Genomic Regions Associated with Milk Composition and Fertility Traits in Spring-Calved Dairy Cows in New Zealand
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Phenotypic Traits
2.3. Descriptive Statistics
2.4. Genotypes and Quality Control
2.5. Genome-Wide Association Analyses
2.6. Candidate Genes and Functional Analysis
3. Results
3.1. Descriptive Statistics
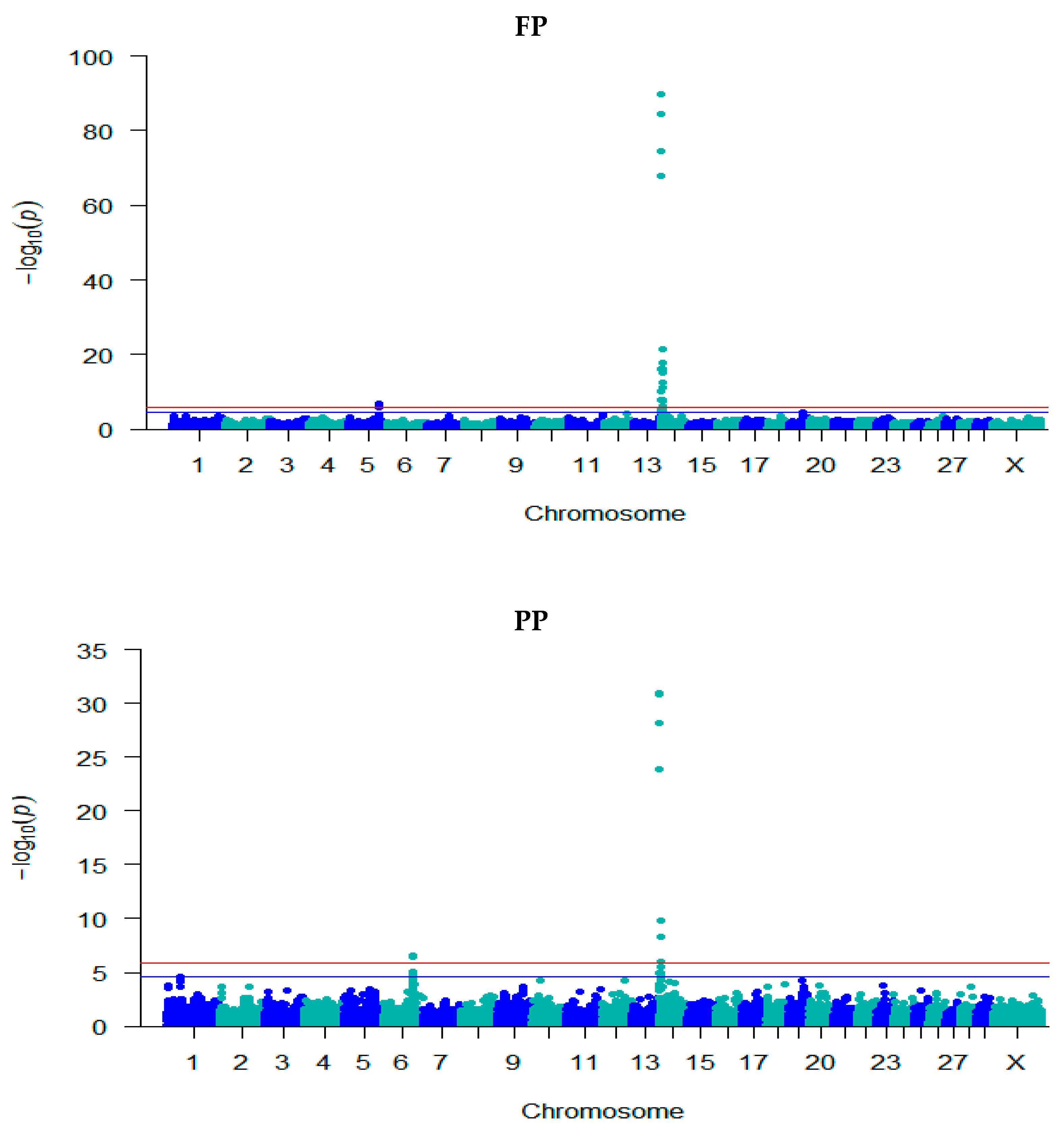
3.2. GWAS for Milk Composition Traits
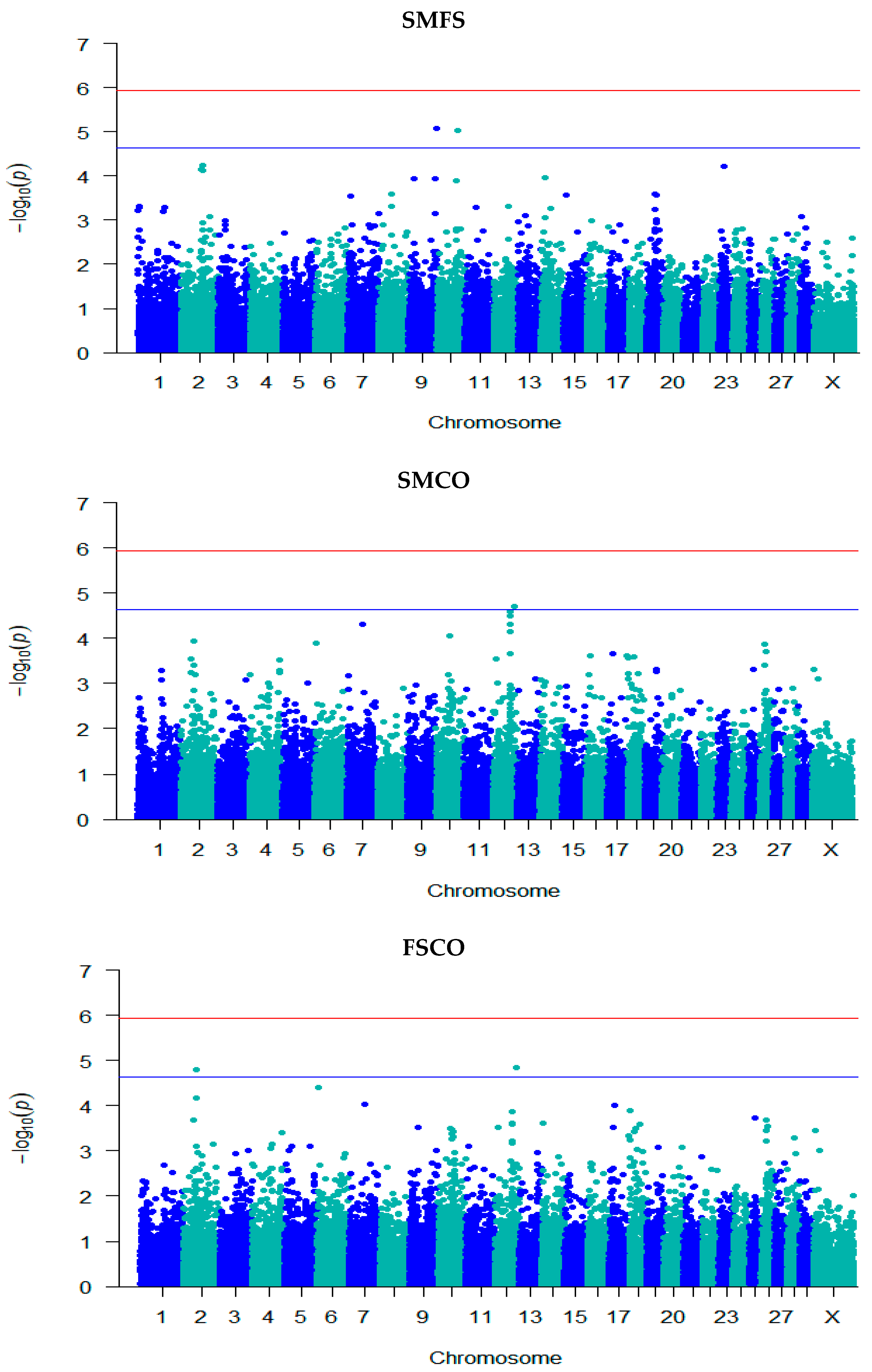
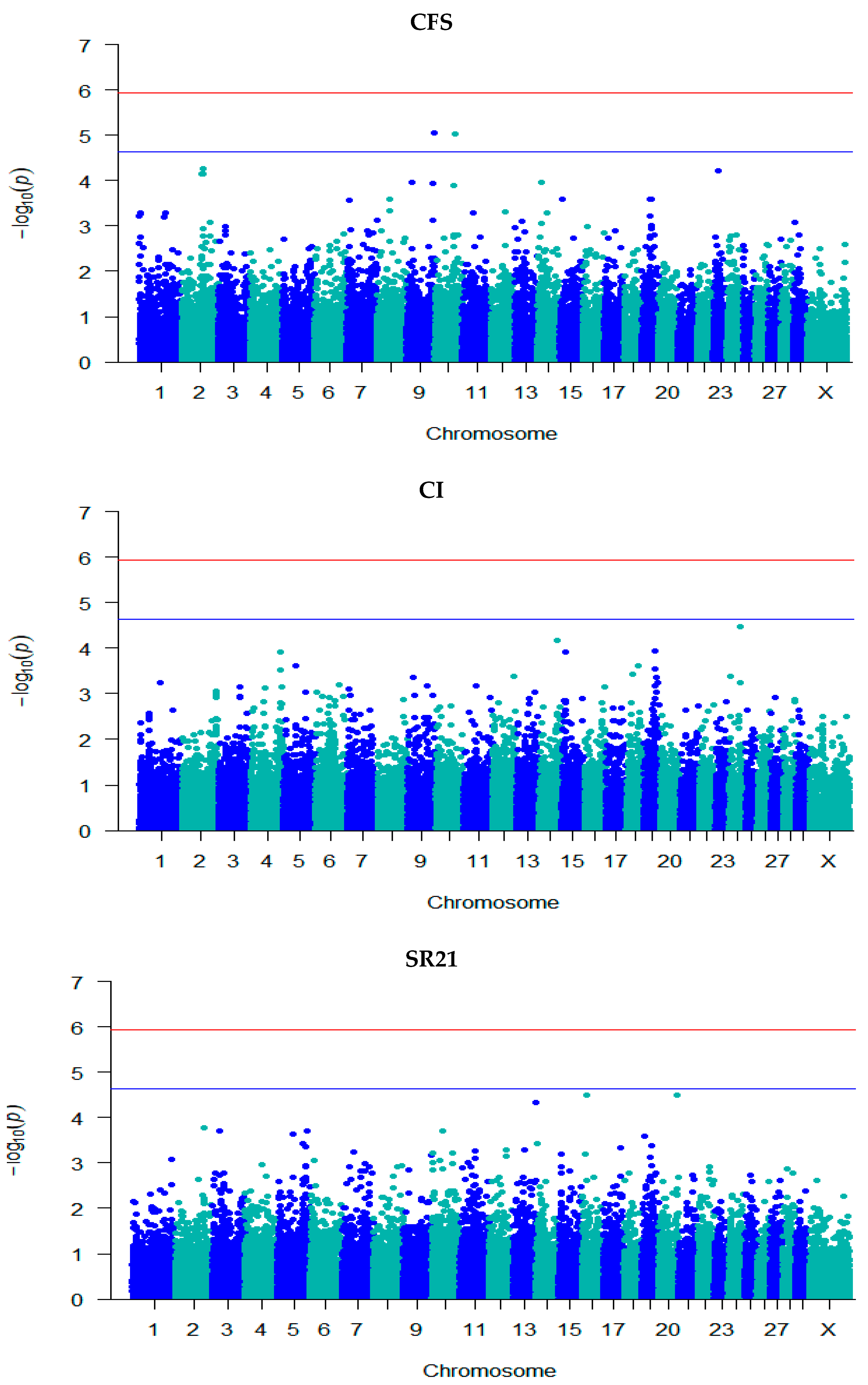
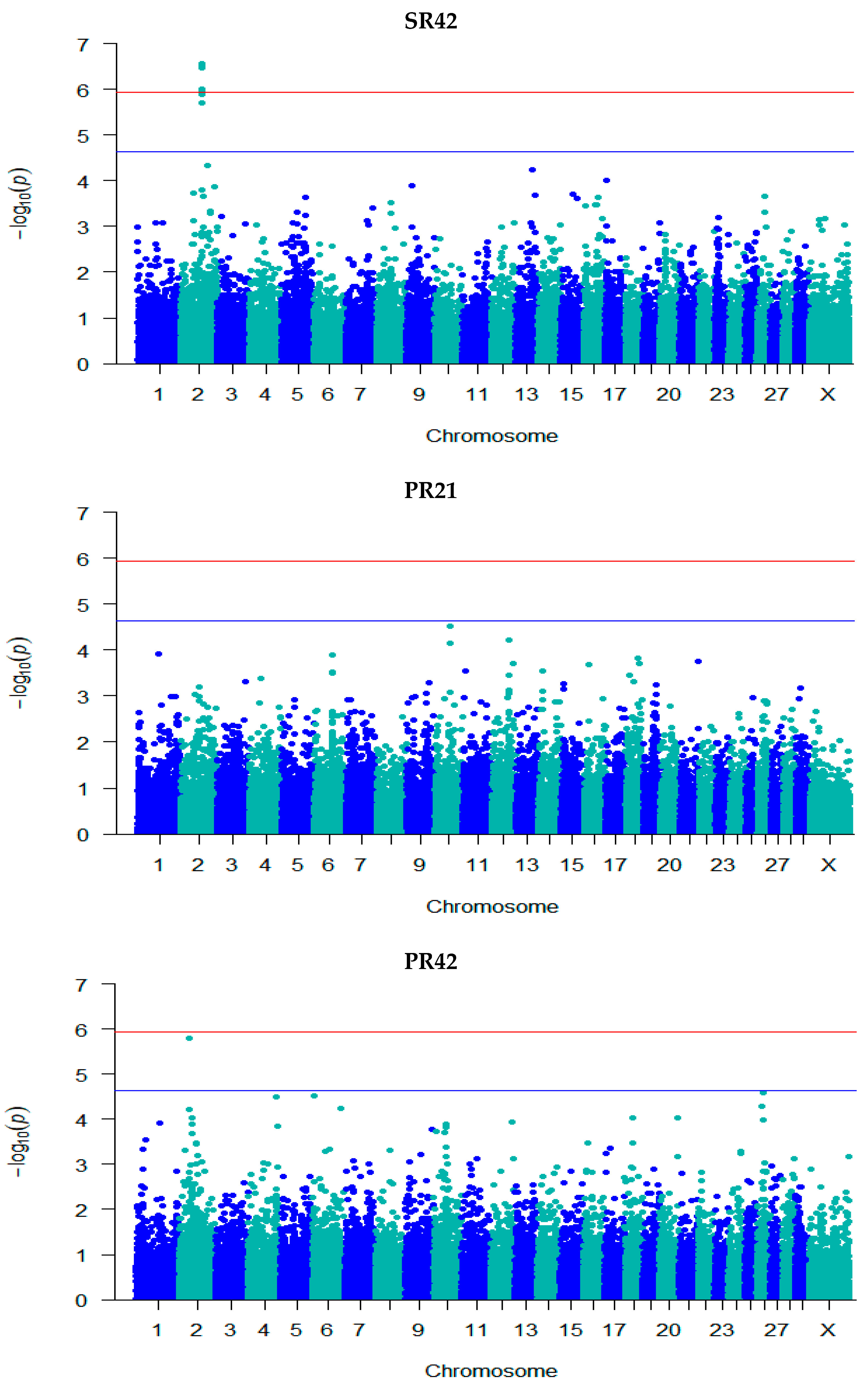
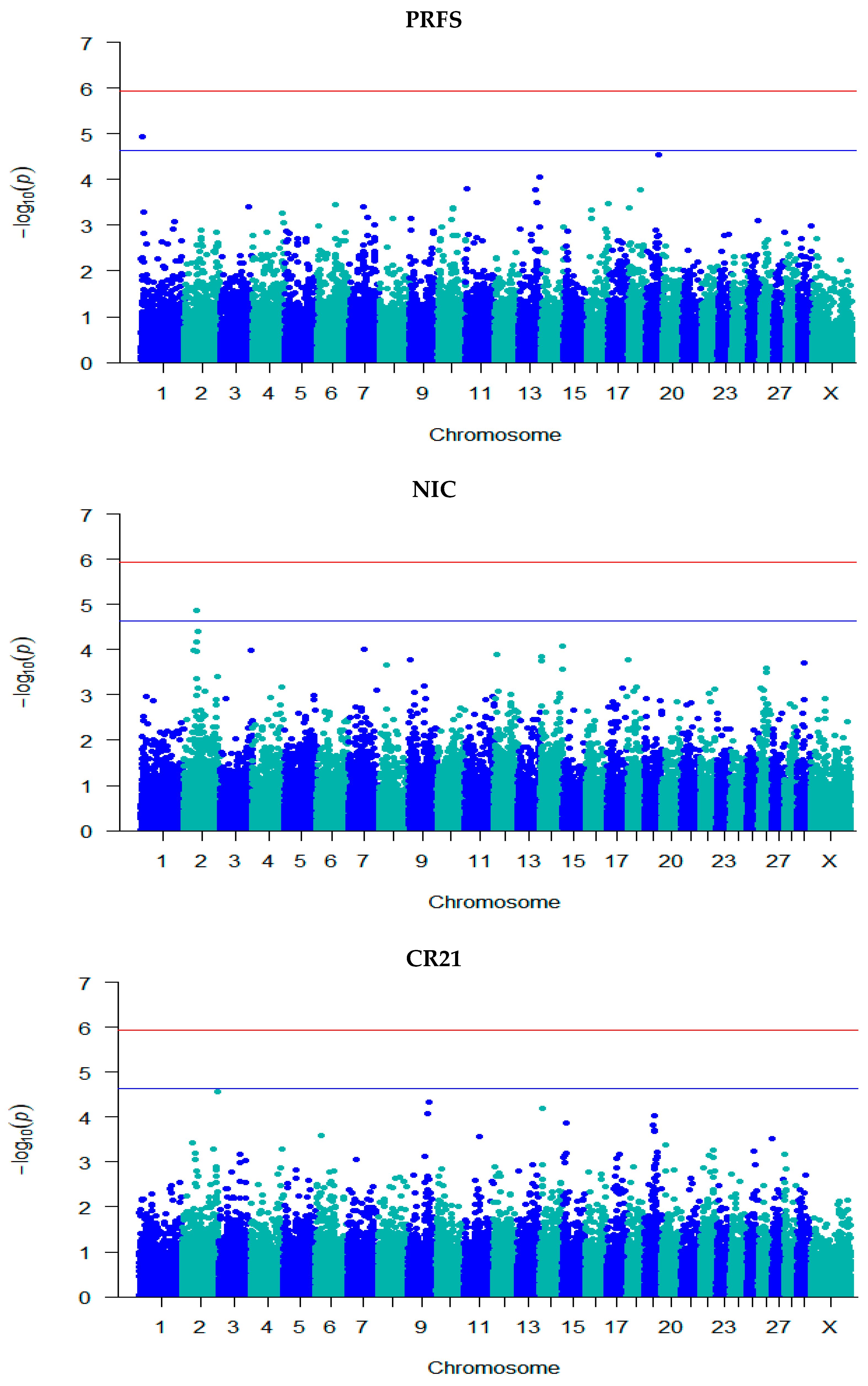
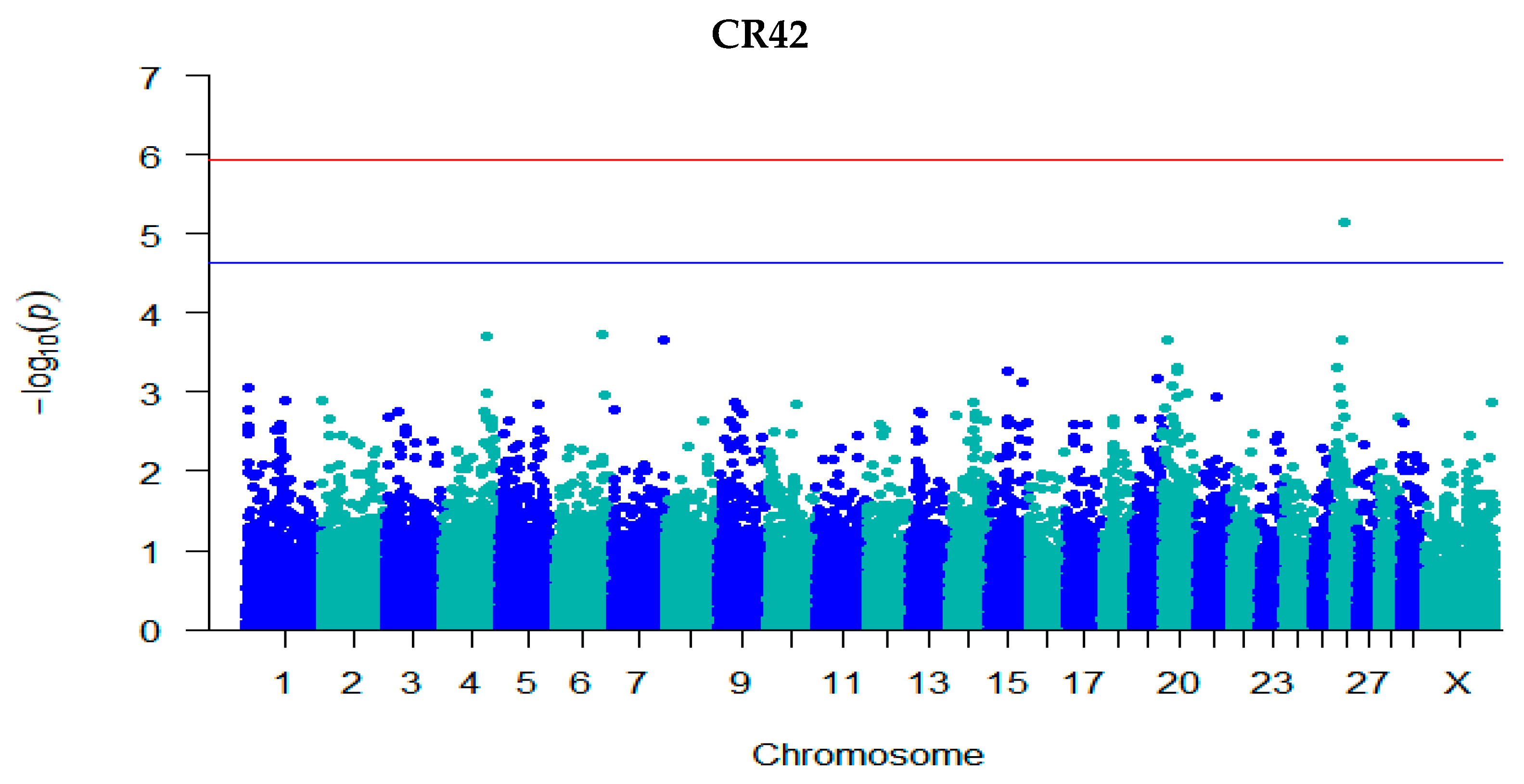
3.3. GWAS for Fertility Traits
4. Discussion
4.1. GWAS for Milk Composition Traits
4.2. GWAS for Fertility Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dairy, N.Z. New Zealand Dairy Statistics 2020-21; Livestock Improvement Corporation Limited & DairyNZ Limited: Hamilton, New Zealand, 2021; Available online: https://www.dairynz.co.nz/publications/dairy-industry/new-zealand-dairy-statistics-2020-21/ (accessed on 30 August 2022).
- Harris, B.L.; Montgomerie, W.A. Fertility breeding values for seasonal dairying. Interbull Bull. 2001, 27, 139–142. [Google Scholar]
- Harris, B.L.; Pryce, J.E.; Xu, Z.Z.; Montgomerie, W.A. Development of new fertility breeding values in the dairy industry. N. Z. Soc. Anim. Prod. 2006, 66, 107–112. [Google Scholar]
- Kerslake, J.I.; Amer, P.R.; O’Neill, P.L.; Wong, S.L.; Roche, J.R.; Phyn, C.V.C. Economic costs of recorded reasons for cow mortality and culling in a pasture-based dairy industry. J. Dairy Sci. 2018, 101, 1795–1803. [Google Scholar] [CrossRef]
- Grosshans, T.; Xu, Z.Z.; Burton, L.J.; Johnson, D.L.; Macmillan, K.L. Performance and genetic parameters for fertility of seasonal dairy cows in New Zealand. Livest. Prod. Sci. 1997, 51, 41–51. [Google Scholar] [CrossRef]
- Jayawardana, J.M.D.R.; Lopez-Villalobos, N.; McNaughton, L.R.; Hickson, R.E. Heritabilities and genetic and phenotypic correlations for milk production and fertility traits of spring-calved once-daily or twice-daily milking cows in New Zealand. J. Dairy Sci. 2023, 106, 1910–1924. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T.; Hayes, B.; Goddard, M. Accelerating improvement of livestock with genomic selection. Annu. Rev. Anim. Biosci. 2013, 1, 221–237. [Google Scholar] [CrossRef]
- Goddard, M.E.; Hayes, B.J. Genomic selection. J. Anim. Breed. Genet. 2007, 124, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.J.; Bowman, P.J.; Chamberlain, A.J.; Goddard, M.E. Invited review: Genomic selection in dairy cattle: Progress and challenges. J. Dairy Sci. 2009, 92, 433–443. [Google Scholar] [CrossRef]
- Schaeffer, L.R. Strategy for applying genome-wide selection in dairy cattle. J. Anim. Breed. Genet. 2006, 123, 218–223. [Google Scholar] [CrossRef]
- Ma, L.; Cole, J.B.; Da, Y.; VanRaden, P.M. Symposium review: Genetics, genome-wide association study, and genetic improvement of dairy fertility traits. J. Dairy Sci. 2019, 102, 3735–3743. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, Y.; Bovenhuis, H. Genome-wide association study for genotype by lactation stage interaction of milk production traits in dairy cattle. J. Dairy Sci. 2020, 103, 5234–5245. [Google Scholar] [CrossRef]
- Boichard, D.; Grohs, C.; Bourgeois, F.; Cerqueira, F.; Faugeras, R.; Neau, A.; Rupp, R.; Amigues, Y.; Boscher, M.Y.; Levéziel, H. Detection of genes influencing economic traits in three French dairy cattle breeds. Genet. Sel. Evol. 2003, 35, 77. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.K.; Sahana, G.; Guldbrandtsen, B.; Lund, M.S. Validation of associations for female fertility traits in Nordic Holstein, Nordic Red and Jersey dairy cattle. BMC Genet. 2014, 15, 8. [Google Scholar] [CrossRef]
- Nayeri, S.; Sargolzaei, M.; Abo-Ismail, M.K.; May, N.; Miller, S.P.; Schenkel, F.; Moore, S.S.; Stothard, P. Genome-wide association for milk production and female fertility traits in Canadian dairy Holstein cattle. BMC Genet. 2016, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.P.; Bastiaansen, J.W.M.; Veerkamp, R.F.; Wijga, S.; Wall, E.; Berglund, B.; Calus, M.P.L. Genome-wide associations for fertility traits in Holstein–Friesian dairy cows using data from experimental research herds in four European countries. Animal 2012, 6, 1206–1215. [Google Scholar] [CrossRef]
- Winkelman, A.M.; Johnson, D.L.; Harris, B.L. Application of genomic evaluation to dairy cattle in New Zealand. J. Dairy Sci. 2015, 98, 659–675. [Google Scholar] [CrossRef]
- Harris, B.L.; Johnson, D.L.; Spelman, J.R. Genomic selection in New Zealand and the implications for national genetic evaluation. In Proceedings of the Interbull Meeting, Niagara Falls, ON, Canada, 16-19 June 2008. [Google Scholar]
- Jayawardana, J.M.D.R.; Lopez-Villalobos, N.; McNaughton, L.R.; Hickson, R.E. Fertility of dairy cows milked once daily or twice daily in New Zealand. J. Dairy Sci. 2022, 105, 8911–8923. [Google Scholar] [CrossRef]
- Burke, C.R.; Fowler, C. Fertility in New Zealand Dairy Herds: Industry Situation and a Way Forward for Improving on-Farm Reproductive Performance. Proc. South Island Dairy Event (SIDE) 2007, 11, 194–202. [Google Scholar]
- DairyNZ. Incalf New Zealand Fertility Focus User’s Guide. 2019. Available online: https://www.dairynz.co.nz/media/5793532/incalf-nz-fertility-focus-users-guide_2020-update-v1.pdf (accessed on 15 June 2021).
- Johnston, D.J.; Bunter, K.L. Days to calving in Angus cattle: Genetic and environmental effects, and covariances with other traits. Livest. Prod. Sci. 1996, 45, 13–22. [Google Scholar] [CrossRef]
- Dickerson, G.E. Inbreeding and heterosis in animals. J. Anim. Sci. 1973, 1973, 54–77. [Google Scholar] [CrossRef]
- SVS. SNP & Variation Suite™ [Software], 8th ed.; Bozeman, M.T., Ed.; SVS Inc.: Boston, MA, USA; Golden Helix Inc.: Bozeman, MT, USA; Available online: https://www.goldenhelix.com/products/SNP_Variation/index.html (accessed on 10 July 2022).
- Gilmour, A.; Gogel, B.; Cullis, B.; Welham, S.; Thompson, R. ASReml User Guide Release 4.1 Structural Specification; VSN International Ltd.: Hemel Hempstead, UK, 2015. [Google Scholar]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef]
- Turner, S.D. qqman: An R Package for Visualizing GWAS Results Using QQ and Manhattan Plots. bioRxiv 2014. Available online: https://www.biorxiv.org/content/10.1101/005165v1 (accessed on 15 September 2022).
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Yates, A.D.; Achuthan, P.; Akanni, W.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; et al. Ensembl 2020. Nucleic Acids Res. 2019, 48, D682–D688. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cutzal, L.X.; Burke, J.L.; Correa-Luna, M.; Lopez-Villalobos, N. A statistical evaluation of associations between reproductive performance and milk composition and animal factors on grazing dairy cows in two New Zealand dairy farms. N. Z. Vet. J. 2022, 70, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Buitenhuis, B.; Janss, L.L.G.; Poulsen, N.A.; Larsen, L.B.; Larsen, M.K.; Sørensen, P. Genome-wide association and biological pathway analysis for milk-fat composition in Danish Holstein and Danish Jersey cattle. BMC Genom. 2014, 15, 1112. [Google Scholar] [CrossRef] [PubMed]
- Meredith, B.K.; Kearney, F.J.; Finlay, E.K.; Bradley, D.G.; Fahey, A.G.; Berry, D.P.; Lynn, D.J. Genome-wide associations for milk production and somatic cell score in Holstein-Friesian cattle in Ireland. BMC Genet. 2012, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Pryce, J.E.; Bolormaa, S.; Chamberlain, A.J.; Bowman, P.J.; Savin, K.; Goddard, M.E.; Hayes, B.J. A validated genome-wide association study in 2 dairy cattle breeds for milk production and fertility traits using variable length haplotypes. J. Dairy Sci. 2010, 93, 3331–3345. [Google Scholar] [CrossRef] [PubMed]
- Raven, L.-A.; Cocks, B.G.; Hayes, B.J. Multibreed genome wide association can improve precision of mapping causative variants underlying milk production in dairy cattle. BMC Genom. 2014, 15, 62. [Google Scholar] [CrossRef]
- Yen, C.L.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic review series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef]
- Pacheco-Pappenheim, S.; Yener, S.; van Valenberg, H.J.F.; Tzompa-Sosa, D.A.; Bovenhuis, H. The DGAT1 K232A polymorphism and feeding modify milk fat triacylglycerol composition. J. Dairy Sci. 2019, 102, 6842–6852. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, J.; Gao, X.; Song, W.; Chen, C.; Yao, D.; Ma, J.; Xu, L.; Ma, Y. Genome-wide association study of milk components in Chinese Holstein cows using single nucleotide polymorphism. Livest. Sci. 2020, 233, 103951. [Google Scholar] [CrossRef]
- Sanchez, M.-P.; Govignon-Gion, A.; Croiseau, P.; Fritz, S.; Hozé, C.; Miranda, G.; Martin, P.; Barbat-Leterrier, A.; Letaïef, R.; Rocha, D.; et al. Within-breed and multi-breed GWAS on imputed whole-genome sequence variants reveal candidate mutations affecting milk protein composition in dairy cattle. Genet. Sel. Evol. 2017, 49, 68. [Google Scholar] [CrossRef] [PubMed]
- Tiplady, K.M.; Lopdell, T.J.; Reynolds, E.; Sherlock, R.G.; Keehan, M.; Johnson, T.J.J.; Pryce, J.E.; Davis, S.R.; Spelman, R.J.; Harris, B.L.; et al. Sequence-based genome-wide association study of individual milk mid-infrared wavenumbers in mixed-breed dairy cattle. Genet. Sel. Evol. 2021, 53, 62. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.; Fang, L.; Liu, S.; Liu, G.E.; Tenesa, A.; Gao, Y.; Mason, B.A.; Chamberlain, A.J.; Goddard, M.E. Genetic score omics regression and multi-trait meta-analysis detect widespread cis-regulatory effects shaping bovine complex traits. bioRxiv 2022. [Google Scholar] [CrossRef]
- Oliveira, H.; Silva, F.; Brito, L.; Jamrozik, J.; Lourenco, D.; Schenkel, F. Genome-wide association study for milk, fat and protein yields in different lactation stages in Canadian Holstein and Jersey cattle. In Proceedings of the World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 11–16 February 2018; pp. 11–16. [Google Scholar]
- Lembeye, F.; Lopez-Villalobos, N.; Burke, J.L.; Davis, S.R. Estimation of genetic parameters for milk traits in cows milked once- or twice-daily in New Zealand. Livest. Sci. 2016, 185, 142–147. [Google Scholar] [CrossRef]
- Sparks, J.D.; Dong, H.H. FoxO1 and hepatic lipid metabolism. Curr. Opin. Lipidol. 2009, 20, 217–226. [Google Scholar] [CrossRef]
- Atashi, H.; Salavati, M.; De Koster, J.; Ehrlich, J.; Crowe, M.; Opsomer, G.; Consortium, G.; Hostens, M. Genome-wide association for milk production and lactation curve parameters in Holstein dairy cows. J. Anim. Breed. Genet. 2020, 137, 292–304. [Google Scholar] [CrossRef]
- Cruz, V.A.R.; Oliveira, H.R.; Brito, L.F.; Fleming, A.; Larmer, S.; Miglior, F.; Schenkel, F.S. Genome-Wide Association Study for Milk Fatty Acids in Holstein Cattle Accounting for the DGAT1 Gene Effect. Animals 2019, 9, 997. [Google Scholar] [CrossRef]
- Bohlouli, M.; Halli, K.; Yin, T.; Gengler, N.; König, S. Genome-wide associations for heat stress response suggest potential candidate genes underlying milk fatty acid composition in dairy cattle. J. Dairy Sci. 2022, 105, 3323–3340. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Peters, S.O.; Akwanji, K.A.; Imumorin, I.G.; Zhao, X. High density genome wide genotyping-by-sequencing and association identifies common and low frequency SNPs, and novel candidate genes influencing cow milk traits. Sci. Rep. 2016, 6, 31109. [Google Scholar] [CrossRef]
- Frąszczak, M.; Szyda, J. Comparison of significant single nucleotide polymorphisms selections in GWAS for complex traits. J. Appl. Genet. 2016, 57, 207–213. [Google Scholar] [CrossRef]
- Freitas, P.H.F.; Oliveira, H.R.; Silva, F.F.; Fleming, A.; Miglior, F.; Schenkel, F.S.; Brito, L.F. Genomic analyses for predicted milk fatty acid composition throughout lactation in North American Holstein cattle. J. Dairy Sci. 2020, 103, 6318–6331. [Google Scholar] [CrossRef]
- Lopdell, T.J.; Tiplady, K.; Struchalin, M.; Johnson, T.J.J.; Keehan, M.; Sherlock, R.; Couldrey, C.; Davis, S.R.; Snell, R.G.; Spelman, R.J.; et al. DNA and RNA-sequence based GWAS highlights membrane-transport genes as key modulators of milk lactose content. BMC Genom. 2017, 18, 968. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.P.; Wolf, V.; El Jabri, M.; Beuvier, E.; Rolet-Répécaud, O.; Gaüzère, Y.; Minéry, S.; Brochard, M.; Michenet, A.; Taussat, S.; et al. Short communication: Confirmation of candidate causative variants on milk composition and cheesemaking properties in Montbéliarde cows. J. Dairy Sci. 2018, 101, 10076–10081. [Google Scholar] [CrossRef] [PubMed]
- Tribout, T.; Croiseau, P.; Lefebvre, R.; Barbat, A.; Boussaha, M.; Fritz, S.; Boichard, D.; Hoze, C.; Sanchez, M.-P. Confirmed effects of candidate variants for milk production, udder health, and udder morphology in dairy cattle. Genet. Sel. Evol. 2020, 52, 55. [Google Scholar] [CrossRef]
- NRC, I. Nutrient Requirements of Dairy Cattle; The National Academies Press: Washington, DC, USA, 2001; Volume 519. [Google Scholar]
- Kirn-Safran, C.B.; D’Souza, S.S.; Carson, D.D. Heparan sulfate proteoglycans and their binding proteins in embryo implantation and placentation. Semin. Cell Dev. Biol. 2008, 19, 187–193. [Google Scholar] [CrossRef]
- Ringvall, M.; Ledin, J.; Holmborn, K.; van Kuppevelt, T.; Ellin, F.; Eriksson, I.; Olofsson, A.-M.; Kjellén, L.; Forsberg, E. Defective Heparan Sulfate Biosynthesis and Neonatal Lethality in Mice LackingN-Deacetylase/N-Sulfotransferase-1. J. Biol. Chem. 2000, 275, 25926–25930. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef]
- Itoh, K.; Sokol, S.Y. Heparan sulfate proteoglycans are required for mesoderm formation in Xenopus embryos. Development 1994, 120, 2703–2711. [Google Scholar] [CrossRef]
- Prinsen, R.T.M.M.; Rossoni, A.; Gredler, B.; Bieber, A.; Bagnato, A.; Strillacci, M.G. A genome wide association study between CNVs and quantitative traits in Brown Swiss cattle. Livest. Sci. 2017, 202, 7–12. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Singh, A.; Panda, S.; Malla, W.A.; Kumar, A.; Dutt, T. Genome-wide elucidation of CNV regions and their association with production and reproduction traits in composite Vrindavani cattle. Gene 2022, 830, 146510. [Google Scholar] [CrossRef]
- Jiang, Z.; Michal, J.J.; Wu, X.L.; Pan, Z.; MacNeil, M.D. The heparan and heparin metabolism pathway is involved in regulation of fatty acid composition. Int. J. Biol. Sci. 2011, 7, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Siebert, L.J. Identifying Genome Associations with Unique Mastitis Phenotypes in Response to Intramammary Streptococcus Uberis Challenge. Ph.D. Thesis, University of Tennessee, Knoxville, Tennessee, 2017. [Google Scholar]
- Watford, M. Glutamine and glutamate: Nonessential or essential amino acids? Anim. Nutr. 2015, 1, 119–122. [Google Scholar] [CrossRef]
- Meijer, G.A.L.; van der Meulen, J.; van Vuuren, A.M. Glutamine is a potentially limiting amino acid for milk production in dairy cows: A hypothesis. Metabolism 1993, 42, 358–364. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Marino, R.; Santillo, A.; Sevi, A. Immune response and milk production of dairy cows fed graded levels of rumen-protected glutamine. Res. Vet. Sci. 2012, 93, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kessel, S.; Stroehl, M.; Meyer, H.H.D.; Hiss, S.; Sauerwein, H.; Schwarz, F.J.; Bruckmaier, R.M. Individual variability in physiological adaptation to metabolic stress during early lactation in dairy cows kept under equal conditions. J. Anim. Sci. 2008, 86, 2903–2912. [Google Scholar] [CrossRef]
- Butler, W.R.; Smith, R.D. Interrelationships Between Energy Balance and Postpartum Reproductive Function in Dairy Cattle. J. Dairy Sci. 1989, 72, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Buettger, C.; Kwagh, J.; Matter, A.; Daikhin, Y.; Nissim, I.B.; Collins, H.W.; Yudkoff, M.; Stanley, C.A.; Matschinsky, F.M. A Signaling Role of Glutamine in Insulin Secretion. J. Biol. Chem. 2004, 279, 13393–13401. [Google Scholar] [CrossRef]
- Newsholme, P.; Bender, K.; Kiely, A.; Brennan, L. Amino acid metabolism, insulin secretion and diabetes. Biochem. Soc. Trans. 2007, 35, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.T.; Marr, A.L.; Pelton, S.H.; Radcliff, R.P.; Lucy, M.C.; Butler, W.R. Insulin restores GH responsiveness during lactation-induced negative energy balance in dairy cattle: Effects on expression of IGF-I and GH receptor 1A. J. Endocrinol. 2003, 176, 205–217. [Google Scholar] [CrossRef]
- Khatib, H.; Huang, W.; Mikheil, D.; Schutzkus, V.; Monson, R.L. Effects of signal transducer and activator of transcription (STAT) genes STAT1 and STAT3 genotypic combinations on fertilization and embryonic survival rates in Holstein cattle. J. Dairy Sci. 2009, 92, 6186–6191. [Google Scholar] [CrossRef]
- Ratcliffe, L.; Mullen, M.; McClure, M.C.; McClure, J.; Kearney, F. 190 Single nucleotide polymorphisms in the signal transducer and regulator of transcription (STAT) genes are associated with milk production, milk composition, and fertility traits in Holstein Friesian cattle. J. Anim. Sci. 2017, 95, 94. [Google Scholar] [CrossRef]
- Walker, C.G.; Littlejohn, M.D.; Mitchell, M.D.; Roche, J.R.; Meier, S. Endometrial gene expression during early pregnancy differs between fertile and subfertile dairy cow strains. Physiol. Genom. 2012, 44, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Darnell, J.E. STATs: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Maj, T.; Chelmonska-Soyta, A. Pleiotropy and redundancy of STAT proteins in early pregnancy. Reprod. Domest. Anim. 2007, 42, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, J.; Li, Q.; Ju, Z.; Li, J.; Shi, F.; Zhong, J.; Wang, C. Novel single nucleotide polymorphisms (SNPs) of the bovine STAT4 gene and their associations with production traits in Chinese Holstein cattle. Afr. J. Biotechnol. 2010, 9, 4003–4008. [Google Scholar]
- Cobanoglu, O.; Zaitoun, I.; Chang, Y.M.; Shook, G.E.; Khatib, H. Effects of the Signal Transducer and Activator of Transcription 1 (STAT1) Gene on Milk Production Traits in Holstein Dairy Cattle. J. Dairy Sci. 2006, 89, 4433–4437. [Google Scholar] [CrossRef]
- Sadri, H.; Mielenz, M.; Morel, I.; Bruckmaier, R.M.; van Dorland, H.A. Plasma leptin and mRNA expression of lipogenesis and lipolysis-related factors in bovine adipose tissue around parturition. J. Anim. Physiol. Anim. Nutr. 2011, 95, 790–797. [Google Scholar] [CrossRef]
- Graber, M.; Kohler, S.; Kaufmann, T.; Doherr, M.G.; Bruckmaier, R.M.; van Dorland, H.A. A field study on characteristics and diversity of gene expression in the liver of dairy cows during the transition period. J. Dairy Sci. 2010, 93, 5200–5215. [Google Scholar] [CrossRef]
- Palombo, V.; Milanesi, M.; Sgorlon, S.; Capomaccio, S.; Mele, M.; Nicolazzi, E.; Ajmone-Marsan, P.; Pilla, F.; Stefanon, B.; D’Andrea, M. Genome-wide association study of milk fatty acid composition in Italian Simmental and Italian Holstein cows using single nucleotide polymorphism arrays. J. Dairy Sci. 2018, 101, 11004–11019. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, R.D.; Jackson, M.R. Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the golgi apparatus. Annu. Rev. Cell Dev. Biol. 1996, 12, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Tenghe, A.M.M.; Bouwman, A.C.; Berglund, B.; Strandberg, E.; de Koning, D.J.; Veerkamp, R.F. Genome-wide association study for endocrine fertility traits using single nucleotide polymorphism arrays and sequence variants in dairy cattle. J. Dairy Sci. 2016, 99, 5470–5485. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.K.; Buitenhuis, B.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Genome-wide association study for female fertility in Nordic Red cattle. BMC Genet. 2015, 16, 110. [Google Scholar] [CrossRef]
- Schulman, N.F.; Sahana, G.; Iso-Touru, T.; McKay, S.D.; Schnabel, R.D.; Lund, M.S.; Taylor, J.F.; Virta, J.; Vilkki, J.H. Mapping of fertility traits in Finnish Ayrshire by genome-wide association analysis. Anim. Genet. 2011, 42, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, G.; Nicolazzi, E.L.; Stella, A.; Biffani, S.; Negrini, R.; Lazzari, B.; Ajmone-Marsan, P.; Williams, J.L. Genome Wide Analysis of Fertility and Production Traits in Italian Holstein Cattle. PLoS ONE 2013, 8, e80219. [Google Scholar] [CrossRef]
- Parker Gaddis, K.L.; Null, D.J.; Cole, J.B. Explorations in genome-wide association studies and network analyses with dairy cattle fertility traits. J. Dairy Sci. 2016, 99, 6420–6435. [Google Scholar] [CrossRef]
- Lander, E.; Kruglyak, L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nat. Genet. 1995, 11, 241–247. [Google Scholar] [CrossRef]

| Trait | Criteria for Trait Calculation |
|---|---|
| Milk composition | |
| FP | Fat percentage, kg fat per kg milk (%) |
| PP | Protein percentage, kg protein per kg milk (%) |
| LP | Lactose percentage, kg lactose per kg milk (%) |
| Fertility | |
| SMFS 1 | The interval from the start of mating to first service (d) |
| SMCO 2 | The interval from the start of mating to conception (d) |
| FSCO 2 | The interval from first service to conception (d) |
| CFS 1 | Interval from calving to first service (d) |
| CI 3 | Interval between two consecutive calvings (d) |
| SR21 | Cows with the first mating date in the first 21 d from the start of mating date were represented as 1, otherwise coded as 0 (binary) |
| SR42 | Cows with the first mating date in the first 42 d from the start of mating date were represented as 1, otherwise coded as 0 (binary) |
| PR21 | Cows conceived in the first 21 d from the start of mating date represented as 1, otherwise coded as 0 (binary) |
| PR42 | Cows conceived in the first 42 d from the start of mating date represented as 1, otherwise coded as 0 (binary) |
| PRFS | Cows conceived to the first AB insemination in the first 21 d from the start of mating date were represented as 1, otherwise coded as 0 (binary) |
| NIC | Cows not in calf at the end of the mating period were represented as 1 and in-calf cows were coded as 0 (binary) |
| CR21 4 | Cows calved in the first 21 d from the planned start of calving date were represented as 1, otherwise coded as 0 (binary) |
| CR42 4 | Cows calved in the first 42 d from the planned start of calving date were represented as 1, otherwise coded as 0 (binary) |
| Trait | No. Records | Mean | SD | CV | Min. | Max. |
|---|---|---|---|---|---|---|
| Yields | ||||||
| Milk | 6381 | 4969 | 1237 | 24.9 | 387 | 8861 |
| Fat | 6381 | 232 | 51 | 21.9 | 22 | 398 |
| Protein | 6381 | 188 | 42 | 22.2 | 19 | 311 |
| Lactose | 6381 | 251 | 63 | 25.1 | 11 | 449 |
| Milk composition | ||||||
| FP% | 6381 | 4.75 | 0.69 | 14.5 | 2.92 | 7.38 |
| PP% | 6381 | 3.83 | 0.32 | 8.3 | 2.96 | 5.14 |
| LP% | 6381 | 5.04 | 0.18 | 3.6 | 2.84 | 5.48 |
| Fertility | ||||||
| SMFS | 6221 | 10.9 | 8.6 | 79.1 | 1 | 71 |
| SMCO | 6221 | 29.6 | 28.4 | 95.8 | 1 | 110 |
| FSCO | 6192 | 19.8 | 27.7 | 139.7 | 1 | 109 |
| CFS | 6221 | 79.6 | 18.0 | 22.6 | 14.0 | 201 |
| CI | 5434 | 368.7 | 21.9 | 5.9 | 261 | 435 |
| SR21(%) | 6221 | 91.4 | 28.0 | 30.7 | 0 | 1 |
| SR42(%) | 6221 | 99.2 | 8.8 | 8.8 | 0 | 1 |
| PR21(%) | 6221 | 54.1 | 49.8 | 92.0 | 0 | 1 |
| PR42(%) | 6221 | 75.9 | 42.8 | 56.4 | 0 | 1 |
| PRFS(%) | 6219 | 47.8 | 49.9 | 104.4 | 0 | 1 |
| NIC(%) | 6381 | 12.2 | 32.7 | 268.4 | 0 | 1 |
| CR21(%) | 4321 | 68.0 | 46.6 | 68.6 | 0 | 1 |
| CR42(%) | 4321 | 91.0 | 28.6 | 31.4 | 0 | 1 |
| Trait | Locus | Chr | Position | −log10 (p) | Effect (SE) | Ref./ MA | Ref. Freq. | Annotation | Gene Name |
|---|---|---|---|---|---|---|---|---|---|
| FP | rs211210569 | 5 | 93,945,738 | 6.76 | − | C/T | 0.62 | Intron | MGST1 |
| rs210744919 | 5 | 93,949,810 | 6.43 | −0.11 (0.02) | G/A | 0.41 | Intron | MGST1 | |
| rs110984572 | 14 | 1,653,693 | 7.85 | −0.17 (0.03) | C/T | 0.89 | upstream | FOXH1 | |
| rs134432442 | 14 | 1,736,599 | 74.54 | −0.33 (0.02) | C/T | 0.52 | missense | CPSF1 | |
| rs211309638 | 14 | 1,757,935 | 10.01 | −0.20 (0.03) | C/T | 0.89 | upstream | ADCK5 | |
| rs137071126 | 14 | 1,765,835 | 84.41 | −0.36 (0.02) | C/G | 0.48 | synonymous | SLC52A2 | |
| rs109421300 | 14 | 1,801,116 | 89.79 | −0.38 (0.02) | T/C | 0.46 | Intron | DGAT1 | |
| rs137787931 | 14 | 1,880,378 | 67.95 | 0.32 (0.02) | T/C | 0.55 | Intron | MROH1 | |
| rs109742607 | 14 | 2,217,163 | 16.15 | 0.16 (0.02) | A/G | 0.69 | Intron | IQANK1 | |
| rs110323635 | 14 | 2,239,085 | 16.15 | −0.16 (0.02) | A/G | 0.31 | missense | MAPK15 | |
| rs109617015 | 14 | 2,386,688 | 17.86 | −0.22 (0.03) | A/G | 0.16 | Intron | ZC3H3 | |
| rs109529219 | 14 | 2,468,020 | 21.47 | −0.22 (0.02) | G/A | 0.22 | Intron | RHPN1 | |
| rs109958270 | 14 | 2,605,493 | 7.81 | 0.12 (0.02) | C/T | 0.78 | intergenic | - | |
| rs110043428 | 14 | 2,790,501 | 12.49 | 0.14 (0.02) | A/G | 0.53 | intergenic | - | |
| rs109476486 | 14 | 2,826,632 | 12.41 | −0.17 (0.02) | T/G | 0.20 | upstream | LYPD2 | |
| rs110545978 | 14 | 3,186,141 | 15.28 | 0.18 (0.02) | T/C | 0.83 | intergenic | - | |
| rs136880486 | 14 | 4,078,923 | 11.13 | −0.13 (0.02) | T/C | 0.28 | upstream | AGO2 | |
| rs110755656 | 14 | 5,274,635 | 7.65 | 0.11 (0.02) | G/T | 0.76 | intergenic | - | |
| rs110359329 | 14 | 7,428,315 | 6.09 | −0.09 (0.02) | A/G | 0.58 | intergenic | - | |
| PP | rs43703015 | 6 | 87,390,576 | 6.52 | 0.05 (0.01) | T/C | 0.61 | missense | CSN3 |
| rs43703016 | 6 | 8,7390,612 | 6.52 | 0.05 (0.01) | C/A | 0.61 | missense | CSN3 | |
| rs110014544 | 6 | 87,390,673 | 6.52 | 0.05 (0.01) | G/A | 0.61 | synonymous | CSN3 | |
| rs109787476 | 6 | 87,390,681 | 6.43 | 0.05 (0.01) | T/A | 0.61 | 3 prime UTR | CSN3 | |
| rs134432442 | 14 | 1,736,599 | 28.13 | −0.09 (0.01) | C/T | 0.52 | missense | CPSF1 | |
| rs137071126 | 14 | 1,765,835 | 30.83 | −0.10 (0.01) | C/G | 0.48 | synonymous | SLC52A2 | |
| rs109421300 | 14 | 1,801,116 | 30.96 | −0.10 (0.01) | T/C | 0.46 | Intron | DGAT1 | |
| rs137787931 | 14 | 1,880,378 | 23.91 | 0.09 (0.01) | T/C | 0.55 | Intron | MROH1 | |
| rs109742607 | 14 | 2,217,163 | 6.59 | 0.04 (0.01) | A/G | 0.69 | Intron | IQANK1 | |
| rs109617015 | 14 | 2,386,688 | 8.31 | −0.07 (0.01) | A/G | 0.16 | Intron | ZC3H3 | |
| rs109529219 | 14 | 2,468,020 | 9.81 | −0.07 (0.01) | G/A | 0.22 | Intron | RHPN1 | |
| rs110545978 | 14 | 3,186,141 | 8.33 | 0.06 (0.01) | T/C | 0.83 | intergenic | - | |
| rs110755656 | 14 | 5,274,635 | 5.94 | 0.05 (0.01) | G/T | 0.76 | intergenic | - | |
| LP | rs378183369 | 29 | 9,563,396 | 8.89 | −0.03 (0.004) | A/G | 0.74 | Intron | PICALM |
| Trait | Locus | Chr | Position | −log10 (p) | SNP Effect (SE) | Ref./ MA | Ref. Freq. | Annotation | Gene Name |
|---|---|---|---|---|---|---|---|---|---|
| SMFS | rs132976072 | 9 | 101,238,301 | 5.08 | 0.06 (0.01) | A/G | 0.48 | Intergenic | - |
| rs110111959 | 10 | 75,105,774 | 5.02 | 0.07 (0.02) | A/G | 0.75 | Intron | KCNH5 | |
| SMCO | rs41635004 | 12 | 77,611,452 | 4.71 | 1.52 × 10−7 (3.55 × 10−8) | A/G | 0.63 | Intron | HS6ST3 |
| FSCO | rs132906739 | 2 | 48,960,169 | 4.81 | −0.05 (0.01) | A/G | 0.57 | Intergenic | - |
| rs109941542 | 2 | 49,095,661 | 4.81 | −0.05 (0.01) | C/T | 0.57 | Intergenic | - | |
| rs41635004 | 12 | 77,611,452 | 4.85 | 0.04 (0.01) | A/G | 0.63 | Intron | HS6ST3 | |
| CFS | rs132976072 | 9 | 101,238,301 | 5.06 | 0.06 (0.01) | A/G | 0.48 | Intergenic | - |
| rs110111959 | 10 | 75,105,774 | 5.02 | 0.07 (0.02) | A/G | 0.75 | Intron | KCNH5 | |
| SR42 | rs135632251 | 2 | 79,410,669 | 5.71 | 0.001 (0.0002) | C/T | 0.94 | Intergenic | - |
| rs134983646 | 2 | 79,474,217 | 5.95 | −0.0005 (9.82 × 10−5) | G/A | 0.19 | Intron | GLS ENSBTAG00000051479 | |
| rs109798660 | 2 | 79,486,672 | 6.47 | −0.001 (9.95 × 10−5) | T/C | 0.19 | Intron | ENSBTAG00000051479 | |
| rs134911740 | 2 | 79,706,385 | 5.99 | −0.0005 (9.81 × 10−5) | G/A | 0.19 | Intergenic | - | |
| rs135975975 | 2 | 79,817,588 | 6.52 | −0.001 (9.94 × 10−5) | G/A | 0.18 | Intron | GLS | |
| rs137812009 | 2 | 79,908,334 | 6.50 | −0.001 (9.90 × 10−5) | T/C | 0.18 | Intron | STAT1 | |
| rs41610299 | 2 | 79,946,595 | 6.57 | −0.001 (9.93 × 10−5) | T/C | 0.18 | Intron | STAT4 | |
| rs380321634 | 2 | 79,975,164 | 5.89 | 0.001 (0.0002) | T/G | 0.94 | Intron | STAT4 | |
| PR42 | rs137030801 | 2 | 39,681,141 | 5.80 | 4.20 × 10−9 (8.72 × 10−10) | T/C | 0.46 | Intron | GPD2 |
| rs109673037 | 26 | 24,432,758 | 4.74 | 0.002 (0.0004) | C/T | 0.42 | Upstream | SH3PXD2A | |
| PRFS | rs132685083 | 1 | 2,262,097 | 4.93 | 0.004 (0.001) | A/G | 0.45 | Intron | EVA1C |
| NIC | rs132906739 | 2 | 48,960,169 | 4.86 | −3.06 × 10−8 (7.04 × 10−9) | A/G | 0.57 | Intergenic | - |
| rs109941542 | 2 | 49,095,661 | 4.86 | −3.06 × 10−8 (7.04 × 10−9) | C/T | 0.57 | Intergenic | - | |
| CR42 | rs41606045 | 26 | 22,526,369 | 5.14 | −0.003 (7.32 × 10−6) | C/T | 0.85 | Intron | ARMH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayawardana, J.M.D.R.; Lopez-Villalobos, N.; McNaughton, L.R.; Hickson, R.E. Genomic Regions Associated with Milk Composition and Fertility Traits in Spring-Calved Dairy Cows in New Zealand. Genes 2023, 14, 860. https://doi.org/10.3390/genes14040860
Jayawardana JMDR, Lopez-Villalobos N, McNaughton LR, Hickson RE. Genomic Regions Associated with Milk Composition and Fertility Traits in Spring-Calved Dairy Cows in New Zealand. Genes. 2023; 14(4):860. https://doi.org/10.3390/genes14040860
Chicago/Turabian StyleJayawardana, J. M. D. R., Nicolas Lopez-Villalobos, Lorna R. McNaughton, and Rebecca E. Hickson. 2023. "Genomic Regions Associated with Milk Composition and Fertility Traits in Spring-Calved Dairy Cows in New Zealand" Genes 14, no. 4: 860. https://doi.org/10.3390/genes14040860
APA StyleJayawardana, J. M. D. R., Lopez-Villalobos, N., McNaughton, L. R., & Hickson, R. E. (2023). Genomic Regions Associated with Milk Composition and Fertility Traits in Spring-Calved Dairy Cows in New Zealand. Genes, 14(4), 860. https://doi.org/10.3390/genes14040860