Comparative Analysis of Codon Usage Patterns in Nuclear and Chloroplast Genome of Dalbergia (Fabaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Processing
2.2. Codon Usage Indicators
2.3. Synonymous Codon Analysis
2.4. ENC-Plot Analysis
2.5. Parity Rule 2 (PR2) Bias Plot Analysis
2.6. Neutrality Plot Analysis
2.7. The Determination of Optimal Codon
2.8. The Correlation between Codon Usage and Gene Expression
2.9. Cluster Analysis and Phylogenetic Tree Construction
3. Results
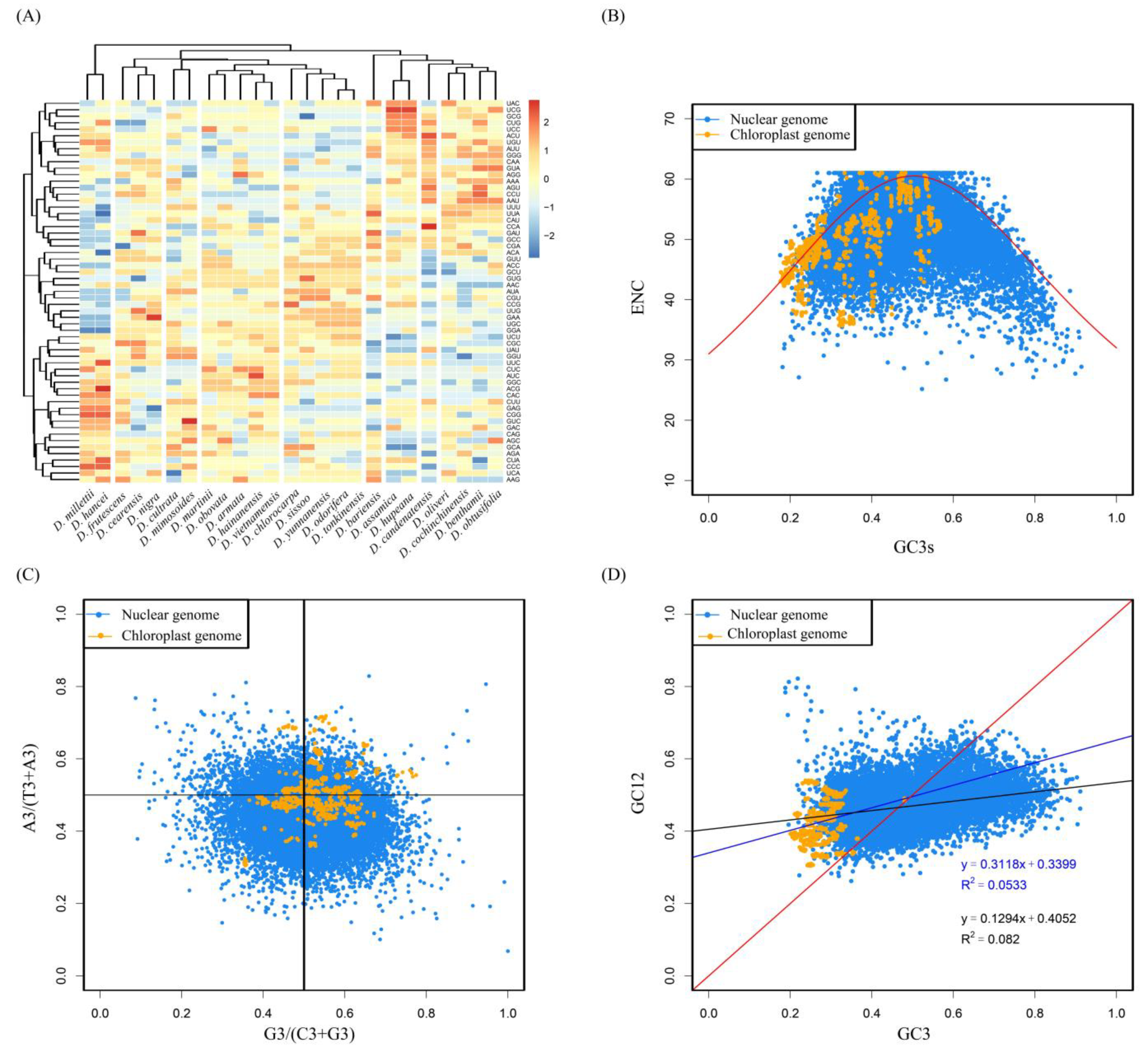
3.1. Analysis of Codon Composition Characteristics
3.2. Synonymous Codon Analysis
3.3. ENC—Plot Analysis
3.4. PR2-Plot Analysis
3.5. Neutrality Plot Analysis
3.6. Optimal Codon Determination
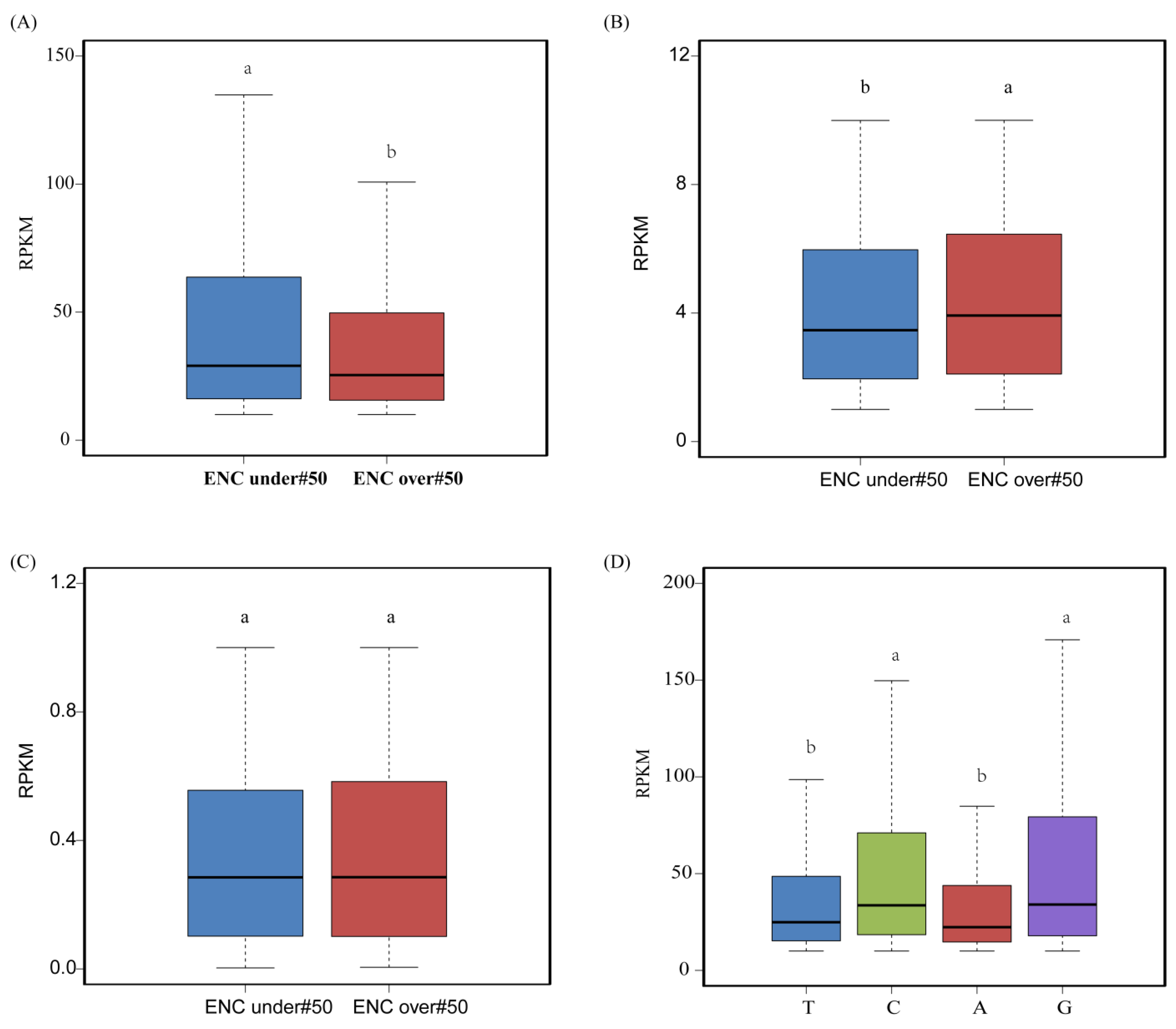
3.7. Relationship between Codon Usage and Gene Expression
3.8. Phylogenetic Relationships of 25 Dalbergia Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ENC | Effective number of codons |
| GC | Total G + C contents of the gene |
| GC1, GC2, GC3 | G + C content at the first, second, and third codon positions |
| GC12 | Average GC content at the first and second codon positions |
| GC3s | G + C content at the third position of synonymous codons |
| ENCexp | Expected ENC value |
| ENCobs | Observed ENC value |
| NCBI | National Centre for Biotechnology |
| PR2 | Parity Rule 2 |
| RSCU | Relative synonymous codon usage |
| T3s, C3s, A3s, G3s | Content of T, C, A and G at the third codon position of synonymous codons |
References
- Saw, P.E.; Xu, X.; Chen, J.; Song, E.W. Non-coding RNAs: The new central dogma of cancer biology. Sci. China Life Sci. 2021, 64, 22–50. [Google Scholar] [CrossRef] [PubMed]
- Hershberg, R.; Petrov, D.A. Selection on Codon Bias. Annu. Rev. Genet. 2008, 42, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Behura, S.K.; Severson, D.W. Comparative analysis of codon usage bias and codon context patterns between dipteran and hymenopteran sequenced genomes. PLoS ONE 2012, 7, e43111. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, Y.; Du, X.; Chen, Z.; Zeng, X.; Chen, Y.; Zhang, H. Patterns of synonymous codon usage bias in the model grass Brachypodium distachyon. Genet. Mol. Res. 2012, 11, 4695–4706. [Google Scholar] [CrossRef]
- Carlini, D.B.; Stephan, W. In vivo introduction of unpreferred synonymous codons into the Drosophila Adh gene results in reduced levels of ADH protein. Genetics 2003, 163, 239–243. [Google Scholar] [CrossRef]
- Roberts, R.J. Restriction and modification enzymes and their recognition sequences. Nucleic. Acids. Res. 1985, 11 (Suppl. S13), r165–r200. [Google Scholar] [CrossRef]
- Archetti, M. Codon usage bias and mutation constraints reduce the level of error minimization of the genetic code. J. Mol. Evol. 2004, 59, 258–266. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, L.; Wang, W.; Zhang, Z.; Du, H.; Qu, Z.; Li, X.Q.; Xiang, H. Differences in Codon Usage Bias between Photosynthesis-Related Genes and Genetic System-Related Genes of Chloroplast Genomes in Cultivated and Wild Solanum Species. Int. J. Mol. Sci. 2018, 19, 3142. [Google Scholar] [CrossRef]
- Monroe, J.G.; Srikant, T.; Carbonell-Bejerano, P.; Becker, C.; Lensink, M.; Exposito-Alonso, M.; Klein, M.; Hildebrandt, J.; Neumann, M.; Kliebenstein, D.; et al. Mutation bias reflects natural selection in Arabidopsis thaliana. Nature 2022, 602, 101–105. [Google Scholar] [CrossRef]
- Ata, G.; Wang, H.; Bai, H.; Yao, X.; Tao, S. Edging on Mutational Bias, Induced Natural Selection from Host and Natural Reservoirs Predominates Codon Usage Evolution in Hantaan Virus. Front. Microbiol. 2021, 12, 699788. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tian, C.; Yang, Y.; Li, Y.; Liu, Q.; Li, Z.; Jin, K. Comparative and Phylogenetic Analysis of Complete Chloroplast Genomes in Leymus (Triticodae, Poaceae). Genes 2022, 13, 1425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Fang, Y.; Wang, X.; Deng, X.; Zhang, X.; Hu, S.; Yu, J. The complete chloroplast and mitochondrial genome sequences of Boea hygrometrica: Insights into the evolution of plant organellar genomes. PLoS ONE 2012, 7, e30531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Liu, Y.; Yuan, Q.; Sun, J.; Guo, L. Chloroplast genome variation and phylogenetic relationships of Atractylodes species. BMC Genom. 2021, 22, 103. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, T.V.; Pavlova, O.A.; Bogomaz, D.I.; Demkovich, A.E.; Lutova, L.A. Molecular markers for plant species identification and phylogenetics. Ecol. Genet. 2011, 9, 32–43. [Google Scholar] [CrossRef]
- Xu, J.; Shen, X.; Liao, B.; Xu, J.; Hou, D. Comparing and phylogenetic analysis chloroplast genome of three Achyranthes species. Sci. Rep. 2020, 10, 10818. [Google Scholar] [CrossRef]
- Yang, L.; Abduraimov, O.; Tojibaev, K.; Shomurodov, K.; Zhang, Y.-M.; Li, W.-J. Analysis of complete chloroplast genome sequences and insight into the phylogenetic relationships of Ferula L. BMC Genom. 2022, 23, 643. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Dong, J.; Zhang, T.; Xiao, H. Comparative chloroplast genomes and phylogenetic analysis of Aquilegia. Appl. Plant Sci. 2021, 9, e11412. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Q.; Wang, C.; Li, F.; Tian, F.; Lu, Y.; Hu, Y.; Yang, H.; Cui, G. Analysis of codon usage patterns of the chloroplast genome in Delphinium grandiflorum L. reveals a preference for AT-ending codons as a result of major selection constraints. PeerJ 2021, 9, e10787. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wong, K.H.; Kong, B.L.; Siu, T.Y.; But, G.W.; Tsang, S.S.; Lau, D.T.; Shaw, P.C. Comparative Analysis of Chloroplast Genomes of Dalbergia Species for Identification and Phylogenetic Analysis. Plants 2022, 11, 1109. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Meng, H.; Yu, Z.; Yang, M.; Wei, J. Dalbergia odorifera: A review of its traditional uses, phytochemistry, pharmacology, and quality control. J. Ethnopharmacol. 2020, 248, 112328. [Google Scholar] [CrossRef]
- Hong, Z.; Li, J.; Liu, X.; Lian, J.; Zhang, N.; Yang, Z.; Niu, Y.; Cui, Z.; Xu, D. The chromosome-level draft genome of Dalbergia odorifera. Gigascience 2020, 9, giaa084. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Panahipour, L.; Gruber, R. Flavonoids from Dalbergia cochinchinensis: Impact on osteoclastogenesis. J. Dent. Sci. 2023, 18, 112–119. [Google Scholar] [CrossRef]
- Hong, Z.; Liao, X.; Ye, Y.; Zhang, N.; Yang, Z.; Zhu, W.; Gao, W.; Sharbrough, J.; Tembrock, L.R.; Xu, D.; et al. A complete mitochondrial genome for fragrant Chinese rosewood (Dalbergia odorifera, Fabaceae) with comparative analyses of genome structure and intergenomic sequence transfers. BMC Genom. 2021, 22, 672. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, M.; Kang, S.; Yang, C.; Meng, H.; Yang, Y.; Zhao, X.; Gao, Z.; Xu, Y.; Jin, Y.; et al. Molecular Mechanism Underlying Mechanical Wounding-Induced Flavonoid Accumulation in Dalbergia odorifera T. Chen, an Endangered Tree That Produces Chinese Rosewood. Genes 2020, 11, 478. [Google Scholar] [CrossRef]
- Lu, J.K.; Li, Z.S.; Yang, F.C.; Wang, S.K.; Liang, J.F.; He, X.H. Concurrent carbon and nitrogen transfer between hemiparasite Santalum album and two N2-fixing hosts in a sandalwood plantation. For. Ecol. Manag. 2020, 464, 118060. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Z.; Meng, X.; Zhang, L.; Liu, Z.; Liu, M.; Zhang, F.; Zhao, J. Codon usage patterns across seven Rosales species. BMC Plant Biol. 2022, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Schalamun, M.; Morales-Suarez, A.; Kainer, D.; Schwessinger, B.; Lanfear, R. Assembly of chloroplast genomes with long- and short-read data: A comparison of approaches using Eucalyptus pauciflora as a test case. BMC Genom. 2018, 19, 977. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Cheng, A.; Wang, M. Analysis of synonymous codon usage in the outer membrane efflux protein gene of Riemerella anatipestifer. In Proceedings of the 2012 5th International Conference on BioMedical Engineering and Informatics, Chongqing, China, 16–18 October 2012; pp. 1147–1152. [Google Scholar]
- Long, S.; Yao, H.; Wu, Q.; Li, G. Analysis of compositional bias and codon usage pattern of the coding sequence in Banna virus genome. Virus Res. 2018, 258, 68–72. [Google Scholar] [CrossRef]
- Sharp, P.M.; Li, W.H. An evolutionary perspective on synonymous codon usage in unicellular organisms. J. Mol. Evol. 1986, 24, 28–38. [Google Scholar] [CrossRef]
- Dilucca, M.; Forcelloni, S.; Georgakilas, A.G.; Giansanti, A.; Pavlopoulou, A. Codon Usage and Phenotypic Divergences of SARS-CoV-2 Genes. Viruses 2020, 12, 498. [Google Scholar] [CrossRef]
- Dilucca, M.; Pavlopoulou, A.; Georgakilas, A.G.; Giansanti, A. Codon usage bias in radioresistant bacteria. Gene 2020, 742, 144554. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xing, H.; Yuan, Y.; Wang, X.; Saeed, M.; Tao, J.; Feng, W.; Zhang, G.; Song, X.; Sun, X. Genome-wide analysis of codon usage bias in four sequenced cotton species. PLoS ONE 2018, 13, e0194372. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Li, Y.; Ji, K.-K.; Zhu, J.; Ling, P.; Zhou, T.; Fan, L.-Y.; Xie, S.-Q. Genome-wide codon usage pattern analysis reveals the correlation between codon usage bias and gene expression in Cuscuta australis. Genomics 2020, 112, 2695–2702. [Google Scholar] [CrossRef]
- Almutairi, M.M. Analysis of chromosomes and nucleotides in rice to predict gene expression through codon usage pattern. Saudi J. Biol. Sci. 2021, 28, 4569–4574. [Google Scholar] [CrossRef]
- Huang, C.; Fu, D.; Wu, X.; Chu, M.; Ma, X.; Jia, C.; Guo, X.; Bao, P.; Yan, P.; Chunnian, L. Characterization of the complete mitochondrial genome of the Bazhou yak (Bos Grunniens). Mitochondrial. DNA B Resour. 2019, 4, 3234–3235. [Google Scholar] [CrossRef]
- Tang, D.; Wei, F.; Cai, Z.; Wei, Y.; Khan, A.; Miao, J.; Wei, K. Analysis of codon usage bias and evolution in the chloroplast genome of Mesona chinensis Benth. Dev. Genes Evol. 2021, 231, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Andargie, M.; Congyi, Z. Genome-wide analysis of codon usage in sesame (Sesamum indicum L.). Heliyon 2022, 8, e08687. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Song, Y.; Jing, L. Analysis of codon usage bias of WRKY transcription factors in Helianthus annuus. BMC Genom. Data 2022, 23, 46. [Google Scholar] [CrossRef]
- He, B.; Dong, H.; Jiang, C.; Cao, F.; Tao, S.; Xu, L.-A. Analysis of codon usage patterns in Ginkgo biloba reveals codon usage tendency from A/U-ending to G/C-ending. Sci. Rep. 2016, 6, 35927. [Google Scholar] [CrossRef]
- Nath Choudhury, M.; Uddin, A.; Chakraborty, S. Codon usage bias and its influencing factors for Y-linked genes in human. Comput. Biol. Chem. 2017, 69, 77–86. [Google Scholar] [CrossRef]
- Zhou, Z.; Dang, Y.; Zhou, M.; Li, L.; Yu, C.-h.; Fu, J.; Chen, S.; Liu, Y. Codon usage is an important determinant of gene expression levels largely through its effects on transcription. Proc. Natl. Acad. Sci. USA 2016, 113, E6117–E6125. [Google Scholar] [CrossRef] [PubMed]
- Ran, W.; Kristensen, D.M.; Koonin, E.V. Coupling between protein level selection and codon usage optimization in the evolution of bacteria and archaea. mBio 2014, 5, e00956-14. [Google Scholar] [CrossRef] [PubMed]
- Southworth, J.; Armitage, P.; Fallon, B.; Dawson, H.; Bryk, J.; Carr, M. Patterns of Ancestral Animal Codon Usage Bias Revealed through Holozoan Protists. Mol. Biol. Evol. 2018, 35, 2499–2511. [Google Scholar] [CrossRef]
- Shen, Z.; Gan, Z.; Zhang, F.; Yi, X.; Zhang, J.; Wan, X. Analysis of codon usage patterns in citrus based on coding sequence data. BMC Genom. 2020, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mazumder, T.H.; Uddin, A. Compositional dynamics and codon usage pattern of BRCA1 gene across nine mammalian species. Genomics 2019, 111, 167–176. [Google Scholar] [CrossRef]
- Deb, B.; Uddin, A.; Chakraborty, S. Codon usage pattern and its influencing factors in different genomes of hepadnaviruses. Arch. Virol. 2020, 165, 557–570. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, Q.; Wang, Y.; Li, M.; Wang, C.; Wang, Z.; Jiao, C.; Xu, C.; Wang, H.; Zhang, Z. Comparative Analysis of Codon Bias in the Chloroplast Genomes of Theaceae Species. Front. Genet. 2022, 13, 824610. [Google Scholar] [CrossRef]
- Barbhuiya, P.A.; Uddin, A.; Chakraborty, S. Understanding the codon usage patterns of mitochondrial CO genes among Amphibians. Gene 2021, 777, 145462. [Google Scholar] [CrossRef]
- Marais, G.; Mouchiroud, D.; Duret, L. Does recombination improve selection on codon usage? Lessons from nematode and fly complete genomes. Proc. Natl. Acad. Sci. USA 2001, 98, 5688–5692. [Google Scholar] [CrossRef]
- Huang, X.; Jiao, Y.; Guo, J.; Wang, Y.; Chu, G.; Wang, M. Analysis of codon usage patterns in Haloxylon ammodendron based on genomic and transcriptomic data. Gene 2022, 845, 146842. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, B.; Li, B.; Zhou, Q.; Wang, G.; Jiang, X.; Wang, C.; Xu, Z. Comparative analysis of codon usage patterns in chloroplast genomes of six Euphorbiaceae species. PeerJ 2020, 8, e8251. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Xue, P. Codon usage pattern and genetic diversity in chloroplast genomes of Panicum species. Gene 2021, 802, 145866. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, L.; Xue, P.; Zhu, M. Comparative Analysis on the Codon Usage Pattern of the Chloroplast Genomes in Malus Species. Biochem. Genet. 2022, 60, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Liang, Y.; Ping, J.; Li, J.; Shi, W.; Su, Y.; Wang, T. Chloroplast gene expression level is negatively correlated with evolutionary rates and selective pressure while positively with codon usage bias in Ophioglossum vulgatum L. BMC Plant Biol. 2022, 22, 580. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Das, S.S.; Rakshit, R. Codon usage pattern and predicted gene expression in Arabidopsis thaliana. Gene 2019, 721, 100012. [Google Scholar] [CrossRef] [PubMed]
- Fuller, Z.L.; Haynes, G.D.; Zhu, D.; Batterton, M.; Chao, H.; Dugan, S.; Javaid, M.; Jayaseelan, J.C.; Lee, S.; Li, M.; et al. Evidence for stabilizing selection on codon usage in chromosomal rearrangements of Drosophila pseudoobscura. G3 2014, 4, 2433–2449. [Google Scholar] [CrossRef] [PubMed]
- Stoletzki, N. The surprising negative correlation of gene length and optimal codon use—Disentangling translational selection from GC-biased gene conversion in yeast. BMC Evol. Biol. 2011, 11, 93. [Google Scholar] [CrossRef]
- Nhung, N.P.; Thu, P.Q.; Chi, N.M.; Dell, B. Vegetative propagation of Dalbergia tonkinensis, a threatened, high-value tree species in South-east Asia. South For. A J. For. Sci. 2019, 81, 195–200. [Google Scholar] [CrossRef]
- Quax, T.E.; Claassens, N.J.; Söll, D.; van der Oost, J. Codon Bias as a Means to Fine-Tune Gene Expression. Mol. Cell 2015, 59, 149–161. [Google Scholar] [CrossRef]





| Indicators | Nuclear Genome | Chloroplast Genome | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Max. | Min. | Mean ± SD | Max. | Min. | |
| T3s | 0.390 ± 0.080 | 0.6854 | 0.0317 | 0.397 ± 0.095 | 0.5588 | 0.1684 |
| C3s | 0.267 ± 0.087 | 0.816 | 0 | 0.216 ± 0.066 | 0.3333 | 0.0649 |
| A3s | 0.311 ± 0.073 | 0.6026 | 0.0242 | 0.404 ± 0.076 | 0.5714 | 0.2079 |
| G3s | 0.283 ± 0.068 | 0.8381 | 0.0476 | 0.253 ± 0.093 | 0.487 | 0.1012 |
| ENC | 51.86 ± 4.410 | 61 | 25.17 | 49.9 ± 6.199 | 35.71 | 61 |
| GC3s | 0.432 ± 0.102 | 0.914 | 0.175 | 0.354 ± 0.108 | 0.567 | 0.176 |
| GC | 0.461 ± 0.050 | 0.695 | 0.259 | 0.390 ± 0.046 | 0.502 | 0.285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-K.; Liu, Y.; Zheng, H.-Y.; Tang, M.-Q.; Xie, S.-Q. Comparative Analysis of Codon Usage Patterns in Nuclear and Chloroplast Genome of Dalbergia (Fabaceae). Genes 2023, 14, 1110. https://doi.org/10.3390/genes14051110
Wang Z-K, Liu Y, Zheng H-Y, Tang M-Q, Xie S-Q. Comparative Analysis of Codon Usage Patterns in Nuclear and Chloroplast Genome of Dalbergia (Fabaceae). Genes. 2023; 14(5):1110. https://doi.org/10.3390/genes14051110
Chicago/Turabian StyleWang, Zu-Kai, Yi Liu, Hao-Yue Zheng, Min-Qiang Tang, and Shang-Qian Xie. 2023. "Comparative Analysis of Codon Usage Patterns in Nuclear and Chloroplast Genome of Dalbergia (Fabaceae)" Genes 14, no. 5: 1110. https://doi.org/10.3390/genes14051110





