Abstract
The columnar growth trait of apple (Malus × domestica Borkh.) is genetically controlled by the Columnar (Co) locus on 10 chromosomes, including several candidate genes. Except for MdCo31, other candidate genes at the Co locus are less elucidated. In this study, a strategy of step-by-step screening was adopted to select 11 candidate genes by experimental cloning, transient expression, and genetic transformation. There existed several SNPs in four genes by sequence alignment in columnar and non-columnar apples. Two genes were detected in the nucleus and three genes in the cell membrane, other genes were located in multiple cellular structures by subcellular location. Ectopic expression demonstrated that more branching occurred in MdCo38-OE by upregulating NtPIN1 and NtGA2ox and enlarged leaves in MdCo41-OE tobaccos by upregulating NtCCDs. Transcripts of MdCo38 and MdCo41 were associated with the Co genotypes in apples. The results indicate that MdCo38 and MdCo41 are involved in the columnar growth phenotype in apple, probably through altering polar auxin transport, active gibberellin levels, and strigolactone biosynthesis.
1. Introduction
Tree architecture is a critical agronomic trait of fruit trees, directly influencing planting density and canopy, and subsequently yield and quality of fruits [1,2]. Columnar apples are characterized by compact growth with less branching and more spurs in the apple (Malus × domestica Borkh.) and are genetically controlled by the Co locus at chromosome 10 in the apple genome [3,4]. The columnar tree form is promising for future orchard production due to its labor-saving characteristics and mechanical management [5]. Therefore, identification of the nature of the Co gene is an effective way to realize gene-targeted apple breeding via genetic transformation.
According to the tree growth habit and their fruit-bearing, apple trees have been divided into four ideotypes: columnar, standard, spur, and tip bearer [6]. The columnar apple is presented by ‘Wijcik McIntosh’ (or ‘Wijcik’), a bud sport from the standard apple cultivar ‘McIntosh’ [5]. Due to the compact growth, short internodes, and few lateral branches, this growth habit is referred to as ‘columnar’ and is controlled by a single dominant Co (columnar) [7,8]. The first linkage analysis of the Co gene is conducted by RAPD (random amplified polymorphic DNA) markers using a segregation population crossed by ‘Rome Beauty’ × ‘White Angel’, mapping the Co at the linkage group 10 [9]. An SSR (Simple Sequence Repeats) maker, SSRco165, is developed from F1 populations of ‘Wijcik’ × ‘NY75411-67’ and ‘Wijcik’ × ‘NY75411-58’, linked to Co at 2.9 cM (centiMorgans) [10]. WB82670, an SSR marker obtained from the progeny of ‘Fuji’ × ‘Tuscan’, is closely linked to Co at 1.8 cM [11]. The genetic distance of a SCAR (Sequence Characterized Amplified Regions) marker, SCAR682, derived from the cross of ‘Fuji spur’בTelamon’, is 2.9 cM to Co [12]. Another SSR marker, CH03d11, is linked to Co at 3.9 cM and developed from ‘Fuji’ × 8H-9-45 and ‘Fuji’ × 5-12786 [13]. The abovementioned DNA molecular markers are widely applied for the genetic mapping of Co.
Several physical maps of Co have been reported since the reference genome of ‘Golden Delicious’ was published [14], such as Bai et al. [15], Baldi et al. [16], Moriya et al. [17], Wolters et al. [18], and Otto et al. [19]. Based on the BAC (bacterial artificial chromosome) library sequencing of several apple varieties and a large number of apple hybrid populations, the Co is finely mapped at the region of 18.51~19.1 Mb on chromosome 10 [15,16,17,18,19]. An 8.2kb retrotransposon, Ty3/Gypsy-44 (referred to as Gy-44), is inserted into the 5′ LTR of another retrotransposon Ty/Gyspy-33 (Gy-33) in the ‘Wijcik’ genome, forming a nested retrotransposon associated with the columnar mutation [19]. Among many predicted genes, MdCo31 (MDP0000687812) encoding 2OG-Fe(II) oxygenases (2-oxoglutarate and Fe(II) dependent oxygenases) is a strong candidate gene for Co [18]. MdCo31, the same as dmr6-like [20], MdDOX-Co [21], and MdCoL [22], is highly expressed in the shoot tips of columnar apples, and is negatively correlated with dwarf growth and shortened internode in apples by preventing 12-hydroxylation of GA12 biosynthesis to reduce active GAs in planta [22,23,24]. Other predicted genes at the Co locus are less deciphered. We suspect that other candidate genes of Co are also involved in columnar growth in apples.
In our previous study, 15 candidate genes covering the Co region (18.51~19.1 Mb) are selected by SRA/EST (Sequence Read Archive/Expressed Sequence Tags) database mining and differential expression in shoot tips of ‘Wijcik’ and ‘McIntosh’ by qRT-PCR analysis. In this study, candidate genes were identified by genomic DNA and cDNA sequencing and sequence alignments between ‘Wijcik’ and ‘McIntosh’ apples. Subcellular localization and tobacco (Nicotiana benthamiana L.) transformation were conducted to analyze the biological functions of these genes. Those candidate genes that led to morphological changes in transgenic plants were verified by gene expression analysis in F1 populations crossed by columnar apples. Screening candidate genes at the Co locus helps to elucidate molecular mechanisms of the columnar mutation and serves as a directional genetic improvement for apple trees.
2. Materials and Methods
2.1. Plant Materials
The columnar apple cultivar ‘Wijicik’, non-columnar apple ‘McIntosh’, and F1 hybrid population of ‘Jinlei No.1’ × ‘Maypole’ cultivated at the Shangzhuang experimental station of China Agricultural Universtity (40°08′ N, 116°11′ E) were used for amplified Co candidate sequences, qRT-PCR, and Co genotype [25]. Tobacco seeds were surface sterilized using 70% ethanol for 30 s and 2% NaClO for 10 min and geminated on MS (Murashige and Skoog) medium plus 30 g/L sucrose, and were used for genetic transformation after the growth of 4–5 leaves. The materials required for qRT-PCR were collected from tobacco leaves and tips 30 days after transplanting in the greenhouse, and the samples were frozen in liquid nitrogen and stored in a −80 °C refrigerator.
2.2. Extraction of DNA and RNA
Total DNA was extracted using a modified CTAB method according to Doyle [26]. DNA purity and concentration were measured using a NanoPhotometer® spectrophotometer (Implen, Westlake Village, CA, USA).
Total RNA was extracted using FastPure® Plant Total RNA Isolation Kit (polysaccharides and polyphenolics-rich) (Vazyme, Nanjing, China). The cDNA was obtained by using the PrimeScriptTM 1st Strand cDNA Synthesis Kit (6110A, TaKaRa, Dalian, China).
2.3. DNA Sequencing and Bioinformatic Analysis
The apple ‘Golden Delicious’ genome (accessed via https://www.rosaceae.org/Analysis/10816131, accessed on 5 July 2022) was used for searching DNA sequences. Twelve specific primer pairs of candidate genes (Table S1) were designed for PCR amplification. Both genomic DNA and cDNA of apple were used as templates. The following PCR parameters were set at 95 °C for 5 min, followed by 40 cycles at 95 °C for 5 s, 56–58 °C for 30 s, and 72 °C for 2 min. The annealing temperature was adjusted according to the primer itself. The PCR products were separated by 1% agarose gel electrophoresis. Sequences of gDNA and cDNA in both ‘McIntosh’ and ‘Wijcik’ were used for alignment analysis by DNAMAN. Phylogenetic analysis was performed by MEGA11.0. The subcellular localization of Co candidate genes was predicted using Plant-mPLoc (accessed via http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/, accessed on 2 January 2023).
2.4. Subcellular Localization of Candidate Genes
The full-length CDS (coding sequence) of MdCo41 was amplified with the forward primer containing an XmaI restriction site and the reverse primer containing HindⅢ restriction sites. Forward primers with an XmaI and reverse primers with an XbaI restriction site were designed to amplify other candidate genes (Table S1). The amplification products without the terminator codon of each candidate gene were ligated to the pSuper1300-GFP vector for constructing the translational GFP fusion constructs. The recombinant plasmid was transferred into Agrobacterium tumefaciens strain GV3101, and incubated at 28 °C, 200 rpm. Bacterial solution was collected when OD600 was at 0.8–1.0, and resuspended using 10 mM MES, 10 mM MgCl2, and 100 mM AS to injection. Tobacco seedlings from two-week-old infected tobaccos were incubated for 1 d in darkness and 2 d in light, and observed by confocal laser scanning microscope (OLYMPUS FLUOVIEW FV3000, Japan Olympus Corporation, Tokyo, Japan).
2.5. Transformation of Tobacco
Tobacco seeds were surface sterilized according to the previous protocols. The CDS of each candidate gene was ligated into a pBI121 vector, and fusion vectors were transformed into tobacco by A. tumefaciens EHA105 and incubated at 28 °C, 200 rpm. Bacterial solution was collected when OD600 was at 0.5–0.8, and resuspended using MS medium plus 30 g/L sucrose and 100 mM AS. Transgenic tobacco discs grown in MS medium were supplemented with 30g/L sucrose, 2 mg/L 6-BA, and 0.2 mg/L IAA for 3d then transferred to MS plus 2 mg/L 6-BA, 0.2 mg/L IAA, 100 mg/L kanamycin, and 250 mg/L cefotaxime, and changed for 15d until regeneration shoots growth.
2.6. Quantitative Real-Time PCR (qRT-PCR)
Ectopic transgenic tobaccos in greenhouse and F1 populations of ‘Jinlei No.1’ × ‘Maypole’ shoots were collected. The qRT-PCR assay was performed using the SuperReal.
PreMix Plus (SYBR Green) (Tiangen, Beijing, China). The qRT-PCR program was as follows: 94 °C for 30 s and then 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The primers used for qRT-PCR were, as seen in Table S1. The relative expression levels in each line were normalized against the expression of the internal standard, NtACTIN, and MdACTIN, respectively. The 2−ΔΔCt method was used to calculate relative gene expression levels [27].
2.7. Statistical Analysis
The plant height, number of branches, leaves area, and internode length of transgenic tobacco were recorded 60 days after the tobacco was transplanted into a greenhouse. Additionally, using SPSS17.0 to analyze the statistical data, differences of p < 0.05 and p < 0.01 were considered significant and extremely significant levels, respectively. Graphs were prepared using GraphPad Prism 9. All of the samples were analyzed in three replicates.
3. Results
3.1. Identification and Sequence Alignments of Co Candidate Genes
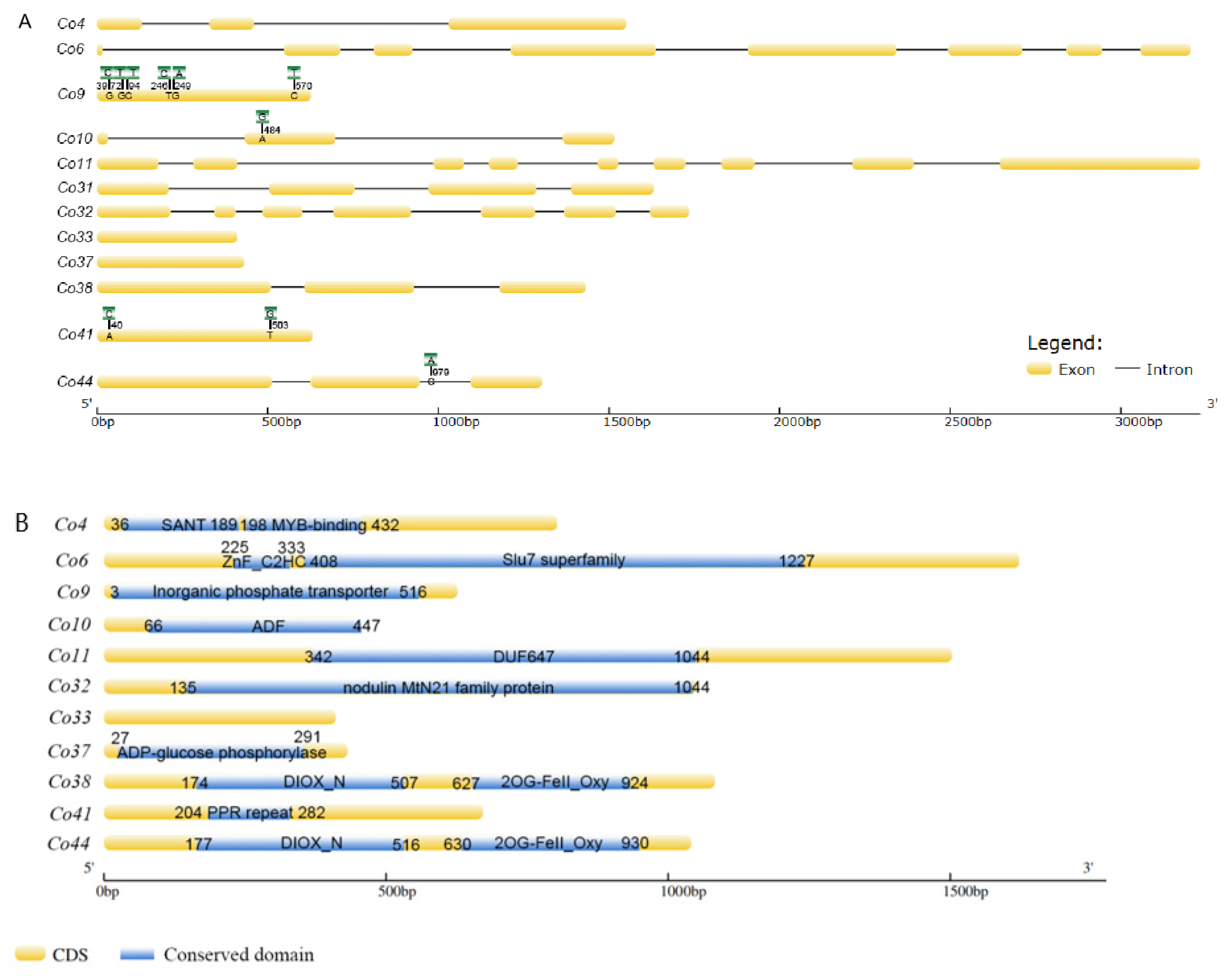
In order to confirm the genomic DNA sequence difference between ‘Wijcik’ (columnar) and ‘McIntosh’ (non-columnar), genomic DNA and cDNA were used to identify candidate genes. Among 15 candidate genes, 12 were isolated from two apple cultivars, including MdCo31 (Figure 1, Table S2), whereas no PCR amplification was obtained for Co27, Co28, and Co30 from both gDNA and cDNA templates. The reasonable explanation was pseudogenes annotated in the apple reference genome. Twelve candidate genes were mapped on chromosome 10 based on their physical positions, and the retrotransposon insertion was in between Co11 and MdCo31 (Figure 1).

Figure 1.
Schematic locations of Co candidate genes and the retrotransposon insertion on chromosome 10 in ‘Wijcik’ apple.
Co9, a putative inorganic phosphate transporter, exhibited six nucleotide variations in CDS by sequence alignments, including two nucleotide transversions and four nucleotide transitions in ‘Wijcik’, compared to ‘McIntosh’ apple (Figure 2A). Only one nucleotide at the 94th bp transit from C to T, leading to a deduced amino acid, Leu to Phe, a non-conserved amino acid, even though this transition was present in the conserved domain (Figure 2B). Co10 encoded a putative actin depolymerization factor (ADF), in which one nucleotide transition (A to G) at the 484th bp of CDS did not change its amino acid sequence (Figure 2). Co41, containing 5 pentatricopetide repeat (PPR) motifs, had two transversions at the 40th (A to C) and 503rd bps (T to G) in the non-conserved domain of the ‘Wijcik’ DNA sequence (Figure 2). Co44 encoded a putative ACO1-like gene that contained DIOX-N (non-haem dioxygenase in the morphine synthesis N-terminal) and 2OG-Fe (Ⅱ) oxygenase domains, in which one nucleotide transverses in the intron of genomic DNA in ‘Wijcik’ and ‘McIntosh’ (Figure 2). No sequence difference was found in seven other genes.
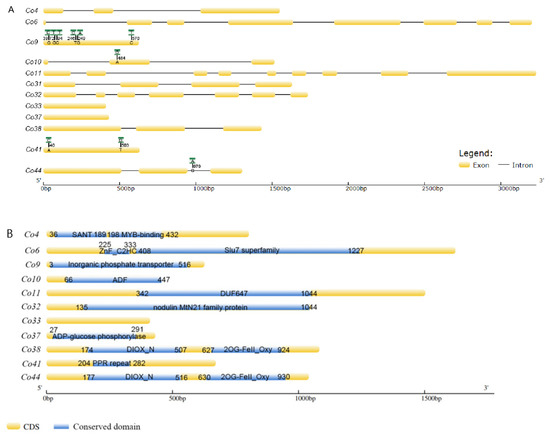
Figure 2.
Schematic structures (A) and conserved domains (B) (blue marked) of candidate genes between ‘Wijcik’ (green marked SNPs) and ‘McIntosh’ (yellow marked) apples.
Co4 encoded a putative MYB transcription factor, containing a SANT (switching-defective protein 3, adaptor 2, nuclear receptor corepressor, and transcription factor IIIB) DNA binding domain and MYB-binding domains. There were Slu7 (pre-mRNA splicing factor) and zinc figure domains in Co6, and the ADF (actin depolymerization factor) domain in Co11. Co32 encoded putative walls are thin 1 (WAT1)-related proteins, including the nodulin MtN21 domain. Co37 functioned probably as ADP-glucose phosphorylase. Co38 comprised DIOX-N and 2OG-Fe (Ⅱ) oxygenase domains, similar to Co44, a homolog with the ACO1-like gene. No conserved domain was predicted in Co33, and its function is unknown (Figure 2B).
3.2. Subcellular Localization Analysis of Co Candidate Genes in Tobacco
To determine the subcellular localization of candidate genes, fusion proteins of Co-GFP were constructed and transiently expressed in N. benthamiana leaves using A. tumefaciens infiltration. Protein localization was examined 48 h after infiltration and representative images are shown in Figure 3. Co4- and Co6-GFP fusion proteins were detected in the nucleus; Co9-, Co33-, and Co38-GFP in cell membranes; Co10-GFP in the cell membrane and nucleus; Co32-GFP in cell membranes and cytoplasm; Co11- and Co41-GFP in the cytoplasm, cell membrane, and nucleus; Co37- and Co44-GFP in the nucleus and cytoplasm (Figure 3). Cellular locations of Co4, Co6, Co9, Co11, Co32, Co37, Co41, and Co44 were consistent with silico prediction, whereas contradictory prediction occurred for Co10 and Co38 (in the cytoplasm), and Co33 (in the nucleus).
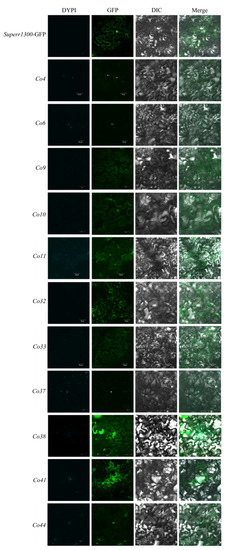
Figure 3.
Subcellular localization of Co candidate genes.
3.3. Ectopic Expression Analysis of Co Candidate Genes in Tobacco
To verify the biological function of candidate genes, four genes (Co32, Co33, Co38, and Co41) upregulated in the columnar apple ‘Wijcik’ were selected for ectopic expression in N. benthamiana. Morphological alterations were observed in Co38-OE and Co41-OE tobaccos (Figure 4 and Figure 5), but no significant difference in Co32-OE and Co33-OE plants was observed compared with wild-type tobacco (WT) and transformants of the pCambia1305 vector (EV) (Figure S1).
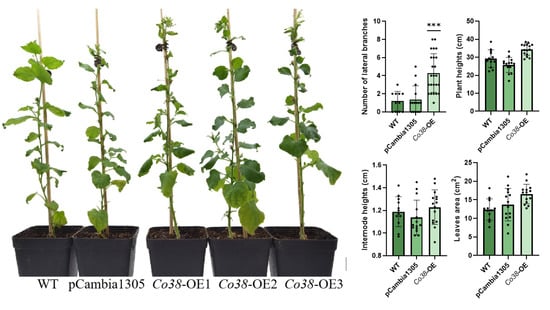
Figure 4.
Phenotypes of transgenic tobacco overexpressed by MdCo38. Bar, 1 cm. *** represents a significant difference of p < 0.0001.
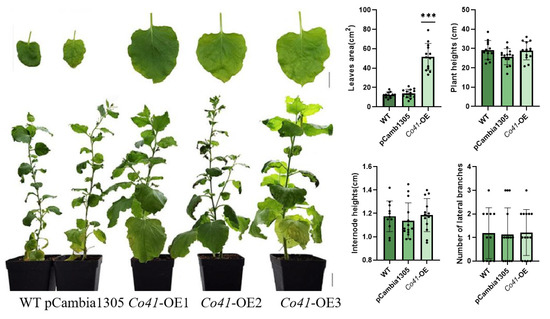
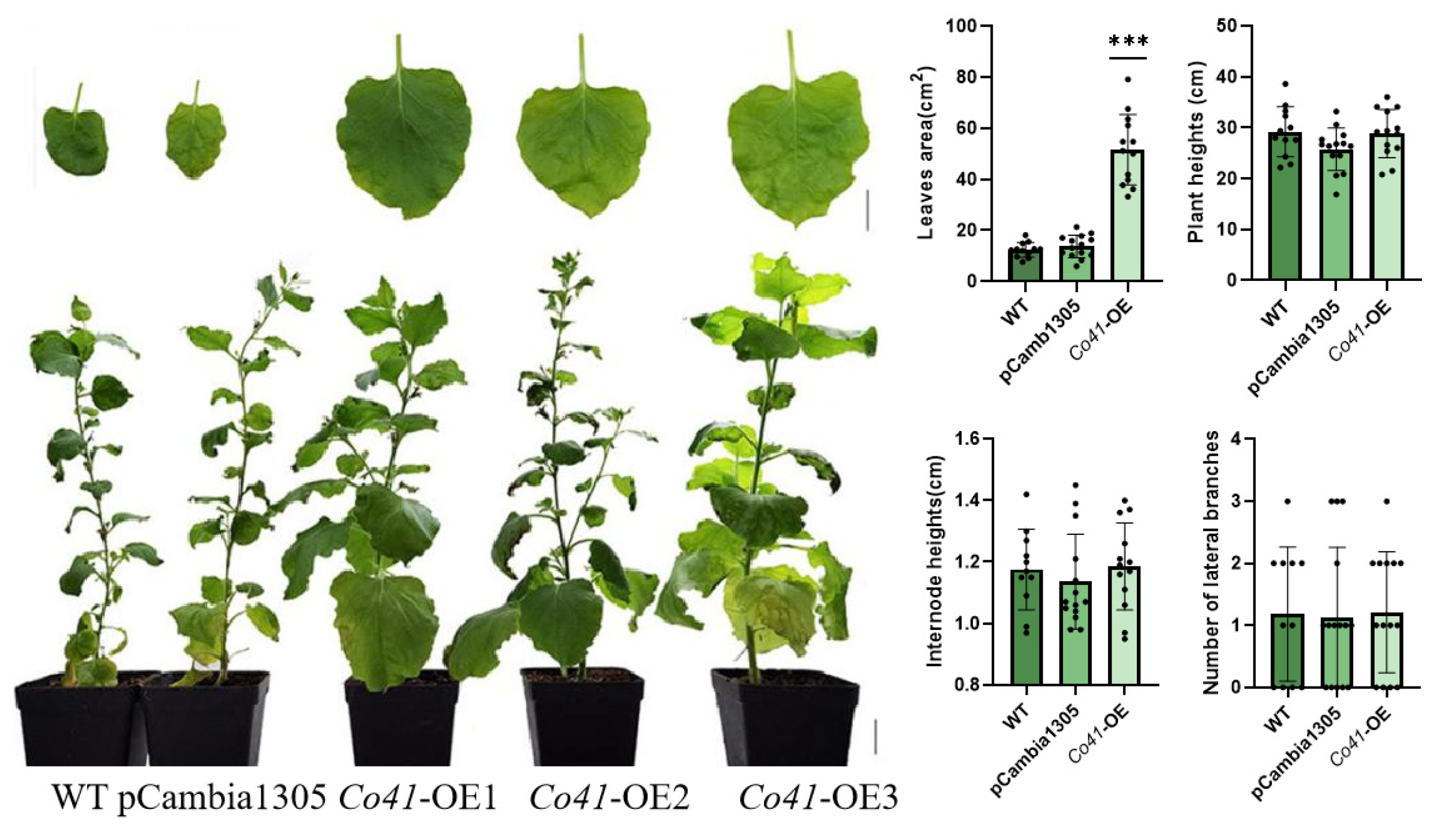
Figure 5.
Phenotypes of transgenic tobacco overexpressed by MdCo41. Bar, 1 cm. *** represents a significant difference of p < 0.0001.
Overexpression of MdCo38 resulted in increased branching in T0 tobaccos. MdCo38-OE plants had branching numbers with an average of 4.29 (±2.14), significantly more than those in WT 1.18 (±1.07) and EV 1.33 (±1.54) plants (Figure 4). No significant changes were observed in transgenic tobaccos for other morphological traits, such as plant height (34.29 cm ± 3.21), internode length (1.23 cm ± 0.15), and leaf size (17.19 cm2 ± 3.52) compared to controls (Figure 4). Overexpression of MdCo41 led to leaf enlargement (51.58 cm2 ± 13.77) compared to that of WT (12.37 cm2 ± 2.89) and empty vector (13.71 cm2 ± 4.37) plants (Figure 5). No obvious alteration in plant height, internode length (1.19 cm ± 0.14), and number of lateral branches (1.21 ± 0.97) occurred in MdCo41-OE plants compared to controls (Figure 5).
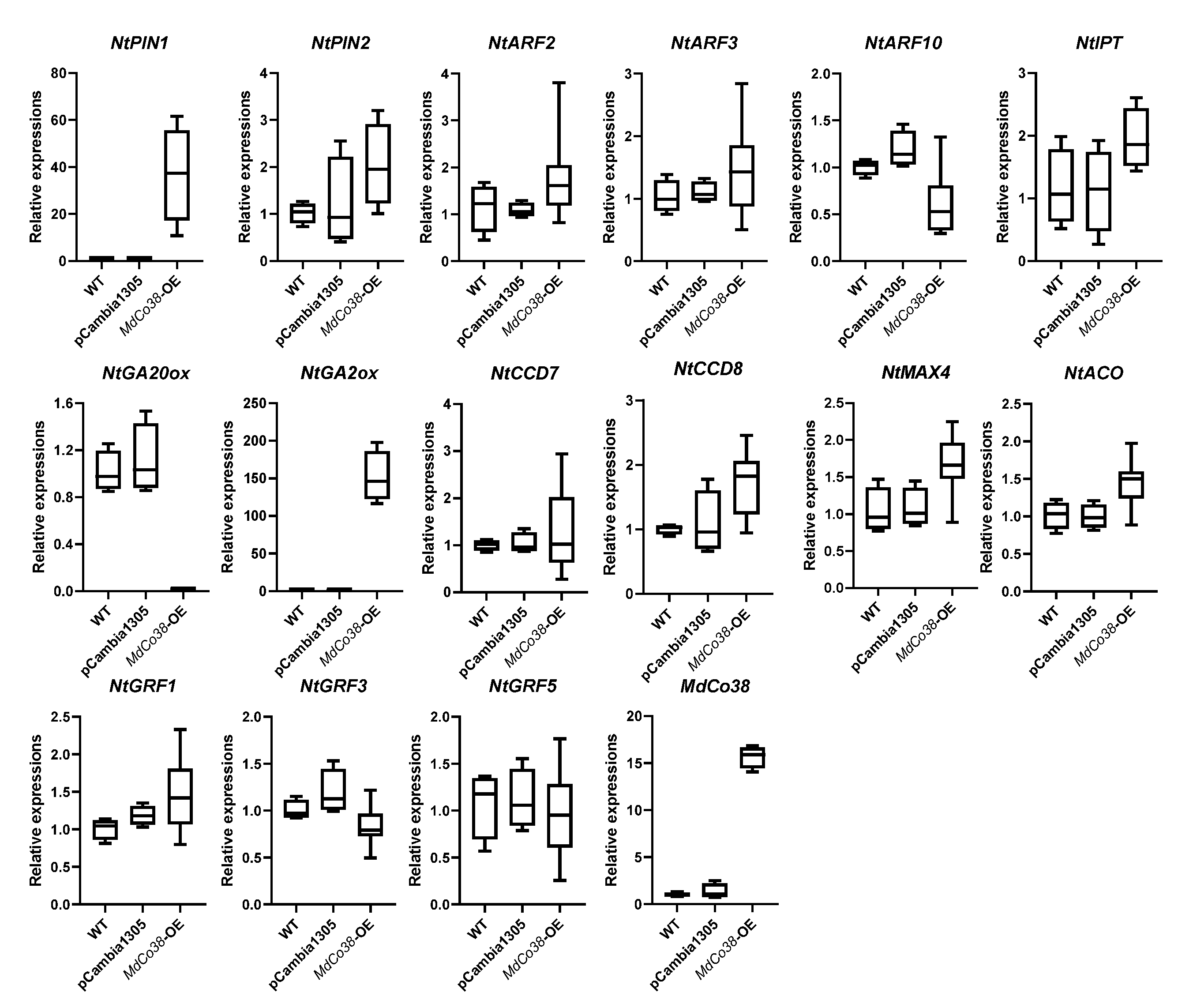
3.4. Branching Was Stimulated by High Expression of NtPIN1 and NtGA2ox in MdCo38-OE Plants
Phylogenetic analysis showed that MdCo38 was clustered with a few ACO-like genes in plants, suggesting a similar function as the ACO (Figure 6). Several key genes related to auxin, gibberellin (GA), and strigolactones (SLs) metabolism were analyzed by qRT-PCR. The auxin efflux carrier gene, NtPIN1, and NtGA2ox were significantly upregulated, but NtGA20ox was downregulated in MdCo38-OE plants. No significant differential expression was detected in transgenic plants, including NtPIN2, NtARF2 (auxin response factor), NtARF3, NtARF10, NtCCD7, NtCCD8, NtACO, NtMAX4, the isopentenyl transferase gene NtIPT, NtGRF1 (growth-regulating factor), NtGRF3, and NtGRF5 (Figure 7). The results indicated that the branching of MdCo38-OE plants was mainly affected by auxin polar transport and active GA levels.

Figure 6.
Phylogenetic analysis of MdCo38 with its homologs in plants.
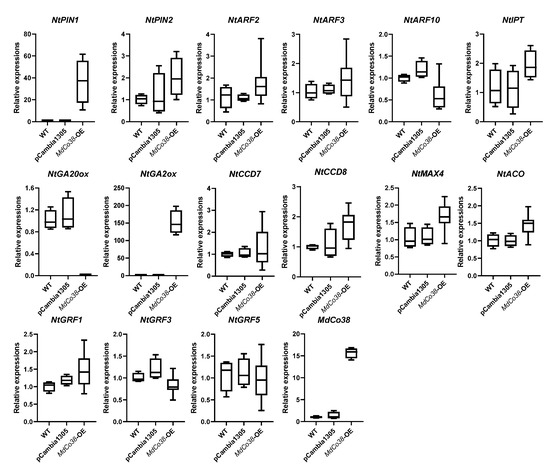
Figure 7.
Expression analysis of several genes related to hormone metabolism and growth regulation factors in MdCo38-OE plant.
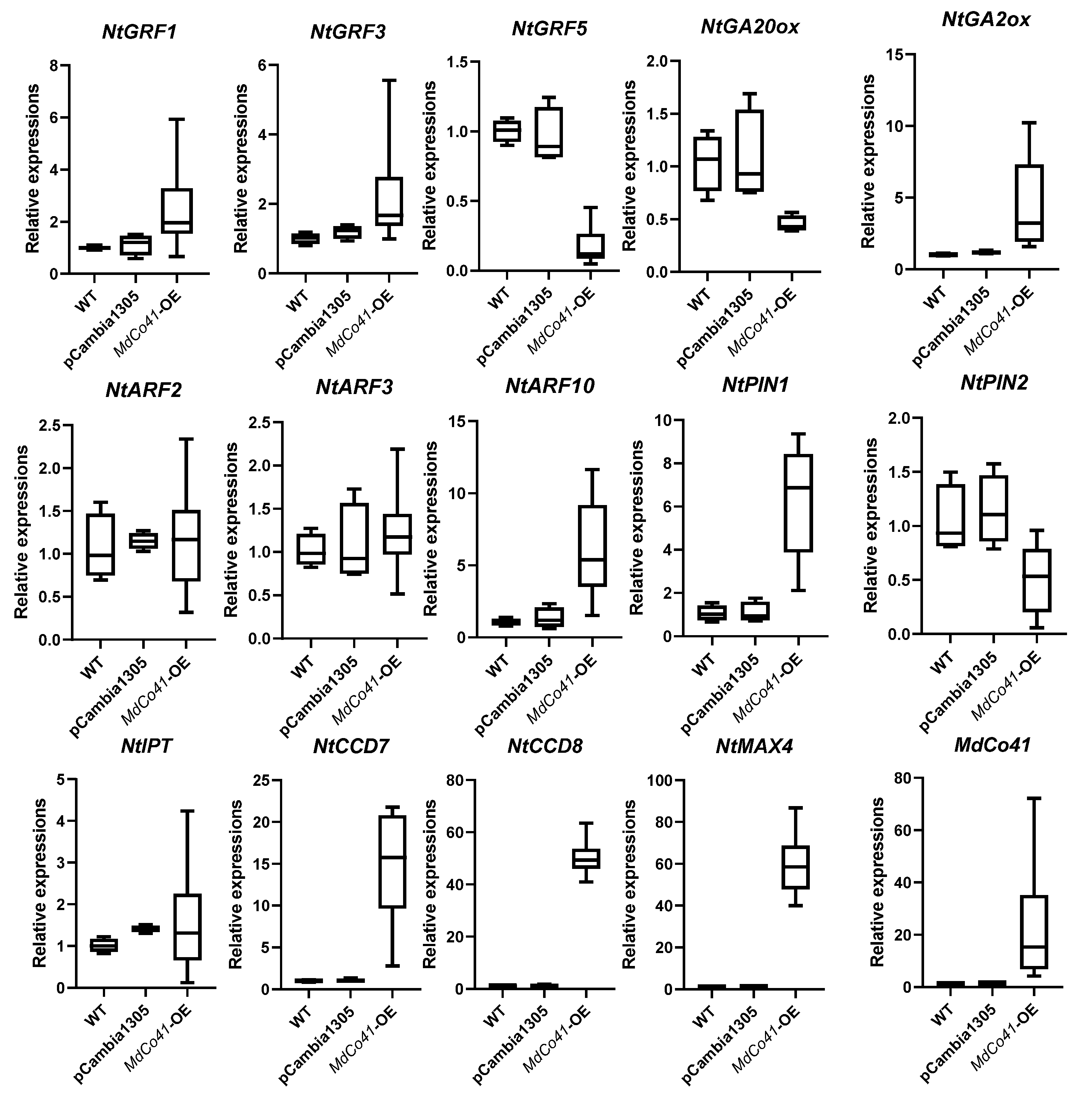
3.5. Leaf Was Enlarged by High Expression of NtCCDs in MdCo41—OE Plants
Phylogenetic analysis showed the MdCo41 homolog with a predicted PPR (XP_028958452.1) in Malus (Figure 8). Analysis of qRT-PCR showed that high expression levels of NtCCD7, NtCCD8, NtMAX4, NtPIN1, and NtARF10 were positively correlated with the expression of MdCo41 (Figure 9). No significant differential expression was observed in transgenic tobaccos, including NtPIN2, NtARF2, NtARF3, NtGA2ox, NtGA20ox, NtIPT, NtGRF1, NtGRF3, and NtGRF5 (Figure 9). The results suggested that the overexpression of MdCo41 altered leaf size by probably regulating the expression of SLs-related genes.

Figure 8.
Phylogenetic analysis of MdCo41 with its homologs in other plants.
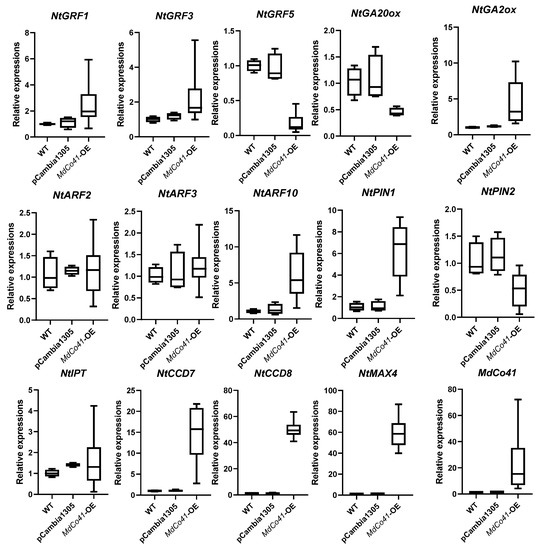
Figure 9.
Expression analysis of several genes related to hormone metabolism and growth regulation factors in MdCo41-OE plants.
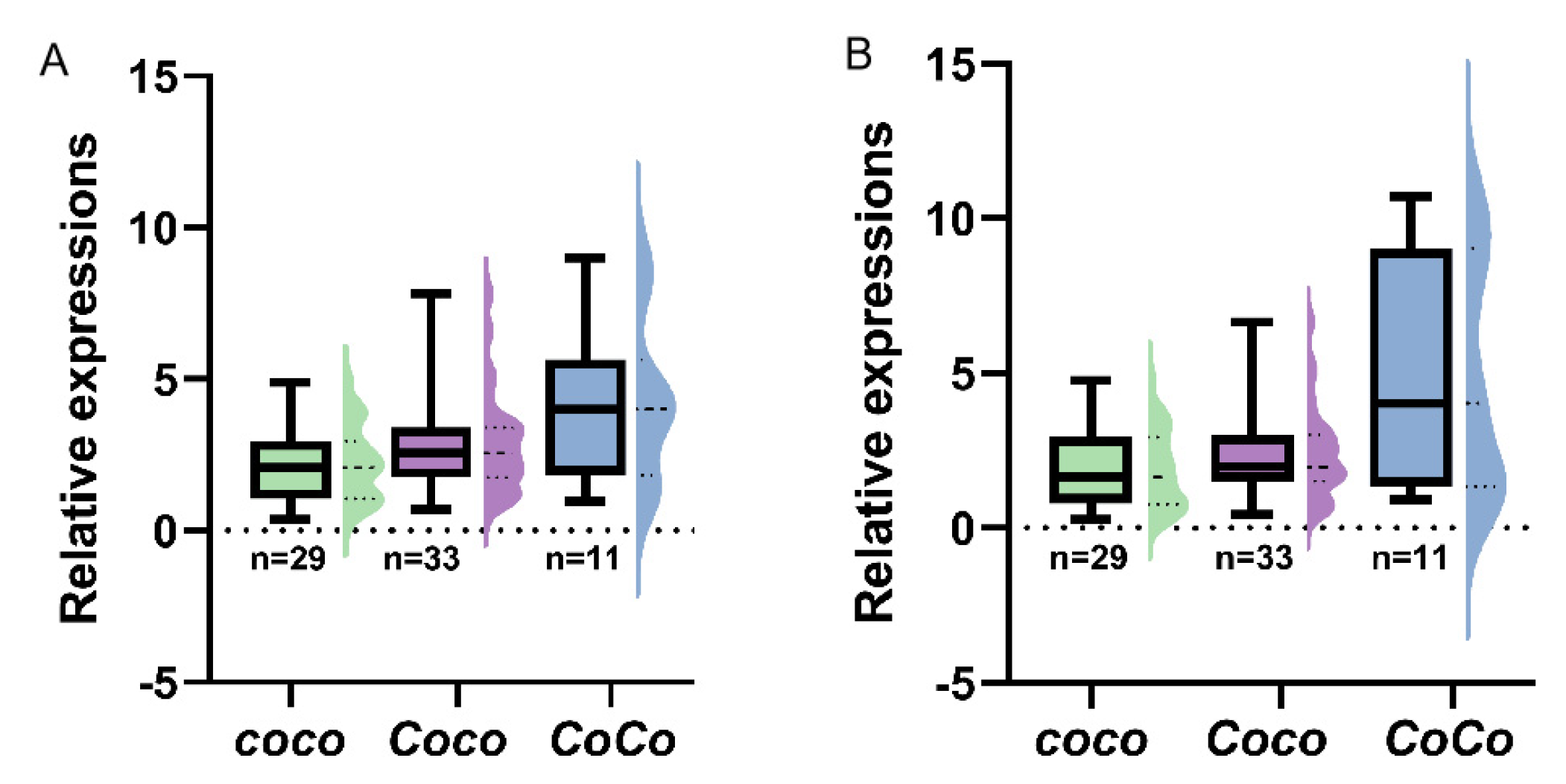
3.6. Expression of MdCo38 and MdCo41 in Apple Cultivars and F1 Generation
In order to analyze transcriptions of MdCo38 and MdCo41 in apples, 68 seedlings were sampled from an F1 population of columnar apples. The genotypes of Co were determined by the retrotransposon specific primers in three, CoCo, Coco, and coco. The expression levels of MdCo38 were 2-fold higher in the CoCo groups, than those in groups of Coco and coco (Figure 10A, Figure S3A). The similar gene expression pattern was found for MdCo41 (Figure 10B, Figure S3B). It proved that MdCo38 and MdCo41 were involved in columnar growth in apple.
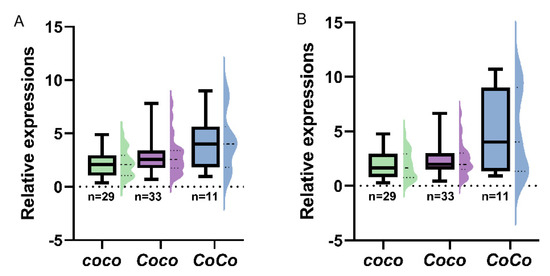
Figure 10.
Expression analysis of MdCo38 (A) and MdCo41 (B) in the F1 population crossed by columnar apples.
4. Discussion
Eleven candidate genes at the Co locus were analyzed by experimental cloning, bioinformatics analysis, and subcellular location analysis. Among these genes, functions of four upregulated expression genes in ‘Wijcik’ apple were characterized by ectopic overexpression of tobacco. Two candidate genes, MdCo38 and MdCo41, led to phenotypic changes in transgenic plants in terms of plant growth.
4.1. Predicted Functions of Genes Downregulated in ‘Wijcik’ Apple
In this study, 11 genes were identified by differential expression analysis between ‘Wijcik’ and ‘McIntosh’ apple cultivars, among which Co4, Co6, Co9, Co10, Co11, Co37, and Co44 were downregulated in ‘Wijcik’ apple. Co4, a member of the MYB transcription factor family, shared high sequence similarity with MYB30 involved in photomorphogenesis and systemic reactive oxygen species (ROS) signaling that is responsive to light stress in Arabidopsis [28,29]. Co6 was predicted as a pre-mRNA-Splicing factor, SLU7, that recognizes the A and G 3′ terminals during the second catalytic step in the pre-mRNA splicing process [30,31]. Co9 shared high homology with other inorganic phosphate transporter proteins 1–4 in plants (Figure S2). AtPht1-4 in Arabidopsis and OsPht1-4 (OsPT4) in rice enhance the accumulation of inorganic phosphate preferentially in roots in response to phosphorus starvation in transgenic plants [32,33]. Homologs of Co10 encode ADF family genes, which involve in plant growth, development, and stress response. MdADF1 transcription accumulates in pollen to promote pollen germination [34]. AtADF6 expresses in the vascular tissue of all organs [35]. OsADF11 persistently expresses in different growth stages of rice, mainly in the stem and leaf blade, and responds to salt and drought [36]. The homolog of Co11 is RUS2 in Arabidopsis, which contains a DUF647 domain and is involved in early seedling morphogenesis and development by maintaining polar auxin transport [37]. Co37 encoded a putative ADP-glucose phosphorylase, localized in the nucleus and cytoplasm (Figure 2B and Figure 3). Its homolog, At5g18200.1, catalyzes adenylyl transfer in the reaction of ADP-glucose with various phosphates [38]. Co44 displayed similar conserved domains but low sequence similarity to Co38 (Figure 6), probably functioning as ACC oxidase. Further experiments are necessary to explain the roles of these genes in apple.
4.2. Functional Analysis of Upregulated Genes in ‘Wijcik’ Apple Co32 and Co33
Heterologous overexpressions of Co32 and Co33 exhibited no morphological changes in transgenic tobaccos, although two genes were upregulated in ‘Wijcik’ apple shoot tips (Figure S1, Table 1). Co32 encoded a plant-specific WAT1-related protein. AtWAT1 in Arabidopsis, a homolog to MtN21 (Medicago truncatula NODULIN21), is essential for auxin homeostasis and secondary cell wall formation. AtWAT1 preferentially accumulates in vascular tissues, including in xylem vascular and fiber development. The loss function of AtWAT1 prevents cell elongation and the formation of secondary cell walls in xylem fibers, which could be restored by applying auxins [39]. Overexpression of Co32 did not change the phenotypes in transgenic tobaccos compared to CK and EV (Figure S1), regarding plant height, internode length, leaf size, and branching. According to previous studies, columnar apples have thicker branches than non-columnar apples [40], indicating Co32’s possible function in cell wall development. To date, the function of Co33 is not reported.

Table 1.
Isolation of predicted genes at the Co locus and expression analysis in apples.
MdCo38 and MdCo41 were highly expressed in columnar apples. Homologs of MdCo38 encode ACO1, the rate-limiting enzyme in ethylene biosynthesis. Overexpression PttACO1 in poplar (Populus tremula × tremuloides) stimulates cambial cell division and inhibits height growth due to elevated endogenous ethylene production [41]. ACO1-deficient mutants in rice (Oryza sativa) exert shorter internodes than WT. Oppositely, the gain-of-function of ACO1 gives rise to elongated internodes [42]. Co41 was grouped in P-class based on the conserved PPR domains [43]. Most of the P-class PPRs are targeted to either mitochondria or plastids, which function principally in RNA processing in plants [43]. A mitochondria P-class gene, PRECOCIOUS1 (POCO1), in Arabidopsis affects mitochondrial RNA editing and functions during flowering time and in the abscisic acid signaling pathway [44]. Arabidopsis pTAC2, targeted to plastids, is required for chlorophyll accumulation; consequently, the ptac2 mutant demonstrates an etiolated appearance and slower growth than the wild type [45]. MdCo41 was distributed in the cell nucleus, cytoplasm, and cell membranes, indicating an analogous function in mediating gene expression in organelles.
4.3. MdCo38 and MdC41 Involved in Columnar Growth in Apple
Plant growth is regulated by a complex network between hormones, especially auxins, cytokinin (CK), GA, and SLs. Several hormone-related genes were selected to analyze the relationship between the phenotype of transgenic plants and gene expression. In tobacco, PIN1/2 is involved in polar auxin transport, and ARF2/3/10 in the auxin signaling pathway. AtPIN1 in Arabidopsis mediates organogenesis and vascular tissue differentiation [46], while AtPIN2 responds to root gravitropic growth by impairing basipetal auxin transport [47]. OsPIN1 deficiency promotes the tillering and elongation of rice by reducing auxin transport [48]. The loss function of AtARF2/3/4 leads to smaller leaves by binding promoters of WOX1 (WUSCHEL-RELATED HOMEOBOX 1) and PRS (PRESSED FLOWER), thus suppressing their expressions in Arabidopsis [49]. Overexpression NtARF10 enlarges the leaf size of transgenic Arabidopsis by increasing cell number and cell size [50]. Our results showed that NtPIN1 was strongly expressed in MdCo38-OE plants, while NtPIN1 and NtARF10 were slightly upregulated in MdCo41-OE plants. This suggests that polar auxin transport exerts morphological changes in transgenic plants.
IPT (isopentenyl transferases) is a rate-limiting enzyme in CK biosynthesis. Overexpression of AtIPT accelerates plant branching by elevating endogenous CK levels [51]. A negative correlation between GA levels and axillary meristem formation in plants has been reported. In peas, gibberellin inhibits the growth and development of lateral branching. Silencing AtGA20ox1 decreases stem and hypocotyl length by reducing endogenous GA levels in transgenic plants [52]. By contrast, the overexpression of OsGA2ox reduces tillering by specifically inactivating C20-GA precursors in rice [53]. Transcripts of NtGA2ox and NtGA20ox changed dramatically in MdCo38-OE but not in MdCo41-OE plants.
Carotenoid cleavage dioxygenase CCDs play an important role in carotenoid metabolism, especially in SLs biosynthesis. CCD7 and CCD8 regulate lateral shoot and root development in plants. AtCCD7 and AtCCD8 influence the growth of lateral buds and roots in Arabidopsis [54]. MAX4 is a homolog of CCD8 in planta. CpMAX1a in Chimonanthus praecox encodes the cytochrome P450 protein (Cyt P450), and its ectopic expression restores branching phenotypes of Arabidopsis max1-3 mutants by upregulating AtBRC1 [55]. In our present study, the SL-related genes NtCCD7, NtCCD8, and NtMAX4 were highly upregulated in MdCo41-OE, but at a low level in Md38-OE plants, meaning that SLs are involved in leaf growth.
GRF plays vital roles in growth and developmental processes such as chloroplast proliferation and regulation of cell size [56]. The atgrf1/2/3 triple mutant exhibits smaller and narrower leaves and shorter petioles; correspondingly, overexpression of AtGRF1 and AtGRF2 leads to leaf enlargement by regulating cell expansion [57]. Likewise, AtGRF5 promotes cell division, inducing leaf enlargement in transgenic plants [58,59]. No significant expression of NtGRFs was observed in transgenic tobaccos.
5. Conclusions
In the present study, two important genes, MdCo38 and MdCo41, were selected from 11 candidate genes at the Co locus. MdCo38 is localized in the cell membrane, and MdCo41 is localized in the nucleus, cytoplasm, and cell membrane. Overexpression of MdCo38 and MdCo41 resulted in branching and enlarged leaf size in transgenic tobaccos, respectively, by regulating auxin, GA, and SLs. Together with MdCo31, MdCo38 and MdCo41 are involved in the columnar growth of apples, which provides new insights into the genetic and molecular regulation of the columnar growth habit, and for further apple breeding through genetic transformation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14050964/s1, Table S1: Primers used in this study; Table S2: Sequence alignments of 11 candidate genes in ‘McIntosh’; Figure S1: Phylogenetic analysis of individual gene at the Co locus mentioned in the study; Figure S2: Phenotypes of transgenic tobacco by overexpressing Co32 (A) and Co33 (B), and analysis of several growth parameters (C~F); Figure S3: Expression analysis of MdCo38 (A) and MdCo41 (B) in apple cultivars and F1 population of ‘Jinlei No.1’ × ‘Maypole’. (XU, GL3, JL1, WL, and WSK represented ‘McIntosh’, ‘Gala 3’, ‘Jin-Lei No.1’, ‘Tuscan’, and ‘Wijcik’, respectively).
Author Contributions
J.G. and Y.C. conceived and managed the experiments. J.G., Y.Z. (Yuan Zhao), Y.C., Y.S., Q.P., Z.Q. and B.Y. conducted the experiments. J.G. and Y.L. wrote the manuscript. Y.Z. (Yuan Zhao) analyzed the experiment. Y.Z. (Yuandi Zhu) reviewed and improved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by the National Natural Science Foundation of China (31672109).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available from the authors upon a reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rom, C.R.; Barritt, B. Spur Development of ‘Delicious’ Apple as Influenced by Position, Wood age, Strain, and Pruning. HortScience 1990, 25, 1578–1581. [Google Scholar] [CrossRef]
- Barritt, B.H. Intensive Orchard Management; Good Fruit Growers: Washington, DC, USA, 1992. [Google Scholar]
- Lespinasse, J.M.; Delort, J.F. Regulation of Fruiting in Apple Role of the Course and Crowned Brindles. Acta Hortic. 1993, 349, 239–245. [Google Scholar] [CrossRef]
- Costes, E.; Lauri, P.É.; Regnard, J.L. Analyzing Fruit Tree Architecture: Implications for Tree Management and Fruit Production. Hortic. Rev. 2006, 32, 1–61. [Google Scholar] [CrossRef]
- Fisher, D.V. The ‘Wijcik Spur McIntosh’. Fruit Var. J. 1995, 49, 212–213. [Google Scholar]
- Lauri, P.; Lespinasse, J.; Térouanne, É. Vegetative Growth and Reproductive Strategies in Apple Fruiting Branches-An Investigation into Various Cultivars. Acta Hortic. 1997, 451, 717–724. [Google Scholar] [CrossRef]
- Lapins, K.O. Segregation of Compact Growth Types in Certain Apple Seedling Progenies. Can. J. Plant Sci. 1969, 49, 765–768. [Google Scholar] [CrossRef]
- Lapins, K.O. Inheritance of Compact Growth Type in Apple. J. Am. Soc. Hortic. Sci. 1976, 101, 133–135. [Google Scholar] [CrossRef]
- Hemmat, M.; Weeden, N.F.; Manganaris, A.G.; Lawson, D.M. Molecular Marker Linkage Map for Apple. J. Hered. 1994, 85, 4–11. [Google Scholar] [CrossRef]
- Conner, P.J.; Brown, S.K.; Weeden, N.F. Randomly Amplified Polymorphic DNA-based Genetic Linkage Maps of Three Apple Cultivars. J. Am. Soc. Hortic. Sci. 1997, 122, 350–359. [Google Scholar] [CrossRef]
- Kim, M.Y.; Song, K.J.; Hwang, J.H.; Shin, Y.U.; Lee, H.J. Development of RAPD and SCAR Markers Linked to the Co Gene Conferring Columnar Growth Habit in Apple (Malus pumila Mill.). J. Am. Soc. Hortic. Sci. 2003, 78, 559–562. [Google Scholar] [CrossRef]
- Tian, Y.K.; Wang, C.H.; Zhang, J.S.; James, C.; Dai, H.Y. Mapping Co, a Gene Controlling the Columnar Phenotype of Apple, with Molecular Markers. Euphytica 2005, 145, 181–188. [Google Scholar] [CrossRef]
- Moriya, S.; Iwanami, H.; Kotoda, N.; Takahashi, S.; Yamamoto, T.; Abe, K. Development of a Marker-Assisted Selection System for Columnar Growth Habit in Apple Breeding. J. Jpn. Soc. Hortic. Sci. 2009, 78, 279–287. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The Genome of the Domesticated Apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Zhu, Y.; Fernandez-Fernandez, F.; Keulemans, J.; Brown, S.; Xu, K. Fine Genetic Mapping of the Co Locus Controlling Columnar Growth Habit in Apple. Mol. Genet. Genom. 2012, 287, 437–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baldi, P.; Wolters, P.J.; Komjanc, M.; Viola, R.; Velasco, R.; Salvi, S. Genetic and Physical Characterisation of the Locus Controlling Columnar Habit in Apple (Malus × domestica Borkh.). Mol. Breed. 2013, 31, 429–440. [Google Scholar] [CrossRef]
- Moriya, S.; Okada, K.; Haji, T.; Yamamoto, T.; Abe, K. Fine Mapping of Co, a Gene Controlling Columnar Growth Habit Located on Apple (Malus × domestica Borkh.) Linkage Group 10. Plant Breed. 2012, 131, 641–647. [Google Scholar] [CrossRef]
- Wolters, P.J.; Schouten, H.J.; Velasco, R.; Si-Ammour, A.; Baldi, P. Evidence for Regulation of Columnar Habit in Apple by a Putative2OG-Fe(II) Oxygenase. New Phytol. 2013, 200, 993–999. [Google Scholar] [CrossRef]
- Otto, D.; Petersen, R.; Brauksiepe, B.; Braun, P.; Schmidt, E.R. The Columnar Mutation (-Co gene) of Apple (Malus × domestica) is Associated with an Integration of a Gypsy-like Retrotransposon. Mol. Breed. 2014, 33, 863–880. [Google Scholar] [CrossRef]
- Petersen, R.; Djozgic, H.; Rieger, B.; Rapp, S.; Schmidt, E.R. Columnar Apple Primary Roots Share Some Features of the Columnar-specific Gene Expression Profile of Aerial Plant Parts as Evidenced by RNA-Seq Analysis. BMC Plant Biol. 2015, 15, 34. [Google Scholar] [CrossRef]
- Kazuma, O.; Masato, W.; Yumiko, T.; Mikiko, K.; Hitoshi, S.; Masaru, N.; Masaharu, M.; Masatoshi, N.; Shigeki, M.; Taku, S.K.A. Columnar Growth Phenotype in Apple Results from Gibberellin Deficiency by Ectopic Expression of a Dioxygenase Gene. Tree Physiol. 2020, 40, 1205–1216. [Google Scholar] [CrossRef]
- Sun, X.; Wen, C.P.; Xu, J.H.; Wang, Y.H.; Zhu, J.; Zhang, Y.G. The Apple Columnar Gene Candidate MdCoL and the AP2/ERF Factor MdDREB2 Positively Regulate ABA Biosynthesis by Ctivating the Expression of MdNCED6/9. Tree Physiol. 2020, 41, 1065–1076. [Google Scholar] [CrossRef]
- Daichi, W.; Ikuo, T.; Naiyanate, J.T.; Sho, M.; Jiang, K.; Masaru, N.; Masato, W.; Tadao, A.; Masaharu, M.; Kazuma, O.; et al. The Apple Gene Responsible for Columnar Tree Shape Reduces the Abundance of Biologically Sctive Gibberellin. Plant J. 2021, 105, 1026–1034. [Google Scholar] [CrossRef]
- Wang, L.M.; Yu, B.Y.; Zhao, Y.N.; Li, Y.Z.; Guo, J.; Zhu, Y.D. A Putative 2OG-Fe(II) Oxygenase’s Response to Gibberellin Deficiency is Related to the Internodal Growth of Columnar Apples. Acta Physiol. Plant. 2021, 43, 70. [Google Scholar] [CrossRef]
- Wang, L.M.; Guo, J.; Chu, Y.; Pan, Q.; Zhu, Y.D. MdCo31 Interacts with an RNA Polymerase II Transcription Subunit 32 to Regulate Dwarf Growth with Short Internodes in Columnar Apple. Plant Sci. 2022, 325, 111496. [Google Scholar] [CrossRef]
- Doley, J. DNA Protocols for Plants. In Molecular Techniques in Taxonomy; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yan, Y.; Li, C.; Dong, X.; Li, H.; Zhang, D.; Zhou, Y.; Jiang, B.; Peng, J.; Qin, X.; Cheng, J.; et al. MYB30 is a Key Negative Regulator of Arabidopsis Photomorphogenic Development that Promotes PIF4 and PIF5 Protein Accumulation in the Light. Plant Cell 2020, 32, 2196–2215. [Google Scholar] [CrossRef]
- Fichman, Y.; Zandalinas, S.I.; Sengupta, S.; Burks, D.; Myers, R.J., Jr.; Azad, R.K.; Mittler, R. MYB30 Orchestrates Systemic Reactive Oxygen Signaling and Plant Acclimation. Plant Physiol. 2020, 184, 666–675. [Google Scholar] [CrossRef]
- Frank, D.; Guthrie, C. An Essential Splicing Factor, SLU7, Mediates 3’ Splice Site Choice in Yeast. Genes Dev. 1992, 6, 2112–2124. [Google Scholar] [CrossRef]
- Brys, A.; Schwer, B. Requirement for SLU7 in Yeast Pre-mRNA Splicing is Dictated by the Distance between the Branchpoint and the 3’ Splice Site. RNA 1996, 2, 707–717. [Google Scholar]
- Karthikeyan, A.S.; Ballachanda, D.N.; Raghothama, K.G. Promoter Deletion Analysis Elucidates the Role of Cis Elements and 5’UTR Intron in Spatiotemporal Regulation of AtPht1;4 Expression in Arabidopsis. Physiol. Plant. 2009, 136, 10–18. [Google Scholar] [CrossRef]
- Ye, Y.; Yuan, J.; Chang, X.J.; Yang, M.; Zhang, L.J.; Lu, K.; Lian, X.M. The Phosphate Transporter Gene OsPht1; 4 is Involved in Phosphate Homeostasis in Rice. PLoS ONE 2015, 10, e0126186. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, S.; Wu, C.; Zhang, Q.; Zhang, Y.; Chen, Q.; Li, Y.; Hao, L.; Gu, Z.; Li, W.; et al. Malus domestica ADF1 Severs Actin Filaments in Growing Pollen Tubes. Funct. Plant Biol. 2017, 44, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Kost, B.; Xia, G.; Chua, N.H. Molecular Identification and Characterization of the Arabidopsis AtADF1, AtADF5 and AtADF6 Genes. Plant Mol. Biol. 2001, 45, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Huang, W.L.; Hong, C.Y.; Lur, H.S.; Chang, M.C. Comprehensive Analysis of Differentially Expressed Rice Actin Depolymerizing Factor Gene Family and Heterologous Overexpression of OsADF3 Confers Arabidopsis thaliana Drought Tolerance. Rice 2012, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Leasure, C.D.; Tong, H.; Yuen, G.; Hou, X.; Sun, X.; He, Z.H. Root UV-B Sensitive2 Acts with Root UV-B Sensitive1 in a Root Ultraviolet B-sensing Pathway. Plant Physiol. 2009, 150, 1902–1915. [Google Scholar] [CrossRef][Green Version]
- Mccoy, J.G.; Arabshahi, A.; Bitto, E.; Bingman, C.A.; Ruzicka, F.J.; Frey, P.A.; Phillips, G.N. Structure and Mechanism of an ADP-glucose Phosphorylase from Arabidopsis thaliana. Biochemistry 2006, 45, 3154–3162. [Google Scholar] [CrossRef][Green Version]
- Ranocha, P.; Dima, O.; Nagy, R.; Felten, J.; Corratgé-Faillie, C.; Novák, O.; Morreel, K.; Lacombe, B.; Martinez, Y.; Pfrunder, S.; et al. Arabidopsis WAT1 is a Vacuolar Auxin Transport Facilitator Required for Auxin Homoeostasis. Nat. Commun. 2013, 4, 2625. [Google Scholar] [CrossRef]
- Okada, K.; Honda, C. Molecular Mechanisms Regulating the Columnar Tree Architecture in Apple. Forests 2022, 13, 1084. [Google Scholar] [CrossRef]
- Love, J.; Björklund, S.; Vahala, J.; Hertzberg, M.; Kangasjärvi, J.; Sundberg, B. Ethylene is an Endogenous Stimulator of Cell Division in the Cambial Meristem of Populus. Proc. Natl. Acad. Sci. USA 2009, 106, 5984–5989. [Google Scholar] [CrossRef]
- Iwamoto, M.; Baba-Kasai, A.; Kiyota, S.; Hara, N.; Takano, M. ACO1, a Gene for Aminocyclopropane-1-carboxylate Oxidase: Effects on Internode Elongation at the Heading Stage in Rice. Plant Cell Environ. 2010, 33, 805–815. [Google Scholar] [CrossRef]
- Manna, S. An Overview of Pentatricopeptide Repeat Proteins and Their Applications. Biochimie 2015, 113, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Emami, H.; Kempken, F. Precocious1 (Poco1), a Mitochondrial Pentatricopeptide Repeat Protein Affects Flowering Time in Arabidopsis thaliana. Plant J. 2019, 100, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Pfalz, J.; Liere, K.; Kandlbinder, A.; Dietz, K.J.; Oelmüller, R. pTAC2, -6, and -12 Are Components of the Transcriptionally Active Plastid Chromosome That Are Required for Plastid Gene Expression. Plant Cell 2006, 18, 176–197. [Google Scholar] [CrossRef]
- Reinhardt, D.; Pesce, E.R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennett, M.; Traas, J.; Friml, J.; Kuhlemeier, C. Regulation of Phyllotaxis by Polar Auxin Transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Guan, C.; Gälweiler, L.; Taenzler, P.; Huijser, P.; Marchant, A.; Parry, G.; Bennett, M.; Wisman, E.; Palme, K. AtPIN2 Defines a Locus of Arabidopsis for Root Gravitropism Control. EMBO J. 1998, 17, 6903–6911. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, L.; Shou, H.; Wu, P. A PIN1 Family Gene, OsPIN1, involved in Auxin-dependent Adventitious Root Emergence and Tillering in Rice. Plant Cell Physiol. 2005, 10, 1674–1681. [Google Scholar] [CrossRef]
- Guan, C.; Wu, B.; Yu, T.; Wang, Q.; Krogan, N.T.; Liu, X.; Jiao, Y. Spatial Auxin Signaling Controls Leaf Flattening in Arabidopsis. Curr. Biol. 2017, 27, 2940–2950. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, R.; Zhou, L.; Wu, X.Y.; Xu, N.; Ma, X.H.; Zhang, Y. Genome-Wide Identification Analysis of the Auxin Response Factors Family in Nicotiana tabacum and the function of NtARF10 in Leaf Size Regulation. J. Plant Biol. 2021, 64, 281–297. [Google Scholar] [CrossRef]
- Miyawaki, K.; Tarkowski, P.; Matsumoto-Kitano, M.; Kato, T.; Sato, S.; Tarkowska, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP Isopentenyltransferases and tRNA Isopentenyltransferases in Cytokinin Biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef]
- Coles, J.P.; Phillips, A.L.; Croker, S.J.; García-Lepe, R.; Lewis, M.J.; Hedden, P. Modification of Gibberellin Production and Plant Development in Arabidopsis by Sense and Antisense Expression of Gibberellin 20-oxidase Genes. Plant J. 1999, 17, 547–556. [Google Scholar] [CrossRef]
- Lo, S.F.; Yang, S.Y.; Chen, K.T.; Hsing, Y.I.; Zeevaart, J.A.; Chen, L.J.; Yu, S.M. A Novel Class of Gibberellin 2-Oxidases Control Semidwarfism, Tillering, and Root Development in Rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The Path from β-carotene to Carlactone, a Strigolactone-like Plant Hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Hua, R.; Wang, X.; Wu, H.F.; Ou, H.; Lu, X.; Huang, Y.; Liu, D.; Sui, S. CpMAX1a, a Cytochrome P450 Monooxygenase Gene of Chimonanthus praecox Regulates Shoot Branching in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 10888. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.J.; Xu, X.; Gong, Z.H.; Tang, Y.W.; Wu, M.B.; Yan, F.; Zhang, X.L.; Zhang, Q.; Yang, F.Q.; Hu, X.W.; et al. Auxin Response Factor 6A Regulates Photosynthesis, Sugar Accumulation, and Fruit Development in Tomato. Hortic. Res. 2019, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Omidbakhshfard, M.A.; Proost, S.; Fujikura, U.; Mueller-roeber, B. Growth Regulating Factors (GRFs): A Small Transcription Factor Family with Important Functions in Plant Biology. Mol. Plant 2015, 8, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, G.; Kim, G.T.; Tsukaya, H. The Transcription Factor AtGRF5 and the Transcription Coactivator AN3 Regulate Cell Proliferation in Leaf Primordia of Arabidopsis thaliana. Plant J. 2005, 43, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Vercruyssen, L.; Tognetti, V.B.; Gonzalez, N.; Van, D.J.; De, M.L.; Bielach, A.; De, R.R.; Van, B.F.; Inzé, D. Growth Regulating Factor 5 Stimulates Arabidopsis Chloroplast Division, Photosynthesis, and Leaf Longevity. Plant Physiol. 2015, 167, 817–832. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).