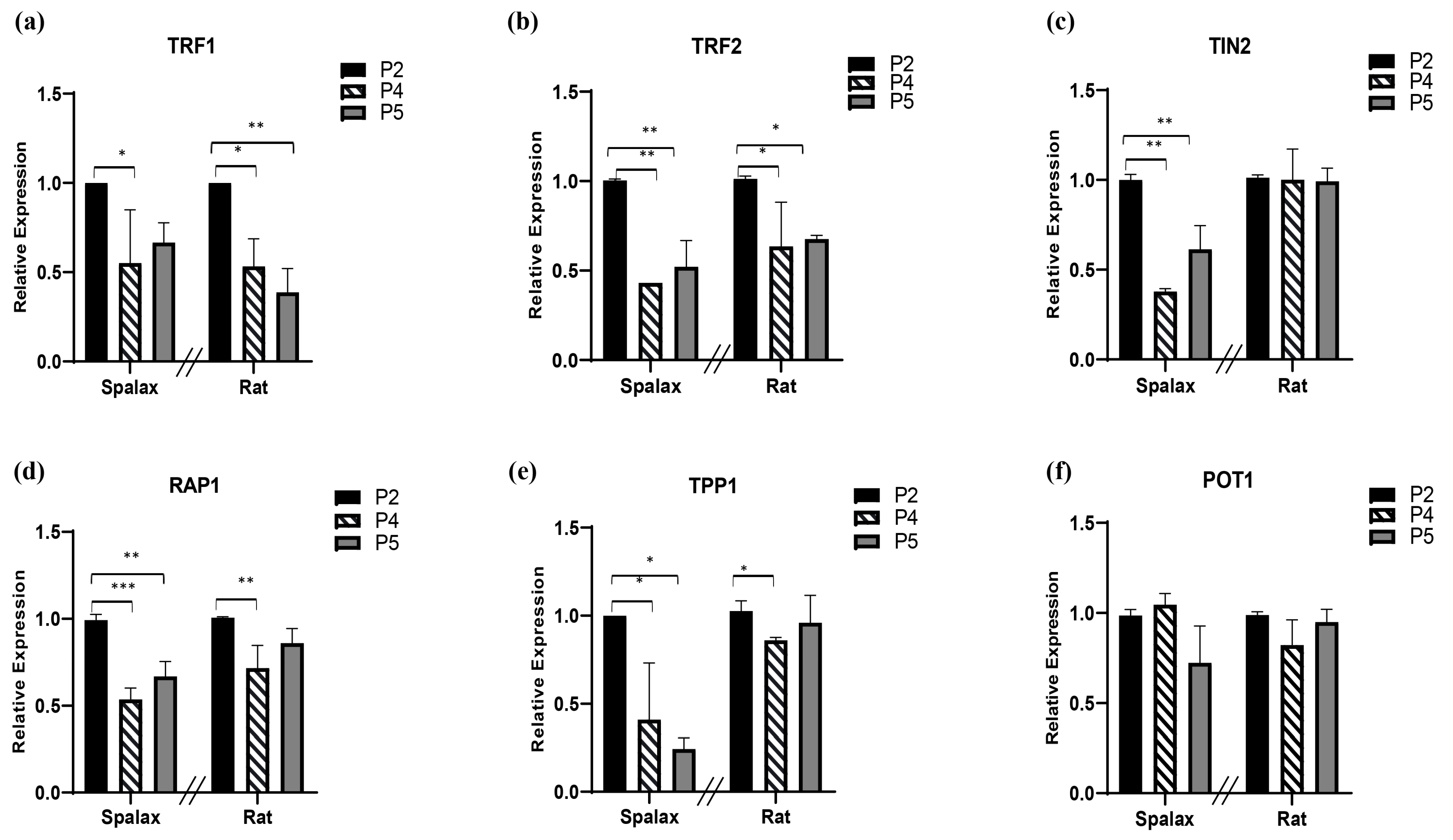
Shelterin is essential for protecting telomeres from undesirable attack and binding of DNA damage response factors. The shelterin core proteins are listed in Table 1. Since loss of one component of this protection complex has detrimental effects on telomere function and stability [34], we next examined the relative RNA expression of these factors in vitro (Figure 3). TRF1 relative transcription declined with passages in both Spalax and rat cells (Figure 3a). The relative expression of both TRF2 and repressor/activator protein 1 RAP1 also decreased with passages in both animals, with more pronounced decline of RAP1 in Spalax cells (Figure 3b,d). TRF1 interacting nuclear protein 2 (TIN2) and TIN2-interacting protein-1 (TPP1) demonstrated a pronounced passage-dependent decline in transcription, mainly in Spalax. TIN2 showed stable transcription in rats along their life span, while TPP1 demonstrated a moderate decline in passage 4 rat cells; however, the expression level increased back to control levels (Figure 3c,e). Yet, protection of telomeres 1 (POT1) relative expression levels remained steady in both animals in all passages (Figure 3f). These results might indicate that the changes in telomere length and shelterin expression show similarities between the long-lived Spalax and the short-lived rat.

Table 1.
Shelterin components and function.
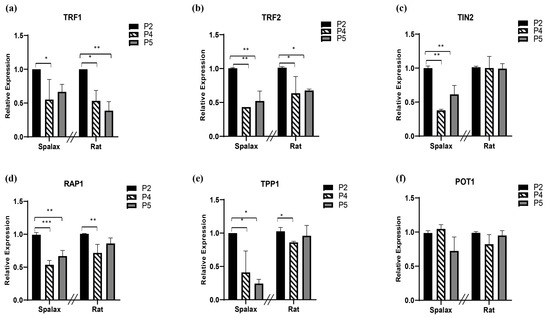
Figure 3.
Levels of relative transcription of shelterin complex core components (a–f) in primary fibroblast cells of Spalax and rats. The mRNA expression rates were quantified in three different passages; passage 2, 4, and 5 (P2, P4 and P5) by using qRT–PCR. Relative transcription levels are presented as mean ± SD of three independent experiments (n = 3, cells from three different individuals of Spalax and rats). * p < 0.05, ** p < 0.01, and *** p < 0.001 (Mann–Whitney U test). Note that the relative standard curve was built for each species separately; therefore, data points of each species can only be compared to its control (1.0). The differences between P4 and P5 were insignificant.
4. Discussion
In this study, we examined the average telomere length and telomerase activity, as well as the formation of telomere associated foci (TAFs) and the mRNA expression levels of the shelterin components in cultured primary cells of Spalax, a long-lived, hypoxia-tolerant, and cancer-resistant rodent. We showed that with cell passages, Spalax fibroblasts demonstrated significant shortening in telomere length, similar to rat cells, and in line with the processes observed earlier in tissues [13]. We also demonstrated that the average telomere length in Spalax fibroblasts was significantly higher than the average length in rats, similar to previously reported results in Spalax muscles [13]. Long telomeres are controversially described in the literature by their association with cancer risk, aging, or longevity. Extremely long telomeres in mice were reported to produce beneficial metabolic effects, low cancer risks, and longevity [35]. Whether the long, seemingly guarded telomeres are one of the driving forces in Spalax longevity and healthy aging remains unclear. It may be speculated that longer telomeres are attributed to telomerase overexpression, which presumably prolongs cell survival; however, we found that Spalax fibroblast telomerase activity was, in fact, lower than that of its counterpart in rats, which further supports our hypothesis that integrity maintenance of the telomeres, rather than telomere elongation, is characteristic of Spalax cells as a strategy that contributes to its long lifespan and supports its unique mode of cellular senescence. It was suggested that long-lived animals have adopted a mechanism [36] whereby the pace of telomere attrition and the activity of the telomerase is the same as that in other, short-lived animals. However, since initially, Spalax exhibits longer and potentially safeguarded telomeres, it seems tempting to speculate that the time it takes to reach critical length/damage that ignites the senescence machinery is longer and therefore, may contribute to their profoundly unique mode of replicative senescence lacking canonical inflammatory response known to accompany the senescent phenotype in all studied species. Therefore, replicative senescence in Spalax cells is seemingly not a direct consequence of telomere attrition and persistent DDR, but is rather determined by other intrinsic, underexplored mechanisms. Another tempting question is whether the downregulation of telomerase activity observed in Spalax cells plays a critical role in its cancer resistance.
Rapid and efficient DNA repair capacity was previously reported in Spalax cells in general [25], including low levels of double strand DNA break marker γ-H2A.X in Spalax senescent cells [14]. In accordance with these reports, here, we show lower telomeric DNA damage manifested as the extremely lower co-immunostaining of γ-H2A.X and telomeric marker TRF2 (Figure 2). This was not surprising, since less damage was shown in senescing Spalax cells [14], along with a higher expression of DNA repair genes [26] and a higher repair capacity of the cells [25]. Another aspect that contributes to genomic integrity in Spalax cells is the extreme abundance of the reduced glutathione antioxidant in Spalax cells under normoxia and hypoxia [37,38]. Taken together, efficient DNA repair and an enhanced antioxidant system may contribute to telomere integrity, thereby allowing the unique mode of senescence in Spalax cells.
We next addressed the complexes responsible for maintaining telomere integrity at the mRNA level (Table 1 and Figure 3) in order to test whether they are expressed differently in Spalax. Many studies have demonstrated that compromised shelterin function has deleterious consequences for the cell, and that telomeres have to bind sufficient amounts of shelterin in order to fully suppress DNA damage signaling and repair [11,39,40]. For instance, studies conducted on mice demonstrated that deletion of each shelterin component, with the exception of RAP1, leads to embryonic lethality (reviewed in [41]). In addition, several studies suggest that shelterin might modulate telomerase activity at the chromosome ends, mainly by acting as a negative regulator [42,43]. To our knowledge, this is the first study to examine shelterin expression levels with cell passages in Spalax. Cell passage-dependent changes in shelterin expression were similar in both Spalax and rat fibroblasts, apart from that of TIN2 and TPP1 (partially) commonly found bonded together, that decreased their levels with cell division in Spalax. Although there was a mild decrease in TPP1 transcription in the rat middle passage (passage 4), a further increase in passage 5 still indicates differences in the senescence phenotype between the two species.
TPP1 is known to elicit a robust damage response at the telomere ends upon deletion, and it is usually required for the recruitment of telomerase, acting as a positive regulator of telomere maintenance [44]. Yet, it may also be involved in telomerase negative regulation, together with POT1, but the precise molecular mechanism of this aspect is still not clear [45]. TIN2 is also a negative regulator of telomere length, where overexpression of TIN2 was found to inhibit telomere elongation [46]. More importantly, TIN2 binds TPP1 and is required for TPP1/POT1′s recruitment of the shelterin complex [47]. The TPP1/POT1 complex requires a connection to the TIN2/TRF1/TRF2 complex in order to repress the ATR DNA damage response. Another TIN2 function is to promote the repression of ATM signaling by TRF2. The fact that the expression levels of these two ATM/ATR repressors decreased with cell division in Spalax may partially support the enhanced activity of DNA repair in Spalax [25]. However, this suggestion requires verification and further studies, since high ATM/ATR activity requires better protection of the telomere ends; furthermore, one of the limitations of this study is that the mRNA expression data presented here may not necessarily represent protein levels; moreover, specific commercial antibodies for Spalax as a non-model organism are not always available, and if so, the identical specificity to the compared species is not guaranteed. This issue might be a subject for future studies.
In summary, our results support that Spalax have evolved strategies for genome protection that apparently include telomere maintenance machinery, together contributing to its longevity and healthy aging. These strategies include a unique mode of senescence not induced by persistent DDR or telomere attrition, but which rather seems to be an independent cell program driven by other types of ‘clocks’. The precise mechanisms of telomere maintenance and the apparently ‘non-canonical senescence clock’ require further investigation in Spalax and other long-lived species as possible requisites for long lifespan and healthy aging.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14040845/s1, Figure S1: Senescence was determined when the cells acquired an enlarged, flattened morphology and showed positive staining for senescence-associated β-galactosidase; Figure S2: Levels of DNA damage in Spalax and rat fibroblasts; Table S1: List of animals and their ages; Table S2: List of primers for telomere length assay; Table S3: Reaction components; Table S4: PCR program; Table S5: List of primers for Spalax shelterin complex; Table S6: List of primers for rat shelterin complex.
Author Contributions
Research, H.A.S.; data analysis, H.A.S.; project administration, I.M., G.A. and I.S.; resources, L.S., D.M.H., G.A. and I.S.; research design and supervision, I.M., G.A. and I.S.; writing, H.A.S.; review and editing of manuscript, D.M.H., G.A. and I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Israel Science Foundation (ISF), personal grant (193/16, to G.A.), and the Israel Science Foundation (grant No. 1935/17, to I.S.).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of University of Haifa (protocol code 671/19 09/12/19).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available in the Methods section and the Supplementary Material of this article.
Acknowledgments
We thank Amani Odeh and Sagie Schif-Zuck for providing technical support. We also thank Yehuda Tzfati’s lab for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, Y.; Liu, X.; Ding, X.; Wang, F.; Geng, X. Telomere and its role in the aging pathways: Telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 2018, 20, 1–16. [Google Scholar] [CrossRef]
- Birch, J.; Barnes, P.J.; Passos, J.F. Mitochondria, telomeres and cell senescence: Implications for lung ageing and disease. Pharmacol. Ther. 2017, 183, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Nabetani, A.; Ishikawa, F. Alternative lengthening of telomeres pathway: Recombination-mediated telomere maintenance mechanism in human cells. J. Biochem. 2010, 149, 5–14. [Google Scholar] [CrossRef]
- Cesare, A.J.; Reddel, R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010, 11, 319–330. [Google Scholar] [CrossRef]
- Lingner, J.; Cooper, J.P.; Cech, T.R. Telomerase and DNA end replication: No longer a lagging strand problem? Science 1995, 269, 1533–1534. [Google Scholar] [CrossRef]
- Greider, C.W. Telomerase is processive. Mol. Cell. Biol. 1991, 11, 4572–4580. [Google Scholar]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef]
- Bandaria, J.N.; Qin, P.; Berk, V.; Chu, S.; Yildiz, A. Shelterin Protects Chromosome Ends by Compacting Telomeric Chromatin. Cell 2016, 164, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence—Size Matters Not. Ebiomedicine 2017, 21, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Adwan Shekhidem, H.; Sharvit, L.; Leman, E.; Manov, I.; Roichman, A.; Holtze, S.; Huffman, D.M.; Cohen, H.Y.; Bernd Hildebrandt, T.; Shams, I.; et al. Telomeres and Longevity: A Cause or an Effect? Int. J. Mol. Sci. 2019, 20, 3233. [Google Scholar] [CrossRef]
- Odeh, A.; Dronina, M.; Domankevich, V.; Shams, I.; Manov, I. Downregulation of the inflammatory network in senescent fibroblasts and aging tissues of the long-lived and cancer-resistant subterranean wild rodent, Spalax. Aging Cell 2019, 19, e13045. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.-Y.; Campisi, J. Tumor Suppressor and Aging Biomarker p16INK4a Induces Cellular Senescence without the Associated Inflammatory Secretory Phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef]
- Ohtani, N.; Brennan, P.; Gaubatz, S.; Sanij, E.; Hertzog, P.; Wolvetang, E.; Ghysdael, J.; Rowe, M.; Hara, E. Epstein-Barr virus LMP1 blocks p16INK4a-RB pathway by promoting nuclear export of E2F4/5. J. Cell Biol. 2003, 162, 173–183. [Google Scholar] [CrossRef]
- Itahana, K.; Dimri, G.; Campisi, J. Regulation of cellular senescence by p53. JBIC J. Biol. Inorg. Chem. 2001, 268, 2784–2791. [Google Scholar] [CrossRef]
- Rhinn, M.; Ritschka, B.; Keyes, W.M. Cellular senescence in development, regeneration and disease. Development 2019, 146, dev151837. [Google Scholar] [CrossRef]
- Schulke, S.; Dreidax, D.; Malik, A.; Burmester, T.; Nevo, E.; Band, M.; Avivi, A.; Hankeln, T. Living with stress: Regulation of antioxidant defense genes in the subterranean, hypoxia-tolerant mole rat, Spalax. Gene 2012, 500, 199–206. [Google Scholar] [CrossRef]
- Avivi, A.; Gerlach, F.; Joel, A.; Reuss, S.; Burmester, T.; Nevo, E.; Hankeln, T. Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax. Proc. Natl. Acad. Sci. USA 2010, 107, 21570–21575. [Google Scholar] [CrossRef] [PubMed]
- Shams, I.; Avivi, A.; Nevo, E. Oxygen and carbon dioxide fluctuations in burrows of subterranean blind mole rats indicate tolerance to hypoxic–hypercapnic stresses. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 142, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Tacutu, R.; Thornton, D.; Johnson, E.; Budovsky, A.; Barardo, D.; Craig, T.; Diana, E.; Lehmann, G.; Toren, D.; Wang, J.; et al. Human Ageing Genomic Resources: New and updated databases. Nucleic Acids Res. 2017, 46, D1083–D1090. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M.; Moreira, D.C.; Rivera-Ingraham, G.A.; Giraud-Billoud, M.; Genaro-Mattos, T.C.; Campos, G. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic. Biol. Med. 2015, 89, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Domankevich, V.; Eddini, H.; Odeh, A.; Shams, I. Resistance to DNA damage and enhanced DNA repair capacity in the hypoxia-tolerant blind mole rat Spalax carmeli. J. Exp. Biol. 2018, 221 Pt 8, jeb174540. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Domankevich, V.; Lijuan, H.; Xiaodong, F.; Korol, A.; Avivi, A.; Shams, I. Genome maintenance and bioenergetics of the long-lived hypoxia-tolerant and cancer-resistant blind mole rat, Spalax: A cross-species analysis of brain transcriptome. Sci. Rep. 2016, 6, 38624. [Google Scholar] [CrossRef]
- Manov, I.; Hirsh, M.; Iancu, T.C.; Malik, A.; Sotnichenko, N.; Band, M.; Avivi, A.; Shams, I. Pronounced cancer resistance in a subterranean rodent, the blind mole-rat, Spalax: In vivo and in vitroevidence. BMC Biol. 2013, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, M.D.; Budagov, T.; Atzmon, G. Telomere length and telomerase activity; a Yin and Yang of cell senescence. J. Vis. Exp. 2013, e50246. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- Shams, I.; Avivi, A.; Nevo, E.; Ivanitskaya, E. Assignment of erythropoietin (EPO) to blind subterranean mole rat chromosome 1q by in situ hybridization. Cytogenet. Genome Res. 2004, 108, 362D. [Google Scholar] [CrossRef]
- Mender, I.; Shay, J.W. Telomerase Repeated Amplification Protocol (TRAP). Bio-Protocol 2015, 5, e1657. [Google Scholar] [CrossRef]
- Takai, H.; Smogorzewska, A.; de Lange, T. DNA Damage Foci at Dysfunctional Telomeres. Curr. Biol. 2003, 13, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.; Jurk, D.; Marques, F.D.; Correia-Melo, C.; Hardy, T.; Gackowska, A.; Anderson, R.; Taschuk, M.; Mann, J.; Passos, J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012, 3, 708. [Google Scholar] [CrossRef]
- Hohensinner, P.J.; Goronzy, J.J.; Weyand, C.M. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2011, 2, 524–537. [Google Scholar] [PubMed]
- Muñoz-Lorente, M.A.; Cano-Martin, A.C.; Blasco, M.A. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 2019, 10, 4723. [Google Scholar] [CrossRef] [PubMed]
- Gutman, D.; Sharvit, L.; Atzmon, G. In Possible Mechanisms for Telomere Length Maintenance in Extremely Old People. Hered. Genet. 2014, 3, e11. [Google Scholar]
- Miskevich, D.; Chaban, A.; Dronina, M.; Abramovich, I.; Gottlieb, E.; Shams, I. Comprehensive Analysis of (13)C6 Glucose Fate in the Hypoxia-Tolerant Blind Mole Rat Skin Fibroblasts. Metabolites 2021, 11, 734. [Google Scholar] [CrossRef]
- Miskevich, D.; Chaban, A.; Dronina, M.; Abramovich, I.; Gottlieb, E.; Shams, I. Glutamine Homeostasis and Its Role in the Adaptive Strategies of the Blind Mole Rat, Spalax. Metabolites 2021, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Diotti, R.; Loayza, D. Shelterin complex and associated factors at human telomeres. Nucleus 2011, 2, 119–135. [Google Scholar] [CrossRef]
- Schmutz, I.; de Lange, T. Shelterin. Curr. Biol. 2016, 26, R397–R399. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Blasco, M.A. Role of shelterin in cancer and aging. Aging Cell 2010, 9, 653–666. [Google Scholar] [CrossRef]
- Loayza, D.; De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 2003, 423, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Smogorzewska, A.; van Steensel, B.; Bianchi, A.; Oelmann, S.; Schaefer, M.R.; Schnapp, G.; de Lange, T. Control of Human Telomere Length by TRF1 and TRF2. Mol. Cell. Biol. 2000, 20, 1659–1668. [Google Scholar] [CrossRef]
- Zemp, I.; Lingner, J. The Shelterin Component TPP1 Is a Binding Partner and Substrate for the Deubiquitinating Enzyme USP7. J. Biol. Chem. 2014, 289, 28595–28606. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Kiselar, J.; Whited, T.L.; Hernandez-Sanchez, W.; Taylor, D.J. POT1-TPP1 differentially regulates telomerase via POT1 His266 and as a function of single-stranded telomere DNA length. Proc. Natl. Acad. Sci. USA 2019, 116, 23527–23533. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.J.; Kim, S.-H.; Tessarollo, L.; Campisi, J.; Hodes, R.J. Telomere-Associated Protein TIN2 Is Essential for Early Embryonic Development through a Telomerase-Independent Pathway. Mol. Cell. Biol. 2004, 24, 6631–6634. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.K.; Kibe, T.; Donigian, J.R.; Frescas, D.; de Lange, T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol. Cell 2011, 44, 647–659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).