IGF2 May Enhance Placental Fatty Acid Metabolism by Regulating Expression of Fatty Acid Carriers in the Growth of Fetus and Placenta during Late Pregnancy in Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Determination of FA Profiles
2.3. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.4. Western Blotting Analysis
2.5. Histomorphological Analysis
2.6. Bisulfite Sequencing Analysis
2.7. Immunofluorescence Analysis
2.8. Construction of IGF2 Overexpression Plasmid
2.9. Cell Culture and Transfection
2.10. Fatty Acid Uptake Assay
2.11. Statistical Analysis
3. Results
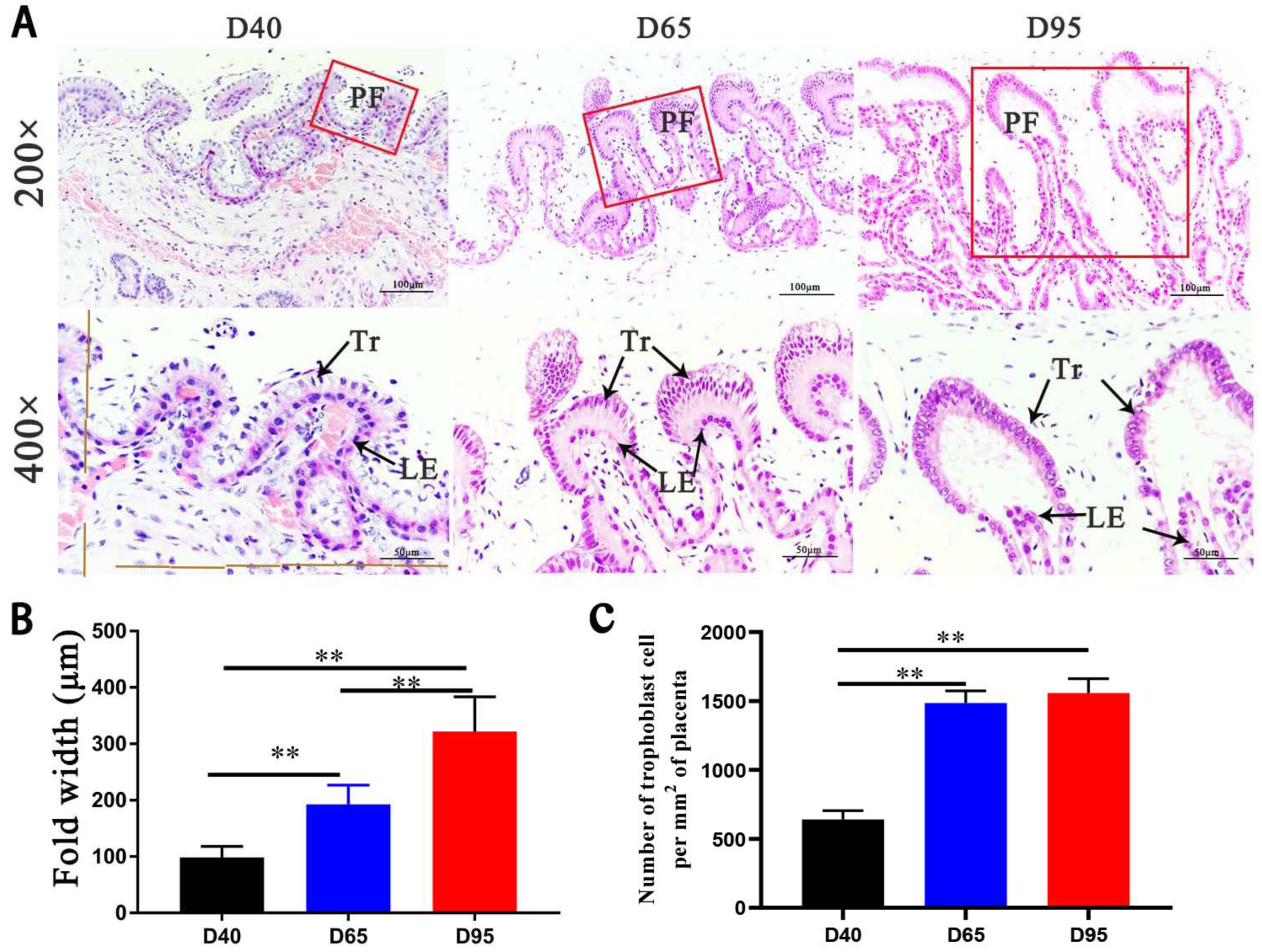
3.1. The Trajectory of Fetal Weight and Placental Morphologies in Pig Pregnancy
3.2. Comparison of FA Concentrations in Porcine Placentae on D40, D65, D95, and Term Pregnancy
3.3. Expression Levels of FA Transport-Related Genes in Porcine Placentae on D40, D65, D95, and Term Pregnancy
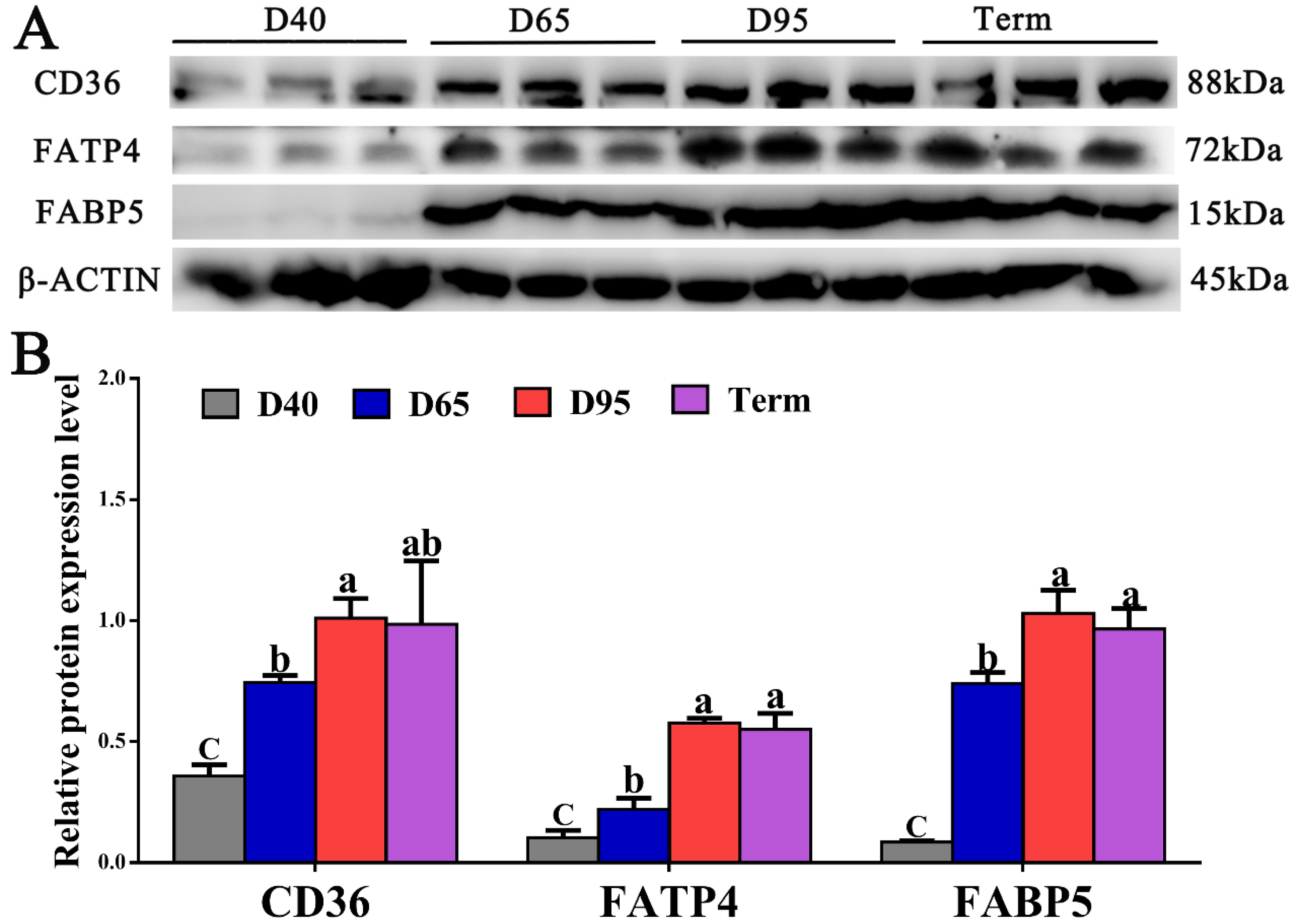
3.4. Localization and Expression of FATP4, FABP5, and CD36 in Pig Placentae
3.5. Imprinting Status of IGF2/H19 Was Altered in Porcine Placentae with Gestation Progress
3.6. Overexpression of IGF2 Enhances Fatty Acid Uptake by Porcine Trophoblast Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aykroyd, B.; Tunster, S.; Sferruzzi-Perri, A. Loss of imprinting of the Igf2-H19 ICR1 enhances placental endocrine capacity via sex-specific alterations in signalling pathways in the mouse. Development 2022, 149, dev199811. [Google Scholar] [CrossRef] [PubMed]
- Napso, T.; Yong, H.; Lopez-Tello, J.; Sferruzzi-Perri, A. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazer, F.; Johnson, G. Pig blastocyst–uterine interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Ren, R.; Cao, J.; Zhao, S.; Yu, M. Genome-wide identification of histone modifications involved in placental development in pigs. Front. Genet. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Duttaroy, A.; Basak, S. Maternal dietary fatty acids and their roles in human placental development. Prostaglandins Leukot. Essent. Fat. Acids 2020, 155, 102080. [Google Scholar] [CrossRef]
- Gil-Sánchez, A.; Koletzko, B.; Larqué, E. Current understanding of placental fatty acid transport. Curr. Opin. Clin. Nutr. 2012, 15, 265–272. [Google Scholar] [CrossRef]
- Larque, E.; Demmelmair, H.; Klingler, M.; De Jonge, S.; Bondy, B.; Koletzko, B. Expression pattern of fatty acid transport protein-1 (FATP-1), FATP-4 and heart-fatty acid binding protein (H-FABP) genes in human term placenta. Early Hum. Dev. 2006, 82, 697–701. [Google Scholar] [CrossRef]
- Larqué, E.; Demmelmair, H.; Gil-Sánchez, A.; Prieto-Sánchez, M.; Blanco, J.; Pagán, A.; Faber, F.; Zamora, S.; Parrilla, J.; Koletzko, B. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 2011, 94 (Suppl. S6), 1908S–1913S. [Google Scholar] [CrossRef] [Green Version]
- Bonen, A.; Chabowski, A.; Luiken, J.; Glatz, J. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: Molecular, biochemical, and physiological evidence. Physiology 2007, 22, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.; Wadsack, C.; Desoye, G. Placental fatty acid transfer. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 78–82. [Google Scholar] [CrossRef]
- Basak, S.; Duttaroy, A. Effects of fatty acids on angiogenic activity in the placental extravillious trophoblast cells. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 155–162. [Google Scholar] [CrossRef]
- Caniçais, C.; Vasconcelos, S.; Ramalho, C.; Marques, C.; Dória, S. Deregulation of imprinted genes expression and epigenetic regulators in placental tissue from intrauterine growth restriction. J. Assist. Reprod. Genet. 2021, 38, 791–801. [Google Scholar] [CrossRef]
- Tabano, S.; Colapietro, P.; Cetin, I.; Grati, F.; Zanutto, S.; Mandò, C.; Antonazzo, P.; Pileri, P.; Rossella, F.; Larizza, L. Epigenetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction. Epigenetics 2010, 5, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Sferruzzi-Perri, A.; Vaughan, O.; Coan, P.; Suciu, M.; Darbyshire, R.; Constancia, M.; Burton, G.; Fowden, A. Placental-Specific Igf2 Deficiency Alters Developmental Adaptations to Undernutrition in Mice. Endocrinology 2011, 152, 3202–3212. [Google Scholar] [CrossRef] [Green Version]
- Thamban, T.; Agarwaal, V.; Khosla, S. Role of genomic imprinting in mammalian development. J. Biosci. 2020, 45, 20. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Tayama, C.; Tomikawa, J.; Akaishi, R.; Kamura, H.; Matsuoka, K.; Wake, N.; Minakami, H.; Kato, K.; Yamada, T. Placenta-specific epimutation at H19-DMR among common pregnancy complications: Its frequency and effect on the expression patterns of H19 and IGF2. Clin. Epigenetics 2019, 11, 113. [Google Scholar] [CrossRef] [Green Version]
- Ao, Z.; Wu, X.; Zhou, J.; Gu, T.; Wang, X.; Shi, J.; Zhao, C.; Cai, G.; Zheng, E.; Liu, D.; et al. Cloned pig fetuses exhibit fatty acid deficiency from impaired placental transport. Mol. Reprod. Dev. 2019, 86, 1569–1581. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, J.; Huan, Y.; Liu, Z.; Yang, C.; Zhang, X.; Mu, Y.; Xia, P.; Liu, Z. Aberrant expression and methylation status of putatively imprinted genes in placenta of cloned piglets. Cell. Reprogram 2010, 12, 213–222. [Google Scholar] [CrossRef]
- Ao, Z.; Li, Z.; Wang, X.; Zhao, C.; Gan, Y.; Wu, X.; Zeng, F.; Shi, J.; Gu, T.; Hong, L. Identification of amniotic fluid metabolomic and placental transcriptomic changes associated with abnormal development of cloned pig fetuses. Mol. Reprod. Dev. 2019, 86, 278–291. [Google Scholar] [CrossRef]
- Hong, L.; Xu, X.; Huang, J.; Lei, M.; Xu, D.; Zhao, S.; Yu, M. Difference in expression patterns of placental cholesterol transporters, ABCA1 and SR-BI, in Meishan and Yorkshire pigs with different placental efficiency. Sci. Rep. 2016, 6, 20503. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.; Li, X.; Wu, G.; Bazer, F.; Burghardt, R.; Bayless, K.; Johnson, G. Mechanotransduction drives morphogenesis to develop folding during placental development in pigs. Placenta 2020, 90, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; He, Y.; Tan, C.; Wu, Z.; Yu, M. HAI-1 regulates placental folds development by influencing trophoblast cell proliferation and invasion in pigs. Gene 2020, 749, 144721. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.; Sferruzzi-Perri, A.; Coan, P.; Constancia, M.; Burton, G. Placental efficiency and adaptation: Endocrine regulation. J. Physiol. 2009, 587, 3459–3472. [Google Scholar] [CrossRef] [PubMed]
- James-Allan, L.; Teal, S.; Powell, T.; Jansson, T. Changes in Placental Nutrient Transporter Protein Expression and Activity Across Gestation in Normal and Obese Women. Reprod. Sci. 2020, 27, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo, S.; Hirschmugl, B.; Wadsack, C.; Desoye, G.; Lewis, R.; Sengers, B. The influence of placental metabolism on fatty acid transfer to the fetus. J. Lipid Res. 2017, 58, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Hanebutt, F.; Demmelmair, H.; Schiessl, B.; Larque, E.; Koletzko, B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin. Nutr. 2008, 27, 685–693. [Google Scholar] [CrossRef]
- Gimeno, R.; Hirsch, D.; Punreddy, S.; Sun, Y.; Ortegon, A.; Wu, H.; Daniels, T.; Stricker-Krongrad, A.; Lodish, H.; Stahl, A. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J. Biol. Chem. 2003, 278, 49512–49516. [Google Scholar] [CrossRef] [Green Version]
- Chassen, S.; Ferchaud-Roucher, V.; Gupta, M.; Jansson, T.; Powell, T. Alterations in placental long chain polyunsaturated fatty acid metabolism in human intrauterine growth restriction. Clin. Sci. 2018, 132, 595–607. [Google Scholar] [CrossRef]
- Duttaroy, A. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakata, T.; Oka, T.; Ogawa, T.; Okamoto, F.; Kusaka, Y.; Sohmiya, K.; Shimamoto, K.; Itakura, K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 2001, 42, 751–759. [Google Scholar] [CrossRef]
- Assumpcao, R.; Mucci, D.; Fonseca, F.; Marcondes, H.; Sardinha, F.; Citelli, M.; Carmo, M. Fatty acid profile of maternal and fetal erythrocytes and placental expression of fatty acid transport proteins in normal and intrauterine growth restriction pregnancies. Prostag. Leukotr. Ess. 2017, 125, 24–31. [Google Scholar] [CrossRef]
- Glatz, J.; Vork, M.; Cistola, D.; van der Vusse, G. Cytoplasmic fatty acid binding protein: Significance for intracellular transport of fatty acids and putative role on signal transduction pathways. Prostaglandins Leukot. Essent. Fat. Acids 1993, 48, 33–41. [Google Scholar] [CrossRef]
- Gómez-Vilarrubla, A.; Mas-Parés, B.; Díaz, M.; Xargay-Torrent, S.; Carreras-Badosa, G.; Jové, M.; Martin-Gari, M.; Bonmatí-Santané, A.; de Zegher, F.; Ibañez, L. Fatty acids in the placenta of appropiate-versus small-for-gestational-age infants at term birth. Placenta 2021, 109, 4–10. [Google Scholar] [CrossRef]
- Roque-Jimenez, J.; Oviedo-Ojeda, M.; Whalin, M.; Lee-Rangel, H.; Relling, A. Eicosapentaenoic and docosahexaenoic acid supplementation during early gestation modified relative abundance on placenta and fetal liver tissue mRNA and concentration pattern of fatty acids in fetal liver and fetal central nervous system of sheep. PLoS ONE 2020, 15, e0235217. [Google Scholar] [CrossRef]
- Makkar, A.; Mishima, T.; Chang, G.; Scifres, C.; Sadovsky, Y. Fatty acid binding protein-4 is expressed in the mouse placental labyrinth, yet is dispensable for placental triglyceride accumulation and fetal growth. Placenta 2014, 35, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Gil-Sanchez, A.; Demmelmair, H.; Parrilla, J.; Koletzko, B.; Larque, E. Mechanisms involved in the selective transfer of long chain polyunsaturated Fatty acids to the fetus. Front. Genet. 2011, 2, 57. [Google Scholar] [CrossRef] [Green Version]
- Godhamgaonkar, A.; Wadhwani, N.; Joshi, S. Exploring the role of LC-PUFA metabolism in pregnancy complications, Prostaglandins. Prostag. Leukotr. Ess. 2020, 163, 102203. [Google Scholar] [CrossRef]
- Aykroyd, B.; Tunster, S.; Sferruzzi-Perri, A. Igf2 deletion alters mouse placenta endocrine capacity in a sexually dimorphic manner. J. Endocrinol. 2020, 246, 93–108. [Google Scholar] [CrossRef]
- Loke, Y.; Galati, J.; Morley, R.; Joo, E.; Novakovic, B.; Li, X.; Weinrich, B.; Carson, N.; Ollikainen, M.; Ng, H.; et al. Association of maternal and nutrient supply line factors with DNA methylation at the imprinted IGF2/H19 locus in multiple tissues of newborn twins. Epigenetics 2013, 8, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Kadlecova, M.; Dobesova, Z.; Zicha, J.; Kunes, J. Abnormal Igf2 gene in Prague hereditary hypertriglyceridemic rats: Its relation to blood pressure and plasma lipids. Mol. Cell Biochem. 2008, 314, 37–43. [Google Scholar] [CrossRef]
- Shen, L.; Li, C.; Wang, Z.; Zhang, R.; Shen, Y.; Miles, T.; Wei, J.; Zou, Z. Early-life exposure to severe famine is associated with higher methylation level in the IGF2 gene and higher total cholesterol in late adulthood: The Genomic Research of the Chinese Famine (GRECF) study. Clin. Epigenetics 2019, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Wang, L.; Liu, M.; Tian, Y.; Chen, T. MiR-210-3p attenuates lipid accumulation and inflammation in atherosclerosis by repressing IGF2. Biosci. Biotechnol. Biochem. 2020, 84, 321–329. [Google Scholar] [CrossRef] [PubMed]




| Genes | Primer Sequences (5′ to 3′) | Product Size (bp) | Amplification Efficiency | GenBank Accession No. |
|---|---|---|---|---|
| FATP1 | F:GTGCTGGTGATGGACGAACTGG | 180 | 98.76% | XM_021076151 |
| R:GCCTGCTTTGCCCTCTACTCCT | ||||
| FATP2 | F:GGACGAGACGCTCACCTATG | 185 | 102.04% | NM_001278777 |
| R:TGTAGTTGAGGCACGCCATG | ||||
| FATP3 | F:AGGTCTCAGCCGAAGTGGATGC | 127 | 104.21% | XM_021089805 |
| R:TGACTGATCCGAGCAGCCTTGG | ||||
| FATP4 | F:TATGGTGTGGAGGTGCCAGGAA | 198 | 103.49% | XM_013993903 |
| R:CCGCAGGTCTGTCTTCTGTAGC | ||||
| FABP3 | F:AGCACCTTCAAGAGCACAGAGA | 105 | 101.65% | NM_001099931 |
| R:GCCTCCATCCAGTGTCACAATG | ||||
| FABP5 | F:GGCAAAGACCTCACCATCAAA | 182 | 96.13% | NM_001039746 |
| R:CTTGTGATTGTGCTCTCCTTCC | ||||
| FABP7 | F:AGGCAGGTGGGAAATGTGACT | 300 | 95.45% | NM_001025229 |
| R:CTCATAGTGGCGAACAGCAACC | ||||
| CD36 | F:AGGACCCTGAGACCCACACA | 116 | 97.34% | NM_001044622 |
| R:TGCCACAGCCAGATTGAGAACA | ||||
| LPL | F:CATTGTGGTGGACTGGCTGTCT | 290 | 95.12% | NM_214286 |
| R:AATCTGCGTCGTCGGGAGAAAG | ||||
| IGF2 | F:CCCCCCTTCCTTCTCTTTCTT | 101 | - | [18] |
| R:GCGACAAGCCTACCTGCAA | ||||
| H19 | F:GGCCGGAGAATGGGAAAGAAGG | 148 | - | [18] |
| R:CGCAGTGCTGCGTGGGAACG | ||||
| β-Actin | F:CCACGAGACCACCTTCAACTC | 131 | 96.56% | DQ845171 |
| R:TGATCTCCTTCTGCATCCTGT |
| Detected Fatty Acids | D40 (n = 8) | D65 (n = 8) | D95 (n = 8) | Term (n = 8) |
|---|---|---|---|---|
| Caproic acid (C6:0) | 1.08 ± 0.03 a | 1.04 ± 0.06 ab | 1.03 ± 0.09 ab | 0.96 ± 0.03 b |
| Caprylic acid (C8:0) | 1.54 ± 0.37 | 1.06 ± 0.19 | 1.47 ± 0.42 | 1.07 ± 0.25 |
| Capric acid (C10:0) | 0.96 ± 0.04 a | 0.94 ± 0.05 ab | 0.94 ± 0.1 ab | 0.84 ± 0.07 b |
| Lauric acid (C12:0) | 1.98 ± 0.11 | 1.92 ± 0.2 | 1.9 ± 0.22 | 1.81 ± 0.11 |
| Tridecanoic acid (C13:0) | 7.04 ± 0.31 | 7.65 ± 0.81 | 7.62 ± 1.07 | 8.12 ± 0.59 |
| Myristic acid (C14:0) | 22.09 ± 1.93 | 25.62 ± 4.48 | 25.95 ± 1.1 | 22.57 ± 2.21 |
| Myristelaidic acid (C14:1T) | 194.55 ± 17.7 a | 161.33 ± 22.08 b | 143.1 ± 4.34 bc | 126.11 ± 9.67 c |
| Myristoleic acid (C14:1) | 42.26 ± 5.18 a | 36.97 ± 3.71 b | 33.63 ± 1.28 b | 35.27 ± 1.27 b |
| Pentadecanoic acid (C15:0) | 13.1 ± 0.36 a | 13.8 ± 4.87 a | 14.31 ± 3.01 a | 5.9 ± 0.58 b |
| Trans-10-Pentadecenoic acid (C15:1T) | 41.81 ± 3.05 | 41.19 ± 4.34 | 40.07 ± 1.52 | 43.79 ± 1.95 |
| cis-10-Pentadecenoic acid (C15:1) | 30.61 ± 3.25 | 30.76 ± 4.03 | 29.02 ± 1.22 | 31.16 ± 1.2 |
| Palmitic acid (C16:0) | 1889.42 ± 233.89 | 1894.75 ± 190.05 | 2049.28 ± 176.39 | 1847.35 ± 139.56 |
| Trans-Palmitelaidic acid (C16:1T) | 77.96 ± 6.74 | 75.84 ± 9.04 | 72.64 ± 3.84 | 78.39 ± 3.07 |
| Palmitoleic acid (C16:1) | 65.69 ± 7.58 | 85.86 ± 18.33 | 92.68 ± 31.75 | 64.61 ± 3.07 |
| Heptadecanoic acid (C17:0) | 25.71 ± 3.06 a | 21.16 ± 7.41 a | 24.03 ± 6.34 a | 12.11 ± 0.69 b |
| Methyl Trans-10-Heptadecenoic Acid (C17:1T) | 28.55 ± 2.06 | 27.76 ± 2.61 | 26.17 ± 1.04 | 27.86 ± 1.15 |
| 10-Heptadecanoic acid(C17:1) | 44.13 ± 5.51 a | 39.47 ± 3.79 ab | 38.06 ± 2.39 b | 36.67 ± 1.32 b |
| Stearic acid(C18:0) | 1172.27 ± 122.3 | 1122.27 ± 137.76 | 1170.75 ± 117.48 | 1016.42 ± 112.09 |
| Petroselaidate (C18:1N12T) | 20.76 ± 1.38 | 20.98 ± 1.87 | 20.1 ± 1 | 19.96 ± 0.72 |
| Elaidate (C18:1N9T) | 8.72 ± 0.59 | 9.16 ± 0.86 | 9.04 ± 0.46 | 9.29 ± 0.29 |
| Transvaccenate (C18:1N7T) | 69.9 ± 3.92 | 69.92 ± 7.09 | 67.77 ± 1.96 | 68.73 ± 2.54 |
| Petroselinic acid (C18:1N12) | 126.95 ± 30.07 b | 154.43 ± 24.74 b | 220.44 ± 59.18 a | 164.89 ± 43.03 ab |
| Oleic acid (C18:1N9C) | 295.12 ± 141.51 d | 749 ± 30.53 b | 1074.14 ± 44 a | 495.38 ± 67.89 c |
| Vaccenic acid (C18:1N7) | 111.01 ± 42.52 c | 246.83 ± 24.95 b | 352.68 ± 16.59 a | 117.76 ± 8.58 c |
| Linoelaidate (C18:2N6T) | 7.28 ± 0.45 | 7.77 ± 0.99 | 8.32 ± 1.26 | 7.61 ± 0.3 |
| 7-Transnonadecenoate (C19:1N12T) | 16.71 ± 0.71 | 17.62 ± 1.89 | 17.26 ± 1.33 | 17 ± 0.95 |
| 10-Transnonadecenoate (C19:1N9T) | 0.64 ± 0.08 a | 0.52 ± 0.08 ab | 0.57 ± 0.12 ab | 0.47 ± 0.05 b |
| Linoleic acid (C18:2N6) | 24.67 ± 11.69 d | 79.63 ± 7.54 b | 116.56 ± 8.69 a | 59.14 ± 5.12 c |
| Arachidic acid (C20:0) | 9.97 ± 0.6 | 10.57 ± 1.98 | 11.4 ± 0.49 | 10 ± 0.82 |
| γ-Linoleic acid (C18:3N6) | 2.96 ± 0.32 | 3.48 ± 0.51 | 3.4 ± 0.39 | 3.06 ± 0.18 |
| Eicosenic-cis-11 acid (C20:1T) | 45.39 ± 3.24 | 45.83 ± 4.74 | 44.58 ± 2.21 | 46.55 ± 2.17 |
| Eicosenic-cis-5 acid (C20:1) | 29 ± 5.24 | 30.32 ± 4.09 | 30.42 ± 3.2 | 27.86 ± 0.84 |
| α-Linoleic acid (C18:3N3) | 1.99 ± 0.13 | 2.09 ± 0.22 | 2.08 ± 0.3 | 2.41 ± 0.32 |
| cis-11,14-Eicosadienoate acid (C20:2) | 8.39 ± 2.45 | 10.58 ± 2.07 | 11.2 ± 2.38 | 8.4 ± 0.26 |
| Behenic acid (C22:0) | 1.51 ± 0.14 b | 3.81 ± 2.53 ab | 4.2 ± 1.44 a | 3.14 ± 0.3 ab |
| cis-8,11,14-Eicosadienoate acid (C20:3N6) | 8.96 ± 3.31 c | 19.6 ± 6.3 ab | 21.95 ± 8.14 a | 12.14 ± 1.65 bc |
| Brassidate (C22:1N9T) | 23.01 ± 1.23 | 22.68 ± 2.61 | 22.99 ± 2.13 | 22.5 ± 3.26 |
| Erucate (C22:1N9) | 22.15 ± 1.2 | 22.74 ± 1.65 | 21.72 ± 1.19 | 22.29 ± 0.91 |
| cis-11,14,17-Eicosadienoate acid (C20:3N3) | 1.86 ± 0.16 | 2.24 ± 0.41 | 2.3 ± 0.32 | 2.16 ± 0.02 |
| Arachidonic acid (C20:4N6) | 25.48 ± 5.68 b | 43.03 ± 13.51 ab | 50.23 ± 22.33 a | 36.75 ± 5.39 ab |
| Tricosanoic acid (C23:0) | 0.57 ± 0.1 | 0.78 ± 0.45 | 0.83 ± 0.26 | 0.57 ± 0.06 |
| Docosanoic acid (C22:2) | 5.38 ± 0.69 | 5.67 ± 0.18 | 5.48 ± 0.33 | 5.56 ± 0.22 |
| Eicosapentaenoic acid (C20:5N3) | 2.2 ± 0.08 b | 3.1 ± 0.32 a | 3.25 ± 0.89 a | 3.61 ± 0.35 a |
| Lignoceric acid (C24:0) | 1.77 ± 0.24 b | 8.32 ± 4.64 a | 9.54 ± 4.56 a | 6.39 ± 0.76 a |
| Nervonic acid (C24:1) | 22.97 ± 5.11 | 26.89 ± 4.96 | 27.86 ± 1.76 | 26.64 ± 1.72 |
| Docosatetraenoic acid (C22:4) | 12.57 ± 1.3 b | 26.2 ± 12.31 a | 22.68 ± 6.84 ab | 20.45 ± 2.25 ab |
| Docosapentaenoic acid (n-6) (C22:5N6) | 16.92 ± 9.41 | 26.53 ± 6.1 | 31.59 ± 14.01 | 20.46 ± 2.89 |
| Docosapentaenoic acid (n-3) (C22:5N3) | 5.63 ± 1.65 b | 9.06 ± 1.43 a | 9.63 ± 2.2 a | 9.34 ± 0.61 a |
| Docosahexaenoic acid (C22:6N3) | 9.9 ± 2.91 b | 15.68 ± 3.68 a | 17.97 ± 6.27 a | 13.1 ± 1.37 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Hu, G.; Zhang, Y.; Ao, Z. IGF2 May Enhance Placental Fatty Acid Metabolism by Regulating Expression of Fatty Acid Carriers in the Growth of Fetus and Placenta during Late Pregnancy in Pigs. Genes 2023, 14, 872. https://doi.org/10.3390/genes14040872
Wu Z, Hu G, Zhang Y, Ao Z. IGF2 May Enhance Placental Fatty Acid Metabolism by Regulating Expression of Fatty Acid Carriers in the Growth of Fetus and Placenta during Late Pregnancy in Pigs. Genes. 2023; 14(4):872. https://doi.org/10.3390/genes14040872
Chicago/Turabian StyleWu, Zhimin, Guangling Hu, Yiyu Zhang, and Zheng Ao. 2023. "IGF2 May Enhance Placental Fatty Acid Metabolism by Regulating Expression of Fatty Acid Carriers in the Growth of Fetus and Placenta during Late Pregnancy in Pigs" Genes 14, no. 4: 872. https://doi.org/10.3390/genes14040872





