Yeast One-Hybrid Screening for Transcription Factors of IbbHLH2 in Purple-Fleshed Sweet Potato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction of Genomic DNA and RNA, Gene Isolation, and Sequence Analysis
2.3. Yeast One-Hybrid Screening
2.4. Yeast One-Hybrid Assay(y1h) Assay
2.5. Yeast Two-Hybridassay (Y2H) Assay
2.6. Dual-Luciferase Assay
2.7. Sub-Cellular Localization Analysis
2.8. Real-Time Quantitative PCR
2.9. Statistical Analysis
3. Results
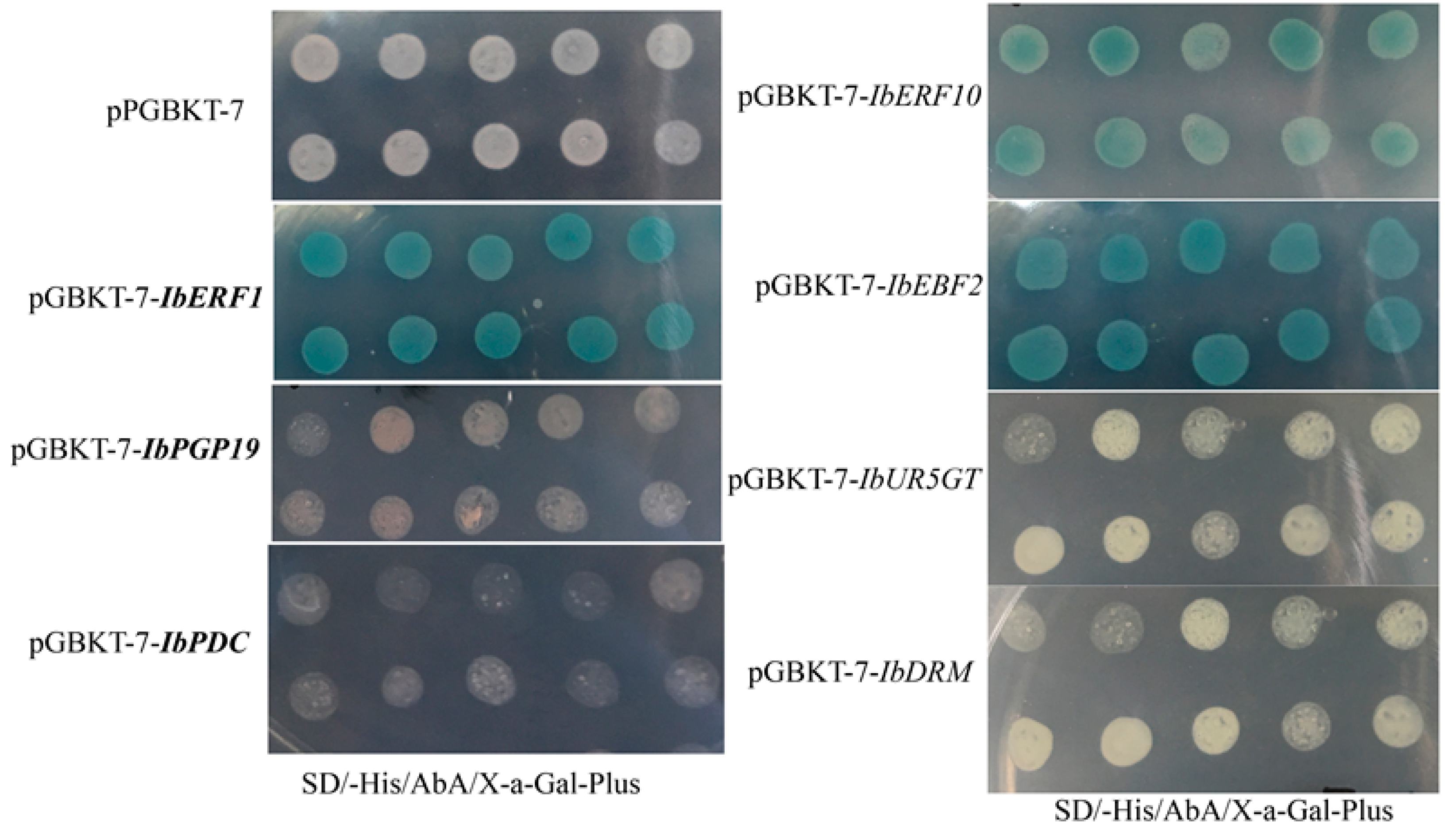
3.1. Screening the Transcription Regulators on the Promoter of IbbHLH2
3.2. Transcriptional Activity of IbERF1, IbERF10, IbEBF2, IbPDC, and IbPGP19
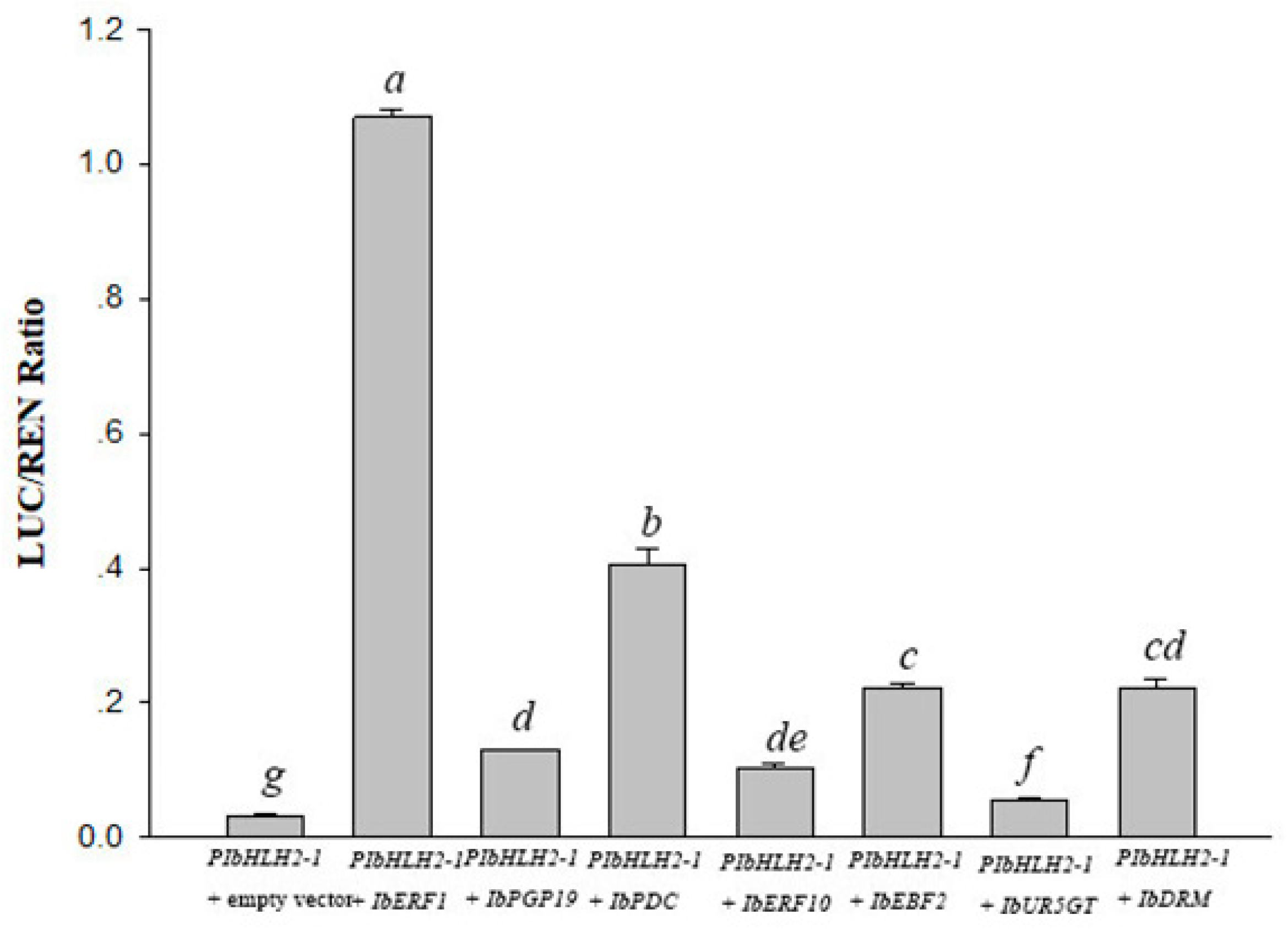
3.3. Interaction between Transcription Regulators and PIbbHLH2-1
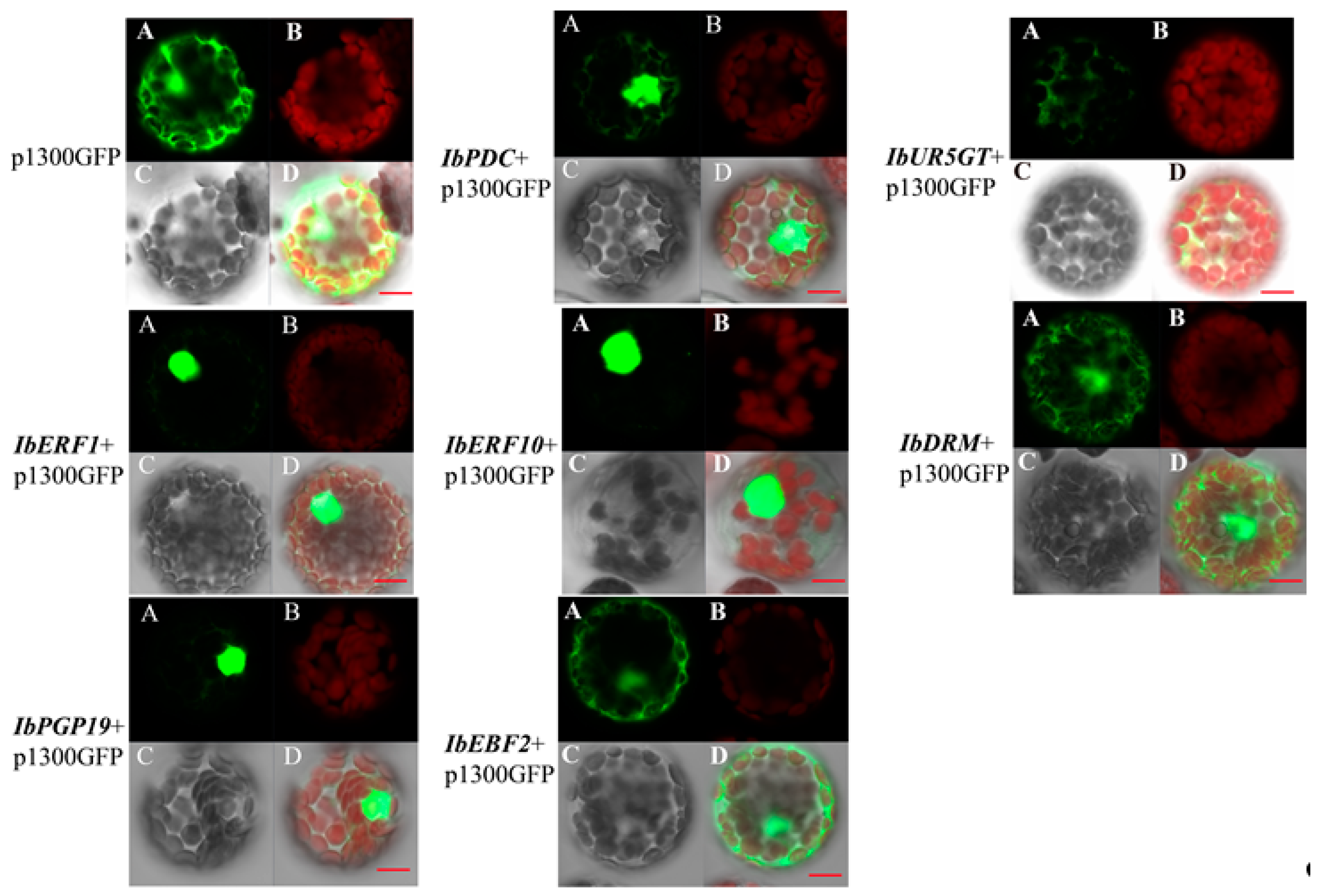
3.4. Sub-Cellular Localization of the Transcription Regulators
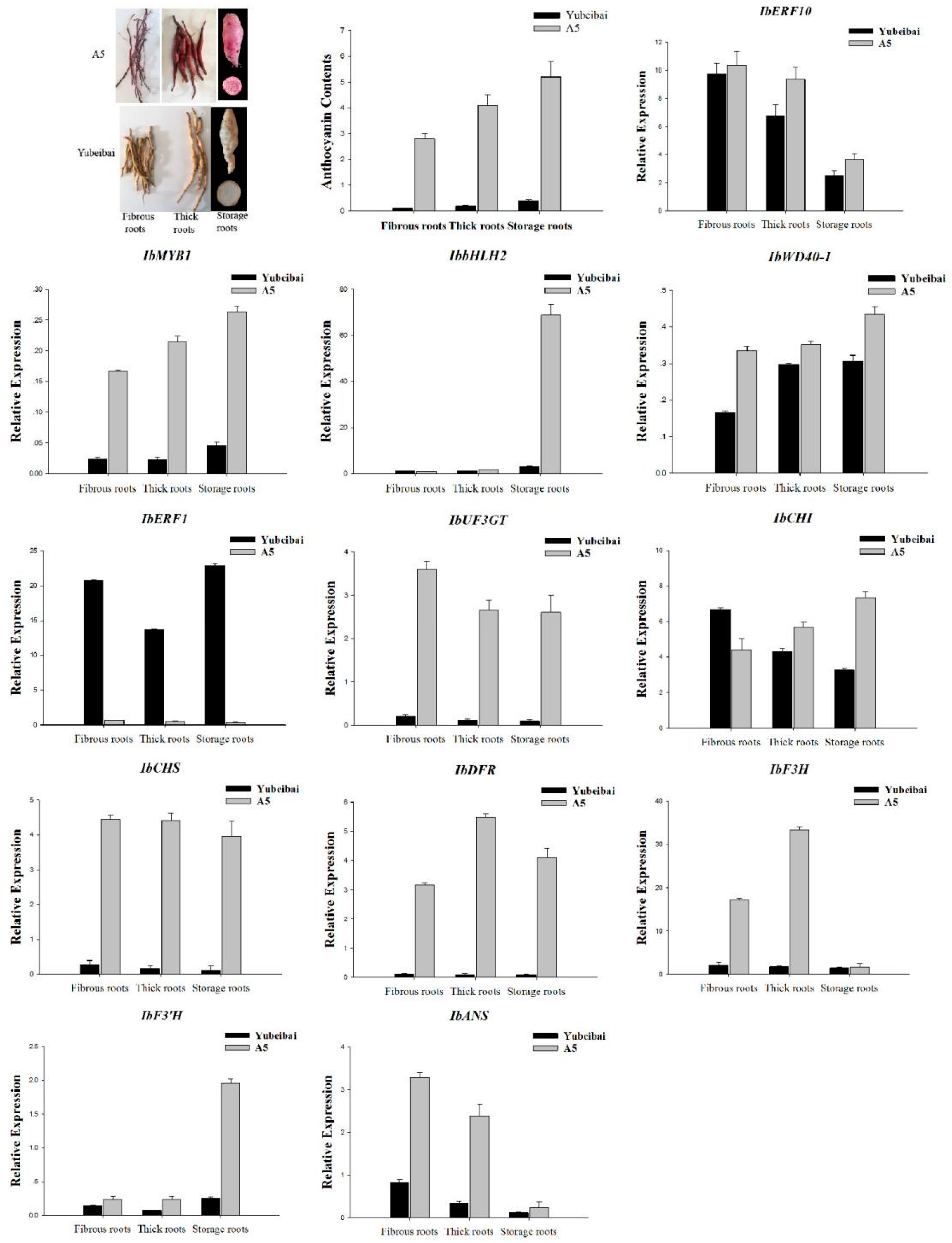
3.5. Expression Characteristics of Transcription Regulators
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Han, W.; Wang, M. Ultraviolet and environmental stresses involved in the induction and regulation of anthocyanin biosynthesis, A review. Afr. J. Biotechnol. 2008, 7, 1–4. [Google Scholar]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Krga, I.; Milenkovic, D. Anthocyanins from sources and bioavailability to cardiovascular-health benefits and molecular mechanisms of action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.; Wei, C. Anthocyanins as promising molecules and dietary bioactive components against diabetes-A review of recent advances. Trends Food Sci. Tech. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Cavallini, E.; Matus, J.T.; Finezzo, L.; Zenoni, S.; Loyola, R.; Guzzo, F.; Schlechter, R.; Ageorges, A.; Arce-Johnson, P.; Tornielli, G.B. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grape vine. Plant Physiol. 2015, 167, 1448–1470. [Google Scholar] [CrossRef]
- Nicola, A.R.; Amanda, R.W.; Mark, M.; John, C.G. Two basic-helix-loop-helix genes (MYC-146 and GL) from Arabidopsis can activate anthocyanin biosynthesis in a white-flowered Matthiola incana mutant. Plant Mol. Biol. 2003, 52, 679–688. [Google Scholar]
- Splet, C.; Quattrocchio, F.; Mol, J.N.M.; Koes, R. Anthocyanin of Petunia encodes a basic helix-loop-helix protein that directly activates trascription of structural anthocyanin genes. Plant Cell 2000, 12, 1619–1631. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, Y.H.; Kim, S.H.; Jeong, Y.J.; Kim, C.Y.; Lee, J.S.; Baef, J.Y.; Ahn, M.J.; Jeong, J.C.; Lee, H.S.; et al. Overexpression of the IbMYB1 gene in an orange-fleshed sweet potato cultivar produces a dual-pigmented transgenic sweet potato with improved antioxidant activity. Physiol. Plant 2015, 153, 525–537. [Google Scholar] [CrossRef]
- Zong, Y.; Li, G.M.; Xi, X.Y.; Sun, X.M.; Li, S.M.; Cao, D.; Zhang, H.G.; Liu, B.L. A bHLH transcription factor TsMYC2 is associated with the blue grain character in triticale (Triticum × Secale). Plant Cell Rep. 2019, 38, 1291–1298. [Google Scholar] [CrossRef]
- Zhao, R.; Song, X.X.; Yang, N.; Chen, L.Q.; Xiang, L.; Liu, X.Q.; Zhao, K. Expression of the subgroup IIIf bHLH transcription factor CpbHLH1 from Chimonanthus praecox (L.) in transgenic model plants inhibits anthocyanin accumulation. Plant Cell Rep. 2020, 39, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Yin, X.R.; Andrew, C.A.; Wang, K.L.; Shi, Y.N.; Huang, Y.J.; Ian, B.F.; Xu, C.J. The role of MrbHLH1 and MrMYB1 in regulating anthocyanin biosynthetic genes in tobacco and Chinese bayberry (Myrica rubra) during anthocyanin biosynthesis. Plant Cell Tiss. Organ. Cult. 2013, 115, 285–298. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Glover, B.J. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005, 10, 63–70. [Google Scholar] [CrossRef] [PubMed]
- An, X.H.; Tian, Y.; Chen, K.Q.; Wang, X.F.; Hao, Y.J. The apple WD40 protein MdTTG1 interacts with bHLH but not MYB proteins to regulate anthocyanin accumulation. J. Plant Physiol. 2012, 169, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.B.; Shen, L.I.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.X.; You, C.X.; Zhang, X.S.; Hao, Y.J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012, 35, 1884–1897. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Du, Z.; Chen, Y.; Li, D.; Deng, S.; Liu, Z.; Yang, L. Effects of anthocyanins on cardio metabolic health, a systematic review and metaanalysis of randomized controlled trials. Adv. Nutr. 2017, 8, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Kui, L.W.; Wang, H.L.; Gu, C.; Dare, A.P.; Espley, R.V.; He, H.P.; Allan, A.C.; Han, Y.P. Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. Plant J. 2015, 82, 105–121. [Google Scholar] [CrossRef]
- Maier, A.; Schrader, A.; Kokkelink, L.; Falke, C.; Welter, B.; Iniesto, E.; Rubio, V.; Uhrig, J.F.; Hulskamp, M.; Hoecker, U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013, 74, 638–651. [Google Scholar] [CrossRef]
- Xie, X.L.; Yin, X.R.; Chen, K.S. Roles of APETALA2/Ethylene responsive factors in regulation of fruit quality. Crit. Rev. Plant Sci. 2016, 35, 120–130. [Google Scholar] [CrossRef]
- Johni, D.; Yogita, N.S.; Banashree, S.; Hari, P.; Deka, B.; Dhanawantari, L.; Channake, S. Ethylene Response Factor (ERF) Family Proteins in Abiotic Stresses and CRISPR–Cas9 Genome Editing of ERFs for Multiple Abiotic Stress Tolerance in Crop Plants, A Review. Mol. Biotech. 2019, 61, 153–172. [Google Scholar]
- Zhuang, J.; Yao, Q.H.; Xiong, A.S.; Zhang, J. Isolation, Phylogeny and Expression Patterns of AP2-Like Genes in Apple (Malus × domestica Borkh). Plant Mol. Biol. Rep. 2011, 29, 209–216. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, M.D.; Wen, C.X.; Xie, X.L.; Tian, W.; Wen, S.Q.; Lu, R.K.; Liu, L.D. Integrated metabolomic and transcriptomic analysis of the anthocyanin regulatory networks in Salvia miltiorrhiza Bge. Flowers. BMC Plant Biol. 2020, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.D.; Liu, L.Q.; Yuan, C.; Guan, J.F. Molecular Characterization of Ethylene Regulated Anthocyanin Biosynthesis in Plums during Fruit Ripening. Plant Mol. Biol. Rep. 2016, 34, 777–785. [Google Scholar] [CrossRef]
- Koyama, T.; Sato, F. The function of ETHYLENE RESPONSE FACTOR genes in the light-induced anthocyanin production of Arabidopsis thaliana leaves. Plant Biotechnol. 2018, 35, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Sun, Y.; Qian, M.; Yang, F.; Ni, J.; Tao, R.; Li, L.; Shu, Q.; Zhang, D.; Teng, Y. Transcriptome analysis of bagging-treated red Chinese sand pear peels reveals light-responsive pathway functions in anthocyanin accumulation. Sci. Rep. 2017, 7, 63. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.F.; Wang, N.; Jiang, S.H.; Fang, H.C.; Zhang, Z.Y.; Yang, G.X. The ethylene response factor MdERF1B regulates anthocyanin and proanthocyanidin biosynthesis in apple. Plant Mol. Biol. 2018, 98, 205–218. [Google Scholar] [CrossRef]
- An, J.P.; Zhang, X.W.; Bi, S.Q.; You, C.X.; Wang, X.F.; Hao, Y.J. The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple. Plant J. 2019, 101, 573–589. [Google Scholar] [CrossRef]
- Ni, J.B.; Bai, S.L.; Zhao, Y.; Qia, M.J.; Tao, R.Y.; Yin, L.; Gao, L. Ethylene response factors Pp4ERF24 and Pp12ERF96 regulate blue lightinduced anthocyanin biosynthesis in ‘Red Zaosu’ pear fruits by interacting with MYB114. Plant Mol. Biol. 2019, 99, 67–78. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yin, X.R.; Xiao, Y.W.; Zhang, Z.Y.; Li, S.J.; Liu, X.F.; Zhang, B. An ETHYLENE RESPONSE FACTOR-MYB transcription complex regulates furaneol biosynthesis by activating QUINONE OXIDOREDUCTASE expression in strawberry. Plant Physiol. 2018, 178, 189–201. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, D.; Chen, Y.; Gao, F. Yeast One-Hybrid Screening for Transcription Factors of IbbHLH2 in Purple-Fleshed Sweet Potato. Genes 2023, 14, 1042. https://doi.org/10.3390/genes14051042
Fu D, Chen Y, Gao F. Yeast One-Hybrid Screening for Transcription Factors of IbbHLH2 in Purple-Fleshed Sweet Potato. Genes. 2023; 14(5):1042. https://doi.org/10.3390/genes14051042
Chicago/Turabian StyleFu, Danwen, Yahui Chen, and Feng Gao. 2023. "Yeast One-Hybrid Screening for Transcription Factors of IbbHLH2 in Purple-Fleshed Sweet Potato" Genes 14, no. 5: 1042. https://doi.org/10.3390/genes14051042
APA StyleFu, D., Chen, Y., & Gao, F. (2023). Yeast One-Hybrid Screening for Transcription Factors of IbbHLH2 in Purple-Fleshed Sweet Potato. Genes, 14(5), 1042. https://doi.org/10.3390/genes14051042



