Characterization of the Liriodendron chinense Pentatricopeptide Repeat (PPR) Gene Family and Its Role in Osmotic Stress Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatment
2.2. Identification of PPR Genes in L. chinense
2.3. Sequence Analysis of LcPPR and Phylogenetic Tree Construction
2.4. Cis-Acting Element Prediction
2.5. Expression Analysis of LcPPR Genes in L. chinense
3. Results
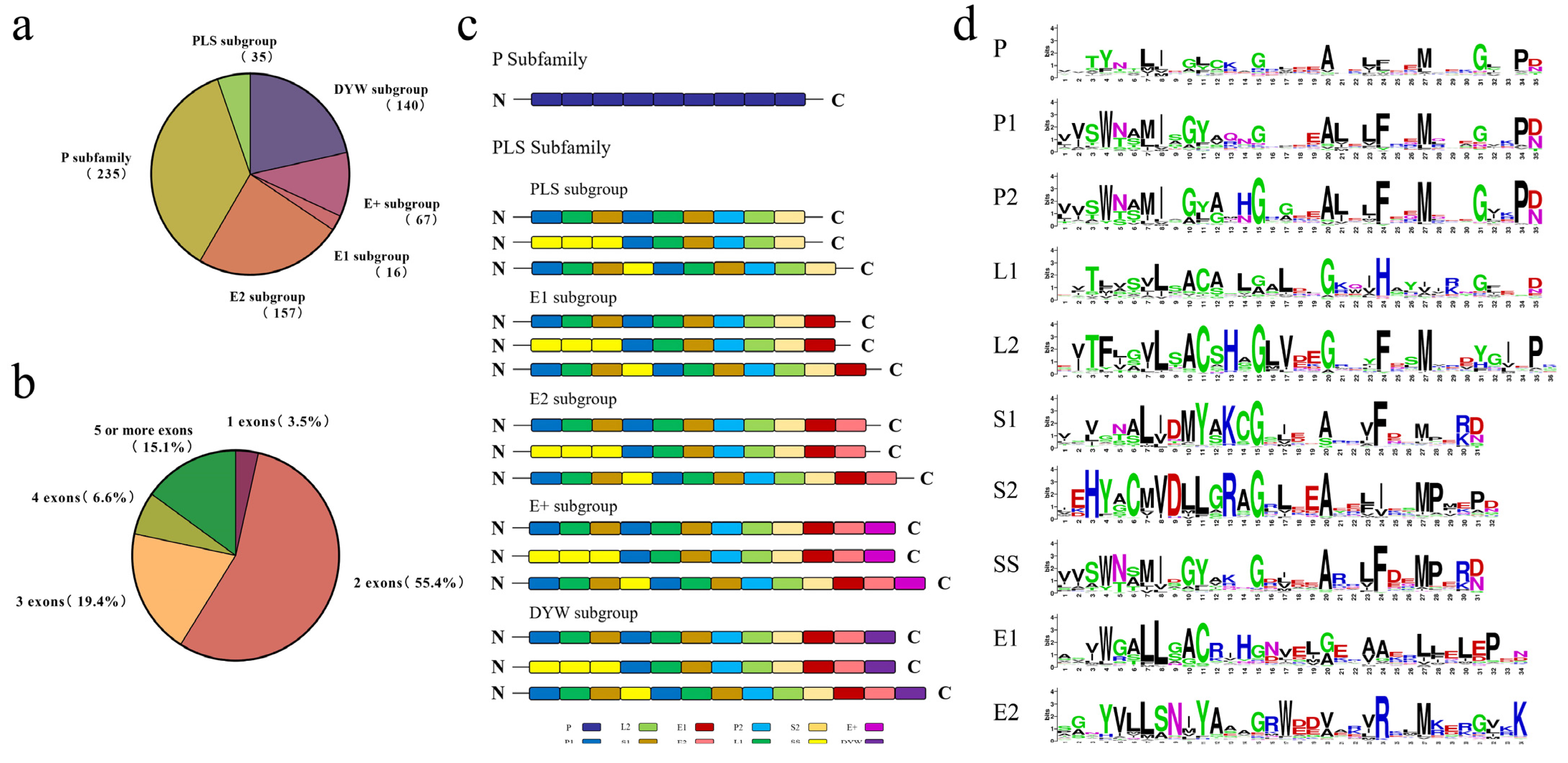
3.1. Genome-Wide Identification of the LcPPR Gene Family in L. chinense
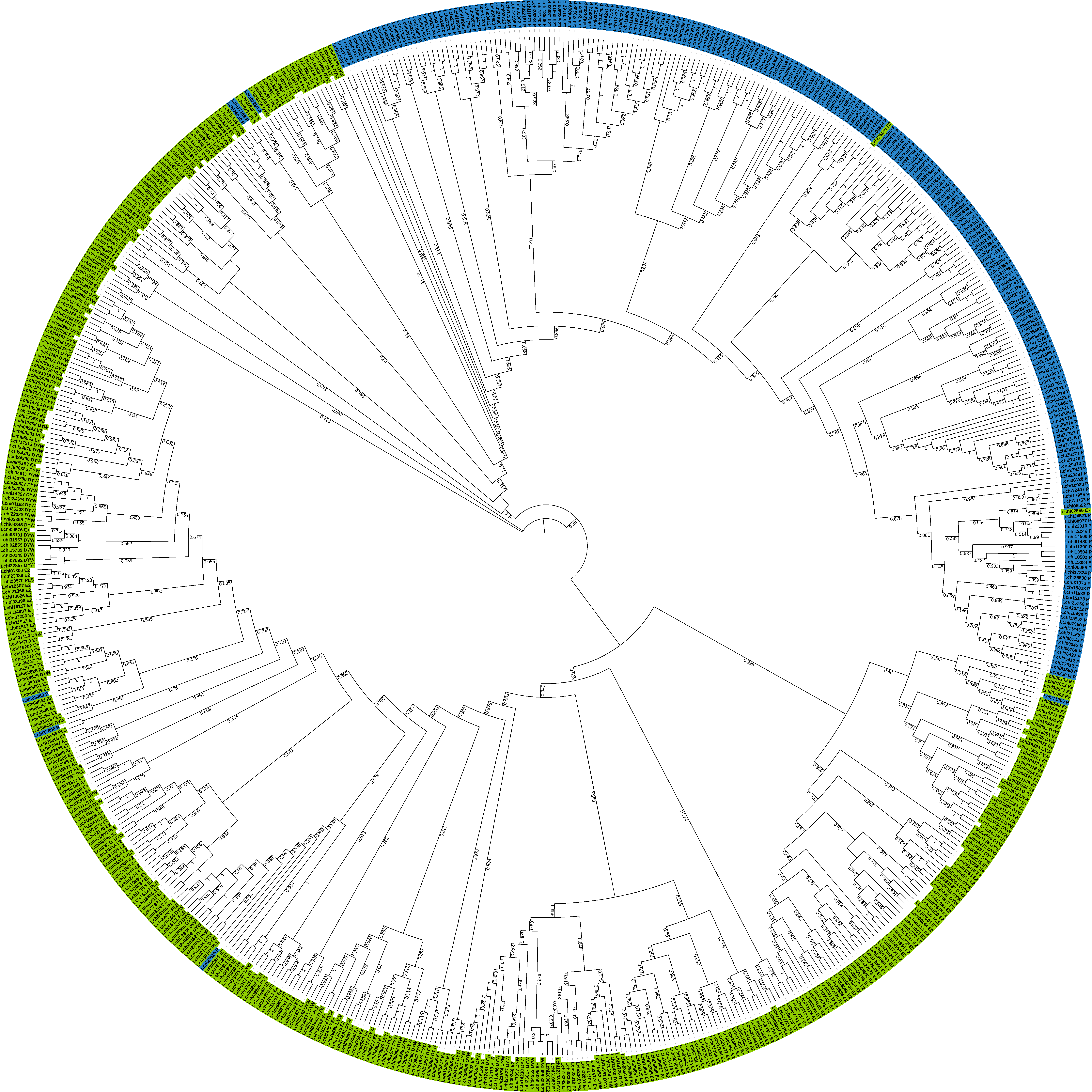
3.2. Phylogenetic Analysis of LcPPR Proteins
3.3. Chromosomal Distribution and Duplication of LcPPR Genes
3.4. Expression Patterns of LcPPR Genes in Different Tissues
3.5. Analysis of the Cis-Acting Elements in LcPPR Gene Promoters
3.6. Expression Analysis of LcPPR Genes under Drought Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lurin, C.; Andres, C.; Aubourg, S.; Bellaoui, M.; Bitton, F.; Bruyere, C.; Caboche, M.; Debast, C.; Gualberto, J.; Hoffmann, B.; et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 2004, 16, 2089–2103. [Google Scholar] [CrossRef]
- Cheng, S.; Gutmann, B.; Zhong, X.; Ye, Y.; Fisher, M.F.; Bai, F.; Castleden, I.; Song, Y.; Song, B.; Huang, J.; et al. Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. Plant J. 2016, 85, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; An, Y.; Xu, P.; Xiao, J. Functioning of PPR Proteins in Organelle RNA Metabolism and Chloroplast Biogenesis. Front. Plant Sci. 2021, 12, 627501. [Google Scholar] [CrossRef] [PubMed]
- Colcombet, J.; Lopez-Obando, M.; Heurtevin, L.; Bernard, C.; Martin, K.; Berthome, R.; Lurin, C. Systematic study of subcellular localization of Arabidopsis PPR proteins confirms a massive targeting to organelles. RNA Biol. 2013, 10, 1557–1575. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Myouga, F.; Motohashi, R.; Shinozaki, K.; Shikanai, T. Conserved domain structure of pentatricopeptide repeat proteins involved in chloroplast RNA editing. Proc. Natl. Acad. Sci. USA 2007, 104, 8178–8183. [Google Scholar] [CrossRef]
- Hayes, M.L.; Dang, K.N.; Diaz, M.F.; Mulligan, R.M. A conserved glutamate residue in the C-terminal deaminase domain of pentatricopeptide repeat proteins is required for RNA editing activity. J. Biol. Chem. 2015, 290, 10136–10142. [Google Scholar] [CrossRef]
- Ichinose, M.; Tasaki, E.; Sugita, C.; Sugita, M. A PPR-DYW protein is required for splicing of a group II intron of cox1 pre-mRNA in Physcomitrella patens. Plant J. 2012, 70, 271–278. [Google Scholar] [CrossRef]
- Tavares-Carreon, F.; Camacho-Villasana, Y.; Zamudio-Ochoa, A.; Shingu-Vazquez, M.; Torres-Larios, A.; Perez-Martinez, X. The pentatricopeptide repeats present in Pet309 are necessary for translation but not for stability of the mitochondrial COX1 mRNA in yeast. J. Biol. Chem. 2008, 283, 1472–1479. [Google Scholar] [CrossRef]
- Schmitz-Linneweber, C.; Small, I. Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 2008, 13, 663–670. [Google Scholar] [CrossRef]
- Bryant, N.; Lloyd, J.; Sweeney, C.; Myouga, F.; Meinke, D. Identification of nuclear genes encoding chloroplast-localized proteins required for embryo development in Arabidopsis. Plant Physiol. 2011, 155, 1678–1689. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, Y.; Li, X.; Zhang, Q.; Chen, L.; Wu, H.; Su, D.; Chen, Y.; Guo, J.; Luo, D.; et al. Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 2006, 18, 676–687. [Google Scholar] [CrossRef]
- Kocabek, T.; Repkova, J.; Dudova, M.; Hoyerova, K.; Vrba, L. Isolation and characterization of a novel semi-lethal Arabidopsis thaliana mutant of gene for pentatricopeptide (PPR) repeat-containing protein. Genetica 2006, 128, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Dahan, J.; Mireau, H. The Rf and Rf-like PPR in higher plants, a fast-evolving subclass of PPR genes. RNA Biol. 2013, 10, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, J.; Zhong, S.; Gu, H.; He, S.; Qu, L.J. Novel DYW-type pentatricopeptide repeat (PPR) protein BLX controls mitochondrial RNA editing and splicing essential for early seed development of Arabidopsis. J. Genet. Genom. 2018, 45, 155–168. [Google Scholar] [CrossRef]
- Yang, D.; Cao, S.K.; Yang, H.; Liu, R.; Sun, F.; Wang, L.; Wang, M.; Tan, B.C. DEK48 Is Required for RNA Editing at Multiple Mitochondrial Sites and Seed Development in Maize. Int. J. Mol. Sci. 2022, 23, 3064. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Yang, J.I.; Moon, S.; Ryu, C.H.; An, K.; Kim, K.M.; Yim, J.; An, G. Rice OGR1 encodes a pentatricopeptide repeat-DYW protein and is essential for RNA editing in mitochondria. Plant J. 2009, 59, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Gillman, J.D.; Bentolila, S.; Hanson, M.R. The petunia restorer of fertility protein is part of a large mitochondrial complex that interacts with transcripts of the CMS-associated locus. Plant J. 2007, 49, 217–227. [Google Scholar] [CrossRef]
- Jo, Y.D.; Ha, Y.; Lee, J.; Park, M.; Bergsma, A.C.; Choi, H.; Goritschnig, S.; Kloosterman, B.; van Dijk, P.J.; Choi, D.; et al. Fine mapping of Restorer-of-fertility in pepper (Capsicum annuum L.) identified a candidate gene encoding a pentatricopeptide repeat (PPR)-containing protein. Theor. Appl. Genet. 2016, 129, 2003–2017. [Google Scholar] [CrossRef]
- Fujii, S.; Suzuki, T.; Giege, P.; Higashiyama, T.; Koizuka, N.; Shikanai, T. The Restorer-of-fertility-like 2 pentatricopeptide repeat protein and RNase P are required for the processing of mitochondrial orf291 RNA in Arabidopsis. Plant J. 2016, 86, 504–513. [Google Scholar] [CrossRef]
- Xie, H.; Peng, X.; Qian, M.; Cai, Y.; Ding, X.; Chen, Q.; Cai, Q.; Zhu, Y.; Yan, L.; Cai, Y. The chimeric mitochondrial gene orf182 causes non-pollen-type abortion in Dongxiang cytoplasmic male-sterile rice. Plant J. 2018, 95, 715–726. [Google Scholar] [CrossRef]
- Tadini, L.; Ferrari, R.; Lehniger, M.; Mizzotti, C.; Moratti, F.; Resentini, F.; Colombo, M.; Costa, A.; Masiero, S.; Pesaresi, P. Trans-splicing of plastid rps12 transcripts, mediated by AtPPR4, is essential for embryo patterning in Arabidopsis thaliana. Planta 2018, 248, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Cushing, D.A.; Forsthoefel, N.R.; Gestaut, D.R.; Vernon, D.M. Arabidopsis emb175 and other ppr knockout mutants reveal essential roles for pentatricopeptide repeat (PPR) proteins in plant embryogenesis. Planta 2005, 221, 424–436. [Google Scholar] [CrossRef] [PubMed]
- de Longevialle, A.F.; Hendrickson, L.; Taylor, N.L.; Delannoy, E.; Lurin, C.; Badger, M.; Millar, A.H.; Small, I. The pentatricopeptide repeat gene OTP51 with two LAGLIDADG motifs is required for the cis-splicing of plastid ycf3 intron 2 in Arabidopsis thaliana. Plant J. 2008, 56, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zou, Y.; Hu, J.; Ding, Y. Genome-wide analysis of the rice PPR gene family and their expression profiles under different stress treatments. BMC Genom. 2018, 19, 720. [Google Scholar] [CrossRef]
- Zhu, Q.; Dugardeyn, J.; Zhang, C.; Takenaka, M.; Kuhn, K.; Craddock, C.; Smalle, J.; Karampelias, M.; Denecke, J.; Peters, J.; et al. SLO2, a mitochondrial pentatricopeptide repeat protein affecting several RNA editing sites, is required for energy metabolism. Plant J. 2012, 71, 836–849. [Google Scholar] [CrossRef]
- Zhu, Q.; Dugardeyn, J.; Zhang, C.; Muhlenbock, P.; Eastmond, P.J.; Valcke, R.; De Coninck, B.; Oden, S.; Karampelias, M.; Cammue, B.P.; et al. The Arabidopsis thaliana RNA editing factor SLO2, which affects the mitochondrial electron transport chain, participates in multiple stress and hormone responses. Mol. Plant 2014, 7, 290–310. [Google Scholar] [CrossRef]
- Jiang, S.C.; Mei, C.; Liang, S.; Yu, Y.T.; Lu, K.; Wu, Z.; Wang, X.F.; Zhang, D.P. Crucial roles of the pentatricopeptide repeat protein SOAR1 in Arabidopsis response to drought, salt and cold stresses. Plant Mol. Biol. 2015, 88, 369–385. [Google Scholar] [CrossRef]
- Zsigmond, L.; Rigo, G.; Szarka, A.; Szekely, G.; Otvos, K.; Darula, Z.; Medzihradszky, K.F.; Koncz, C.; Koncz, Z.; Szabados, L. Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol. 2008, 146, 1721–1737. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, Z.; Zou, X.; Xu, Y.; Peng, L.; Hu, J.; Lin, H. Silencing of rice PPR gene PPS1 exhibited enhanced sensibility to abiotic stress and remarkable accumulation of ROS. J. Plant Physiol. 2021, 258–259, 153361. [Google Scholar] [CrossRef]
- Liu, J.M.; Zhao, J.Y.; Lu, P.P.; Chen, M.; Guo, C.H.; Xu, Z.S.; Ma, Y.Z. The E-Subgroup Pentatricopeptide Repeat Protein Family in Arabidopsis thaliana and Confirmation of the Responsiveness PPR96 to Abiotic Stresses. Front. Plant Sci. 2016, 7, 1825. [Google Scholar] [CrossRef]
- Xing, H.; Fu, X.; Yang, C.; Tang, X.; Guo, L.; Li, C.; Xu, C.; Luo, K. Genome-wide investigation of pentatricopeptide repeat gene family in poplar and their expression analysis in response to biotic and abiotic stresses. Sci. Rep. 2018, 8, 2817. [Google Scholar] [CrossRef]
- O’Toole, N.; Hattori, M.; Andres, C.; Iida, K.; Lurin, C.; Schmitz-Linneweber, C.; Sugita, M.; Small, I. On the expansion of the pentatricopeptide repeat gene family in plants. Mol. Biol. Evol. 2008, 25, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Sugita, M. An Overview of Pentatricopeptide Repeat (PPR) Proteins in the Moss Physcomitrium patens and Their Role in Organellar Gene Expression. Plants 2022, 11, 2279. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y.; Messing, J. Genome-wide analysis of pentatricopeptide-repeat proteins of an aquatic plant. Planta 2016, 244, 893–899. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Lu, P.; Li, W.; Chen, M.; Guo, C.; Ma, Y. Genome-wide investigation and expression analyses of the pentatricopeptide repeat protein gene family in foxtail millet. BMC Genom. 2016, 17, 840. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hao, Z.; Guang, X.; Zhao, C.; Wang, P.; Xue, L.; Zhu, Q.; Yang, L.; Sheng, Y.; Zhou, Y.; et al. Liriodendron genome sheds light on angiosperm phylogeny and species–pair differentiation. Nat. Plants 2019, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, K.; Wang, X.; Yang, P. Morphological and proteomic analysis reveal the role of pistil under pollination in Liriodendron chinense (Hemsl.) Sarg. PLoS ONE 2014, 9, e99970. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, D.; Long, X.; Hao, Z.; Lu, Y.; Zhou, Y.; Peng, Y.; Cheng, T.; Shi, J.; Chen, J. Agrobacterium-Mediated Genetic Transformation of Embryogenic Callus in a Liriodendron Hybrid (L. Chinense x L. Tulipifera). Front. Plant Sci. 2022, 13, 802128. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, S.; Zhu, L.; Wang, D.; Liu, Y.; Liu, S.; Zhang, J.; Hao, Z.; Lu, Y.; Cheng, T.; et al. Characterization of the Liriodendron Chinense MYB Gene Family and Its Role in Abiotic Stress Response. Front. Plant Sci. 2021, 12, 641280. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yuan, W.; Qiu, S.; Shi, J. Selection of reference genes for gene expression analysis in Liriodendron hybrids’ somatic embryogenesis and germinative tissues. Sci. Rep. 2021, 11, 4957. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Sugita, M.; Ichinose, M.; Ide, M.; Sugita, C. Architecture of the PPR gene family in the moss Physcomitrella patens. RNA Biol. 2013, 10, 1439–1445. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, Y.; Hua, J. Genomewide identification of PPR gene family and prediction analysis on restorer gene in Gossypium. J. Genet. 2018, 97, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Subburaj, S.; Tu, L.; Lee, K.; Park, G.; Lee, H.; Chun, J.; Lim, Y.; Park, M.; McGregor, C.; Lee, G. A Genome-Wide Analysis of the Pentatricopeptide Repeat (PPR) Gene Family and PPR-Derived Markers for Flesh Color in Watermelon (Citrullus lanatus). Genes 2020, 11, 1125. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Meng, Y.; Xiao, Y.; Zhao, J.; Xiao, B.; An, C.; Gao, Y. PPR proteins in the tea plant (Camellia sinensis) and their potential roles in the leaf color changes. Sci. Hortic. 2022, 293, 110745. [Google Scholar] [CrossRef]
- Wei, K.; Han, P. Pentatricopeptide repeat proteins in maize. Mol. Breed. 2016, 36, 170. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The Evolutionary Fate and Consequences of Duplicate Genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Kalladan, R.; Lasky, J.R.; Chang, T.Z.; Sharma, S.; Juenger, T.E.; Verslues, P.E. Natural variation identifies genes affecting drought-induced abscisic acid accumulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, 11536–11541. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]


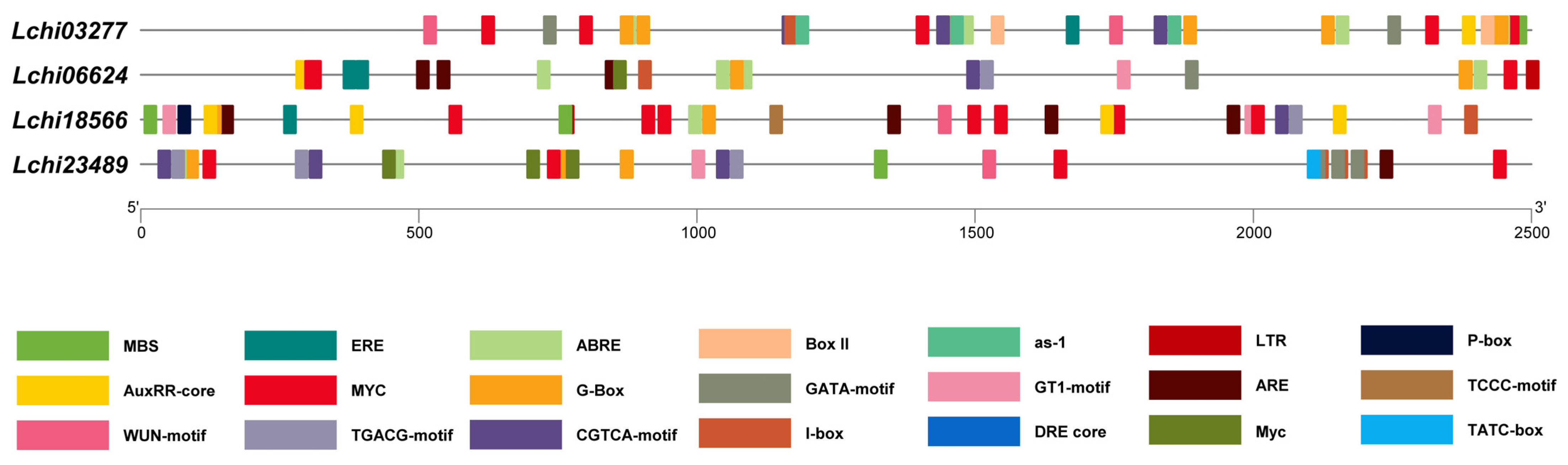

| Lchi03277 | Lchi06624 | Lchi18566 | Lchi23489 | |||
|---|---|---|---|---|---|---|
| Hormone-Related | MeJA | CGTCA motif | 3 | 1 | 1 | 3 |
| TGACG motif | 3 | 1 | 1 | 3 | ||
| Auxin | AuxRR core | 1 | 1 | 4 | 0 | |
| Gibberellin | TATC box | 0 | 0 | 0 | 1 | |
| GARE motif | 0 | 0 | 0 | 0 | ||
| P-box | 0 | 0 | 1 | 0 | ||
| ABA | ABRE | 7 | 5 | 1 | 4 | |
| Ethylene | ERE | 1 | 2 | 1 | 0 | |
| Environment-Related | Light | G-box | 10 | 4 | 2 | 6 |
| Box II | 2 | 0 | 0 | 0 | ||
| GATA motif | 2 | 1 | 0 | 3 | ||
| I-box | 1 | 1 | 1 | 6 | ||
| GT1 motif | 0 | 1 | 3 | 1 | ||
| TCCC motif | 0 | 0 | 1 | 0 | ||
| ATCT motif | 0 | 0 | 0 | 0 | ||
| Wound | WUN motif | 2 | 0 | 1 | 1 | |
| Anaerobic induction | ARE | 0 | 4 | 4 | 1 | |
| Drought | MBS | 1 | 0 | 2 | 1 | |
| MYC | 6 | 4 | 8 | 7 | ||
| DRE core | 0 | 1 | 0 | 1 | ||
| as-1 | 3 | 1 | 1 | 3 | ||
| Low temperature | LTR | 0 | 1 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Wang, D.; Xue, G.; Zheng, X.; Lu, Y.; Shi, J.; Hao, Z.; Chen, J. Characterization of the Liriodendron chinense Pentatricopeptide Repeat (PPR) Gene Family and Its Role in Osmotic Stress Response. Genes 2023, 14, 1125. https://doi.org/10.3390/genes14061125
Ma X, Wang D, Xue G, Zheng X, Lu Y, Shi J, Hao Z, Chen J. Characterization of the Liriodendron chinense Pentatricopeptide Repeat (PPR) Gene Family and Its Role in Osmotic Stress Response. Genes. 2023; 14(6):1125. https://doi.org/10.3390/genes14061125
Chicago/Turabian StyleMa, Xiaoxiao, Dandan Wang, Guoxia Xue, Xueyan Zheng, Ye Lu, Jisen Shi, Zhaodong Hao, and Jinhui Chen. 2023. "Characterization of the Liriodendron chinense Pentatricopeptide Repeat (PPR) Gene Family and Its Role in Osmotic Stress Response" Genes 14, no. 6: 1125. https://doi.org/10.3390/genes14061125






