Screening Candidate Genes at the Co Locus Conferring to the Columnar Growth Habit in Apple (Malus × Domestica Borkh.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction of DNA and RNA
2.3. DNA Sequencing and Bioinformatic Analysis
2.4. Subcellular Localization of Candidate Genes
2.5. Transformation of Tobacco
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
3.1. Identification and Sequence Alignments of Co Candidate Genes
3.2. Subcellular Localization Analysis of Co Candidate Genes in Tobacco
3.3. Ectopic Expression Analysis of Co Candidate Genes in Tobacco
3.4. Branching Was Stimulated by High Expression of NtPIN1 and NtGA2ox in MdCo38-OE Plants
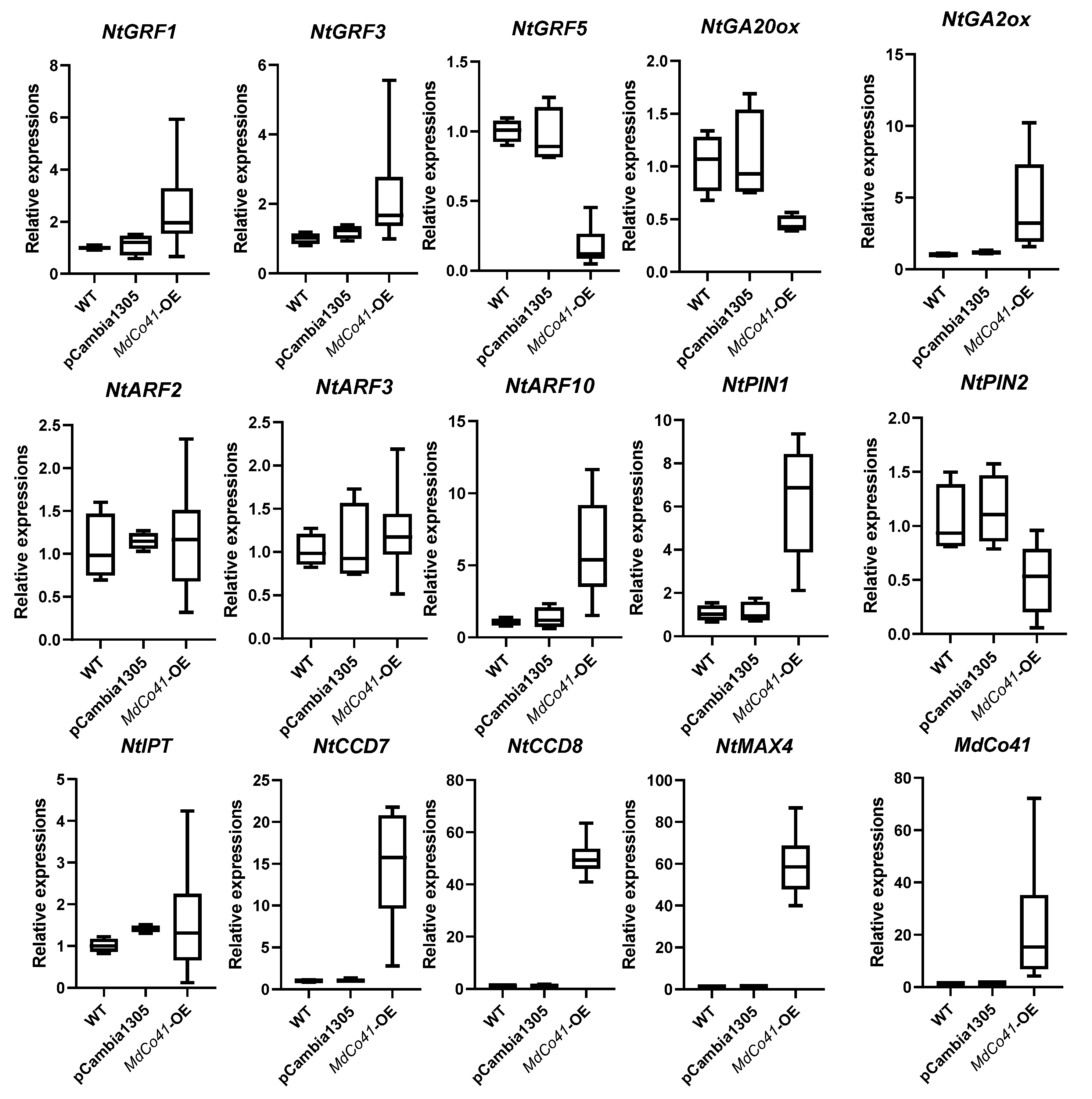
3.5. Leaf Was Enlarged by High Expression of NtCCDs in MdCo41—OE Plants
3.6. Expression of MdCo38 and MdCo41 in Apple Cultivars and F1 Generation
4. Discussion
4.1. Predicted Functions of Genes Downregulated in ‘Wijcik’ Apple
4.2. Functional Analysis of Upregulated Genes in ‘Wijcik’ Apple Co32 and Co33
4.3. MdCo38 and MdC41 Involved in Columnar Growth in Apple
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rom, C.R.; Barritt, B. Spur Development of ‘Delicious’ Apple as Influenced by Position, Wood age, Strain, and Pruning. HortScience 1990, 25, 1578–1581. [Google Scholar] [CrossRef]
- Barritt, B.H. Intensive Orchard Management; Good Fruit Growers: Washington, DC, USA, 1992. [Google Scholar]
- Lespinasse, J.M.; Delort, J.F. Regulation of Fruiting in Apple Role of the Course and Crowned Brindles. Acta Hortic. 1993, 349, 239–245. [Google Scholar] [CrossRef]
- Costes, E.; Lauri, P.É.; Regnard, J.L. Analyzing Fruit Tree Architecture: Implications for Tree Management and Fruit Production. Hortic. Rev. 2006, 32, 1–61. [Google Scholar] [CrossRef]
- Fisher, D.V. The ‘Wijcik Spur McIntosh’. Fruit Var. J. 1995, 49, 212–213. [Google Scholar]
- Lauri, P.; Lespinasse, J.; Térouanne, É. Vegetative Growth and Reproductive Strategies in Apple Fruiting Branches-An Investigation into Various Cultivars. Acta Hortic. 1997, 451, 717–724. [Google Scholar] [CrossRef]
- Lapins, K.O. Segregation of Compact Growth Types in Certain Apple Seedling Progenies. Can. J. Plant Sci. 1969, 49, 765–768. [Google Scholar] [CrossRef]
- Lapins, K.O. Inheritance of Compact Growth Type in Apple. J. Am. Soc. Hortic. Sci. 1976, 101, 133–135. [Google Scholar] [CrossRef]
- Hemmat, M.; Weeden, N.F.; Manganaris, A.G.; Lawson, D.M. Molecular Marker Linkage Map for Apple. J. Hered. 1994, 85, 4–11. [Google Scholar] [CrossRef]
- Conner, P.J.; Brown, S.K.; Weeden, N.F. Randomly Amplified Polymorphic DNA-based Genetic Linkage Maps of Three Apple Cultivars. J. Am. Soc. Hortic. Sci. 1997, 122, 350–359. [Google Scholar] [CrossRef]
- Kim, M.Y.; Song, K.J.; Hwang, J.H.; Shin, Y.U.; Lee, H.J. Development of RAPD and SCAR Markers Linked to the Co Gene Conferring Columnar Growth Habit in Apple (Malus pumila Mill.). J. Am. Soc. Hortic. Sci. 2003, 78, 559–562. [Google Scholar] [CrossRef]
- Tian, Y.K.; Wang, C.H.; Zhang, J.S.; James, C.; Dai, H.Y. Mapping Co, a Gene Controlling the Columnar Phenotype of Apple, with Molecular Markers. Euphytica 2005, 145, 181–188. [Google Scholar] [CrossRef]
- Moriya, S.; Iwanami, H.; Kotoda, N.; Takahashi, S.; Yamamoto, T.; Abe, K. Development of a Marker-Assisted Selection System for Columnar Growth Habit in Apple Breeding. J. Jpn. Soc. Hortic. Sci. 2009, 78, 279–287. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The Genome of the Domesticated Apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Zhu, Y.; Fernandez-Fernandez, F.; Keulemans, J.; Brown, S.; Xu, K. Fine Genetic Mapping of the Co Locus Controlling Columnar Growth Habit in Apple. Mol. Genet. Genom. 2012, 287, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Baldi, P.; Wolters, P.J.; Komjanc, M.; Viola, R.; Velasco, R.; Salvi, S. Genetic and Physical Characterisation of the Locus Controlling Columnar Habit in Apple (Malus × domestica Borkh.). Mol. Breed. 2013, 31, 429–440. [Google Scholar] [CrossRef]
- Moriya, S.; Okada, K.; Haji, T.; Yamamoto, T.; Abe, K. Fine Mapping of Co, a Gene Controlling Columnar Growth Habit Located on Apple (Malus × domestica Borkh.) Linkage Group 10. Plant Breed. 2012, 131, 641–647. [Google Scholar] [CrossRef]
- Wolters, P.J.; Schouten, H.J.; Velasco, R.; Si-Ammour, A.; Baldi, P. Evidence for Regulation of Columnar Habit in Apple by a Putative2OG-Fe(II) Oxygenase. New Phytol. 2013, 200, 993–999. [Google Scholar] [CrossRef]
- Otto, D.; Petersen, R.; Brauksiepe, B.; Braun, P.; Schmidt, E.R. The Columnar Mutation (-Co gene) of Apple (Malus × domestica) is Associated with an Integration of a Gypsy-like Retrotransposon. Mol. Breed. 2014, 33, 863–880. [Google Scholar] [CrossRef]
- Petersen, R.; Djozgic, H.; Rieger, B.; Rapp, S.; Schmidt, E.R. Columnar Apple Primary Roots Share Some Features of the Columnar-specific Gene Expression Profile of Aerial Plant Parts as Evidenced by RNA-Seq Analysis. BMC Plant Biol. 2015, 15, 34. [Google Scholar] [CrossRef]
- Kazuma, O.; Masato, W.; Yumiko, T.; Mikiko, K.; Hitoshi, S.; Masaru, N.; Masaharu, M.; Masatoshi, N.; Shigeki, M.; Taku, S.K.A. Columnar Growth Phenotype in Apple Results from Gibberellin Deficiency by Ectopic Expression of a Dioxygenase Gene. Tree Physiol. 2020, 40, 1205–1216. [Google Scholar] [CrossRef]
- Sun, X.; Wen, C.P.; Xu, J.H.; Wang, Y.H.; Zhu, J.; Zhang, Y.G. The Apple Columnar Gene Candidate MdCoL and the AP2/ERF Factor MdDREB2 Positively Regulate ABA Biosynthesis by Ctivating the Expression of MdNCED6/9. Tree Physiol. 2020, 41, 1065–1076. [Google Scholar] [CrossRef]
- Daichi, W.; Ikuo, T.; Naiyanate, J.T.; Sho, M.; Jiang, K.; Masaru, N.; Masato, W.; Tadao, A.; Masaharu, M.; Kazuma, O.; et al. The Apple Gene Responsible for Columnar Tree Shape Reduces the Abundance of Biologically Sctive Gibberellin. Plant J. 2021, 105, 1026–1034. [Google Scholar] [CrossRef]
- Wang, L.M.; Yu, B.Y.; Zhao, Y.N.; Li, Y.Z.; Guo, J.; Zhu, Y.D. A Putative 2OG-Fe(II) Oxygenase’s Response to Gibberellin Deficiency is Related to the Internodal Growth of Columnar Apples. Acta Physiol. Plant. 2021, 43, 70. [Google Scholar] [CrossRef]
- Wang, L.M.; Guo, J.; Chu, Y.; Pan, Q.; Zhu, Y.D. MdCo31 Interacts with an RNA Polymerase II Transcription Subunit 32 to Regulate Dwarf Growth with Short Internodes in Columnar Apple. Plant Sci. 2022, 325, 111496. [Google Scholar] [CrossRef]
- Doley, J. DNA Protocols for Plants. In Molecular Techniques in Taxonomy; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yan, Y.; Li, C.; Dong, X.; Li, H.; Zhang, D.; Zhou, Y.; Jiang, B.; Peng, J.; Qin, X.; Cheng, J.; et al. MYB30 is a Key Negative Regulator of Arabidopsis Photomorphogenic Development that Promotes PIF4 and PIF5 Protein Accumulation in the Light. Plant Cell 2020, 32, 2196–2215. [Google Scholar] [CrossRef]
- Fichman, Y.; Zandalinas, S.I.; Sengupta, S.; Burks, D.; Myers, R.J., Jr.; Azad, R.K.; Mittler, R. MYB30 Orchestrates Systemic Reactive Oxygen Signaling and Plant Acclimation. Plant Physiol. 2020, 184, 666–675. [Google Scholar] [CrossRef]
- Frank, D.; Guthrie, C. An Essential Splicing Factor, SLU7, Mediates 3’ Splice Site Choice in Yeast. Genes Dev. 1992, 6, 2112–2124. [Google Scholar] [CrossRef]
- Brys, A.; Schwer, B. Requirement for SLU7 in Yeast Pre-mRNA Splicing is Dictated by the Distance between the Branchpoint and the 3’ Splice Site. RNA 1996, 2, 707–717. [Google Scholar]
- Karthikeyan, A.S.; Ballachanda, D.N.; Raghothama, K.G. Promoter Deletion Analysis Elucidates the Role of Cis Elements and 5’UTR Intron in Spatiotemporal Regulation of AtPht1;4 Expression in Arabidopsis. Physiol. Plant. 2009, 136, 10–18. [Google Scholar] [CrossRef]
- Ye, Y.; Yuan, J.; Chang, X.J.; Yang, M.; Zhang, L.J.; Lu, K.; Lian, X.M. The Phosphate Transporter Gene OsPht1; 4 is Involved in Phosphate Homeostasis in Rice. PLoS ONE 2015, 10, e0126186. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, S.; Wu, C.; Zhang, Q.; Zhang, Y.; Chen, Q.; Li, Y.; Hao, L.; Gu, Z.; Li, W.; et al. Malus domestica ADF1 Severs Actin Filaments in Growing Pollen Tubes. Funct. Plant Biol. 2017, 44, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Kost, B.; Xia, G.; Chua, N.H. Molecular Identification and Characterization of the Arabidopsis AtADF1, AtADF5 and AtADF6 Genes. Plant Mol. Biol. 2001, 45, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Huang, W.L.; Hong, C.Y.; Lur, H.S.; Chang, M.C. Comprehensive Analysis of Differentially Expressed Rice Actin Depolymerizing Factor Gene Family and Heterologous Overexpression of OsADF3 Confers Arabidopsis thaliana Drought Tolerance. Rice 2012, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Leasure, C.D.; Tong, H.; Yuen, G.; Hou, X.; Sun, X.; He, Z.H. Root UV-B Sensitive2 Acts with Root UV-B Sensitive1 in a Root Ultraviolet B-sensing Pathway. Plant Physiol. 2009, 150, 1902–1915. [Google Scholar] [CrossRef]
- Mccoy, J.G.; Arabshahi, A.; Bitto, E.; Bingman, C.A.; Ruzicka, F.J.; Frey, P.A.; Phillips, G.N. Structure and Mechanism of an ADP-glucose Phosphorylase from Arabidopsis thaliana. Biochemistry 2006, 45, 3154–3162. [Google Scholar] [CrossRef]
- Ranocha, P.; Dima, O.; Nagy, R.; Felten, J.; Corratgé-Faillie, C.; Novák, O.; Morreel, K.; Lacombe, B.; Martinez, Y.; Pfrunder, S.; et al. Arabidopsis WAT1 is a Vacuolar Auxin Transport Facilitator Required for Auxin Homoeostasis. Nat. Commun. 2013, 4, 2625. [Google Scholar] [CrossRef]
- Okada, K.; Honda, C. Molecular Mechanisms Regulating the Columnar Tree Architecture in Apple. Forests 2022, 13, 1084. [Google Scholar] [CrossRef]
- Love, J.; Björklund, S.; Vahala, J.; Hertzberg, M.; Kangasjärvi, J.; Sundberg, B. Ethylene is an Endogenous Stimulator of Cell Division in the Cambial Meristem of Populus. Proc. Natl. Acad. Sci. USA 2009, 106, 5984–5989. [Google Scholar] [CrossRef]
- Iwamoto, M.; Baba-Kasai, A.; Kiyota, S.; Hara, N.; Takano, M. ACO1, a Gene for Aminocyclopropane-1-carboxylate Oxidase: Effects on Internode Elongation at the Heading Stage in Rice. Plant Cell Environ. 2010, 33, 805–815. [Google Scholar] [CrossRef]
- Manna, S. An Overview of Pentatricopeptide Repeat Proteins and Their Applications. Biochimie 2015, 113, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Emami, H.; Kempken, F. Precocious1 (Poco1), a Mitochondrial Pentatricopeptide Repeat Protein Affects Flowering Time in Arabidopsis thaliana. Plant J. 2019, 100, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, J.; Liere, K.; Kandlbinder, A.; Dietz, K.J.; Oelmüller, R. pTAC2, -6, and -12 Are Components of the Transcriptionally Active Plastid Chromosome That Are Required for Plastid Gene Expression. Plant Cell 2006, 18, 176–197. [Google Scholar] [CrossRef]
- Reinhardt, D.; Pesce, E.R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennett, M.; Traas, J.; Friml, J.; Kuhlemeier, C. Regulation of Phyllotaxis by Polar Auxin Transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Guan, C.; Gälweiler, L.; Taenzler, P.; Huijser, P.; Marchant, A.; Parry, G.; Bennett, M.; Wisman, E.; Palme, K. AtPIN2 Defines a Locus of Arabidopsis for Root Gravitropism Control. EMBO J. 1998, 17, 6903–6911. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, L.; Shou, H.; Wu, P. A PIN1 Family Gene, OsPIN1, involved in Auxin-dependent Adventitious Root Emergence and Tillering in Rice. Plant Cell Physiol. 2005, 10, 1674–1681. [Google Scholar] [CrossRef]
- Guan, C.; Wu, B.; Yu, T.; Wang, Q.; Krogan, N.T.; Liu, X.; Jiao, Y. Spatial Auxin Signaling Controls Leaf Flattening in Arabidopsis. Curr. Biol. 2017, 27, 2940–2950. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, R.; Zhou, L.; Wu, X.Y.; Xu, N.; Ma, X.H.; Zhang, Y. Genome-Wide Identification Analysis of the Auxin Response Factors Family in Nicotiana tabacum and the function of NtARF10 in Leaf Size Regulation. J. Plant Biol. 2021, 64, 281–297. [Google Scholar] [CrossRef]
- Miyawaki, K.; Tarkowski, P.; Matsumoto-Kitano, M.; Kato, T.; Sato, S.; Tarkowska, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP Isopentenyltransferases and tRNA Isopentenyltransferases in Cytokinin Biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef]
- Coles, J.P.; Phillips, A.L.; Croker, S.J.; García-Lepe, R.; Lewis, M.J.; Hedden, P. Modification of Gibberellin Production and Plant Development in Arabidopsis by Sense and Antisense Expression of Gibberellin 20-oxidase Genes. Plant J. 1999, 17, 547–556. [Google Scholar] [CrossRef]
- Lo, S.F.; Yang, S.Y.; Chen, K.T.; Hsing, Y.I.; Zeevaart, J.A.; Chen, L.J.; Yu, S.M. A Novel Class of Gibberellin 2-Oxidases Control Semidwarfism, Tillering, and Root Development in Rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The Path from β-carotene to Carlactone, a Strigolactone-like Plant Hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Hua, R.; Wang, X.; Wu, H.F.; Ou, H.; Lu, X.; Huang, Y.; Liu, D.; Sui, S. CpMAX1a, a Cytochrome P450 Monooxygenase Gene of Chimonanthus praecox Regulates Shoot Branching in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 10888. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.J.; Xu, X.; Gong, Z.H.; Tang, Y.W.; Wu, M.B.; Yan, F.; Zhang, X.L.; Zhang, Q.; Yang, F.Q.; Hu, X.W.; et al. Auxin Response Factor 6A Regulates Photosynthesis, Sugar Accumulation, and Fruit Development in Tomato. Hortic. Res. 2019, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Omidbakhshfard, M.A.; Proost, S.; Fujikura, U.; Mueller-roeber, B. Growth Regulating Factors (GRFs): A Small Transcription Factor Family with Important Functions in Plant Biology. Mol. Plant 2015, 8, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, G.; Kim, G.T.; Tsukaya, H. The Transcription Factor AtGRF5 and the Transcription Coactivator AN3 Regulate Cell Proliferation in Leaf Primordia of Arabidopsis thaliana. Plant J. 2005, 43, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Vercruyssen, L.; Tognetti, V.B.; Gonzalez, N.; Van, D.J.; De, M.L.; Bielach, A.; De, R.R.; Van, B.F.; Inzé, D. Growth Regulating Factor 5 Stimulates Arabidopsis Chloroplast Division, Photosynthesis, and Leaf Longevity. Plant Physiol. 2015, 167, 817–832. [Google Scholar] [CrossRef] [PubMed]

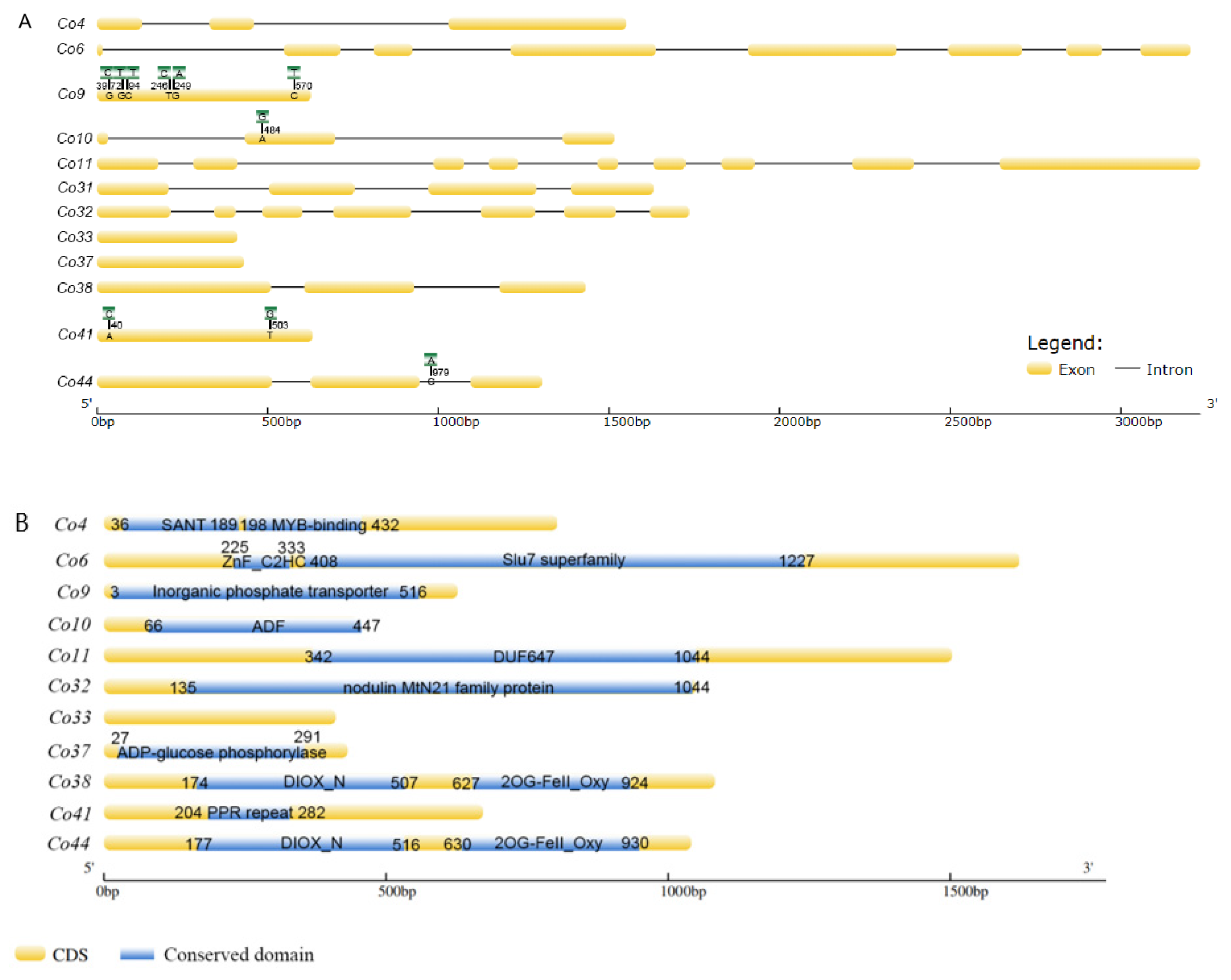
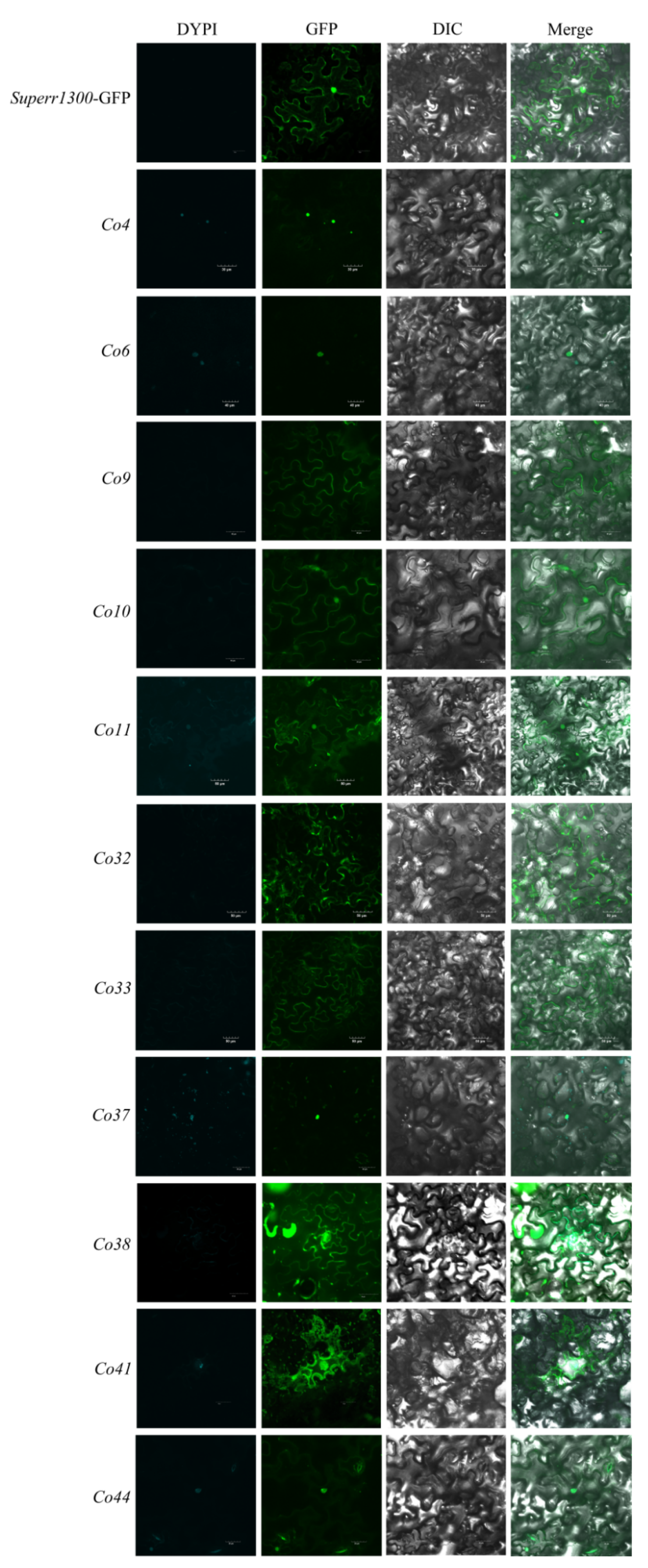
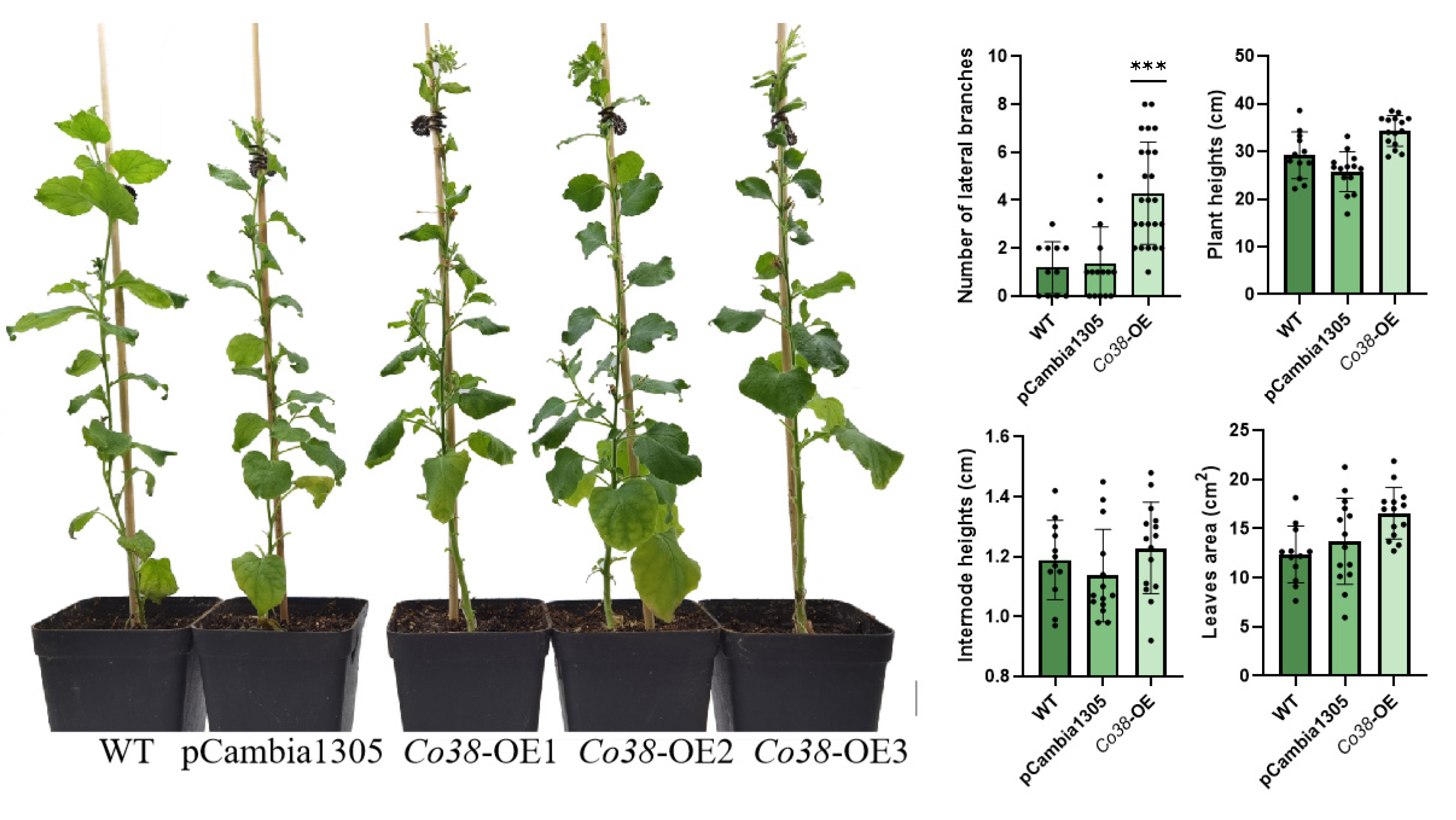


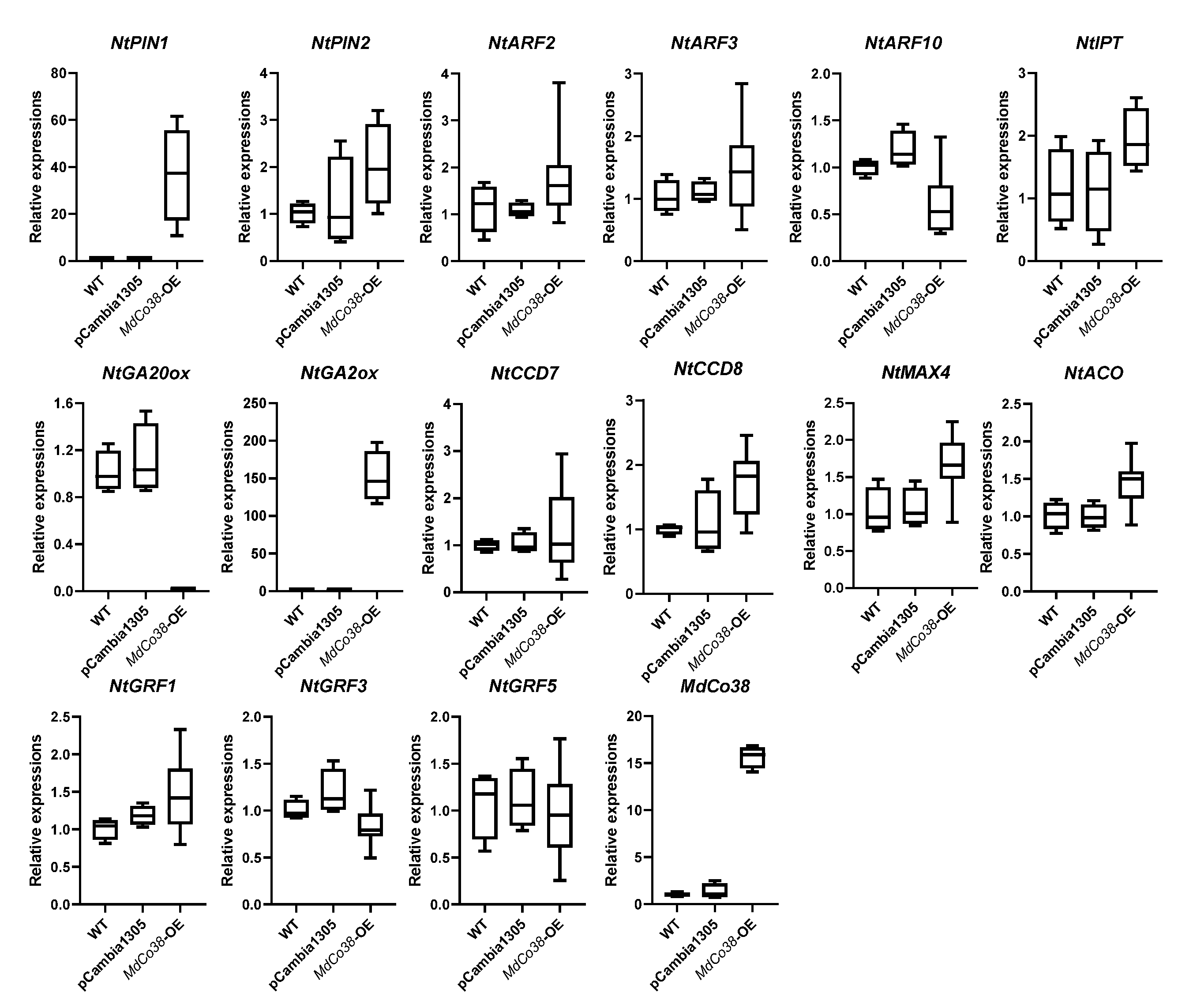


| Serial No. of Predicted Genes | Reported Genes | Genome No. | Putative Function | Gene Expression through SRA/EST Mining | Differential Expression in Shoot Tips of Columnar Apple | The Length of Genes in Genomic DNA and cDNA |
|---|---|---|---|---|---|---|
| Co4 | MdCo4 [18] | MDP0000897594 | MYB Transcription factor 30- like | Fruit, root, shoot tip | 1.6-fold down regulated | gDNA 1573 bp/cDNA 804 bp |
| Co6 | MDP0000326311 | Pre-mRNA-splicing factor SLU7 | Fruit, leaf, root, shoot tip | 2.8-fold down regulated | gDNA3204bp/cDNA1623bp | |
| Co9 | MdCo9 [18] | MDP0000942873 | Inorganic phosphate transporter1-4 | Fruit, leaf, root, shoot tip | 4.1-fold down regulated | gDNA 626bp/cDNA 626bp |
| Co10 | MdCo8 [18] | MDP0000329966 | Actin depolymerization factor (ADF) | Fruit, leaf, root, shoot tip | 2.1-fold down regulated | gDNA1516bp/cDNA450bp |
| Co11 | MdCo6 [18] | MDP0000284965 | Root UV-B sensitive protein (RUS) | Fruit, leaf, root, shoot tip | 1.6-fold down regulated | gDNA 3232bp/cDNA1503bp |
| Co31 | MdCo31/32 [18] | MDP0000687812 | 2OG-Fe(Ⅱ)oxygenase | Root, shoot tip | 62-fold up regulated | gDNA1631bp/cDNA1020bp |
| Co32 | MdCo39 [18] | MDP0000912170 | Walls are thin 1(WAT1)-related protein | Fruit, leaf, root, shoot tip | 2-fold up regulated | gDNA1735bp/cDNA1050bp |
| Co33 | MdCo40 [18] | MDP0000912172 | Unknown | Fruit, leaf, root, shoot tip | 14-fold up regulated | gDNA 411 bp/cDNA 411 bp |
| Co37 | MDP0000934869 | A putative ADP-glucose phosphorylase | Fruit, leaf, root, shoot tip | 2.9-fold down regulated | Gdna 432 bp/cDNA 432bp | |
| Co38 | 100016-gene [18] | MDP0000163720 | 1-aminocyclopropane-1-carboxylate oxidase (ACO)—like | Fruit, leaf, root, shoot tip | 4.7-fold up regulated | gDNA1437 bp/cDNA1083 bp |
| Co41 | MDP0000684988 | Pentatricopetide repeat (PPR) | Fruit, leaf, shoot tip | 34.8-fold up regulated | gDNA 672 bp/cDNA 672 bp | |
| Co44 | 95062-gene [18] | MDP0000878773 | ACO-like | Fruit, leaf, root, shoot tip | 2.3-fold down regulated | gDNA1304 bp/cDNA 1041 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Zhao, Y.; Chu, Y.; Li, Y.; Song, Y.; Pan, Q.; Qiu, Z.; Yu, B.; Zhu, Y. Screening Candidate Genes at the Co Locus Conferring to the Columnar Growth Habit in Apple (Malus × Domestica Borkh.). Genes 2023, 14, 964. https://doi.org/10.3390/genes14050964
Guo J, Zhao Y, Chu Y, Li Y, Song Y, Pan Q, Qiu Z, Yu B, Zhu Y. Screening Candidate Genes at the Co Locus Conferring to the Columnar Growth Habit in Apple (Malus × Domestica Borkh.). Genes. 2023; 14(5):964. https://doi.org/10.3390/genes14050964
Chicago/Turabian StyleGuo, Jing, Yuan Zhao, Yu Chu, Yuru Li, Yuqi Song, Qi Pan, Zhannan Qiu, Boyang Yu, and Yuandi Zhu. 2023. "Screening Candidate Genes at the Co Locus Conferring to the Columnar Growth Habit in Apple (Malus × Domestica Borkh.)" Genes 14, no. 5: 964. https://doi.org/10.3390/genes14050964
APA StyleGuo, J., Zhao, Y., Chu, Y., Li, Y., Song, Y., Pan, Q., Qiu, Z., Yu, B., & Zhu, Y. (2023). Screening Candidate Genes at the Co Locus Conferring to the Columnar Growth Habit in Apple (Malus × Domestica Borkh.). Genes, 14(5), 964. https://doi.org/10.3390/genes14050964







