Identification of ZmBK2 Gene Variation Involved in Regulating Maize Brittleness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Map-Based Cloning of the Brittle Gene
2.3. Allelic Tests of bk2
2.4. Stalk Stiffness Test and Measurement of Stalk and Leaf Composition
2.5. Subcellular Localization
2.6. Phylogenetic Analysis and Protein Modeling
2.7. Transcriptome Analysis
2.8. RNA Extraction and RT-qPCR
2.9. Putative Cell Wall Regulatory Network
2.10. Phylogenetic Analysis of ZmBK2
3. Results
3.1. Brittle Mutant Decreased Maize Tissue Strength
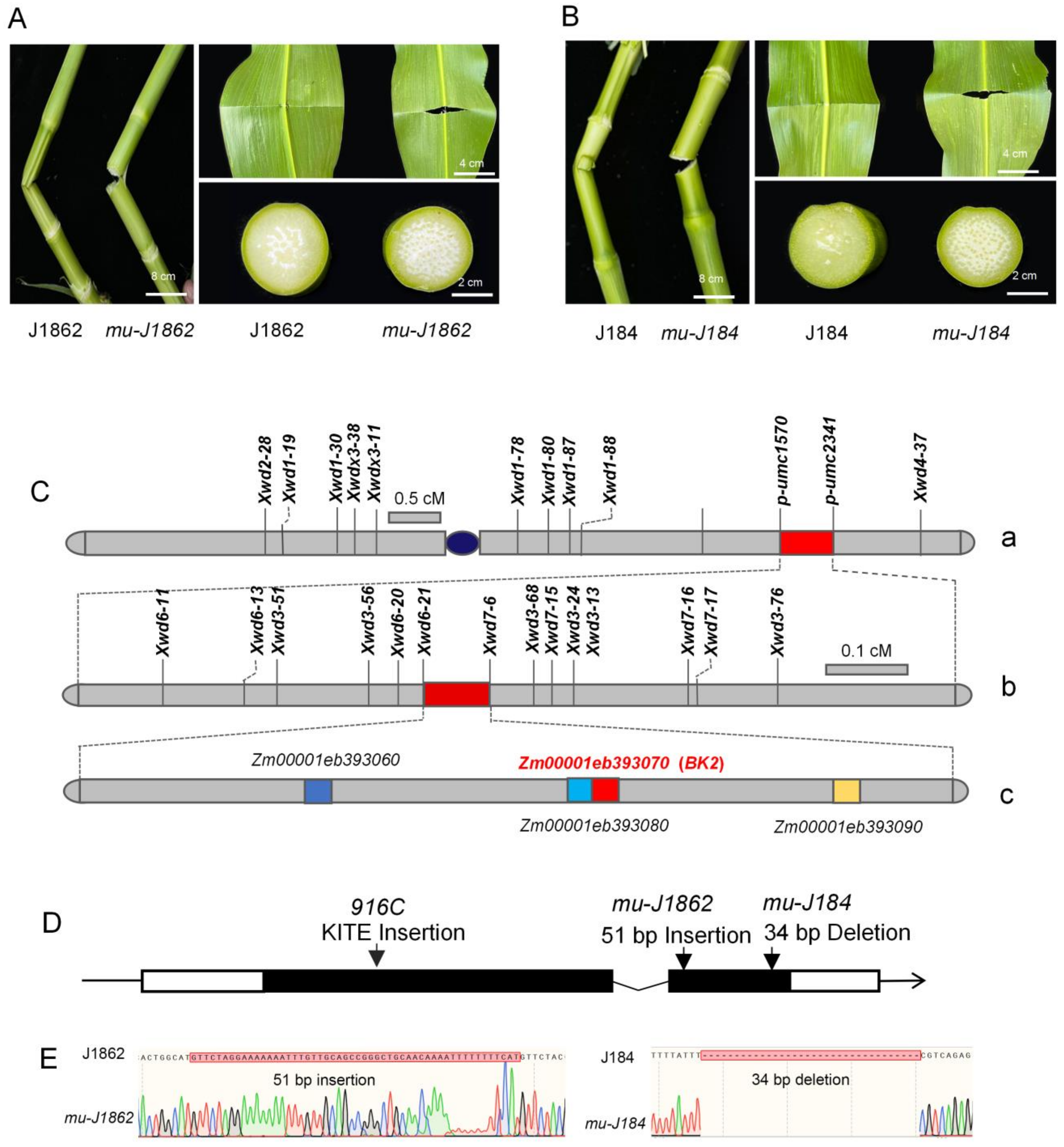
3.2. Genetic Analysis and Map-Based Cloning of the Brittle Gene
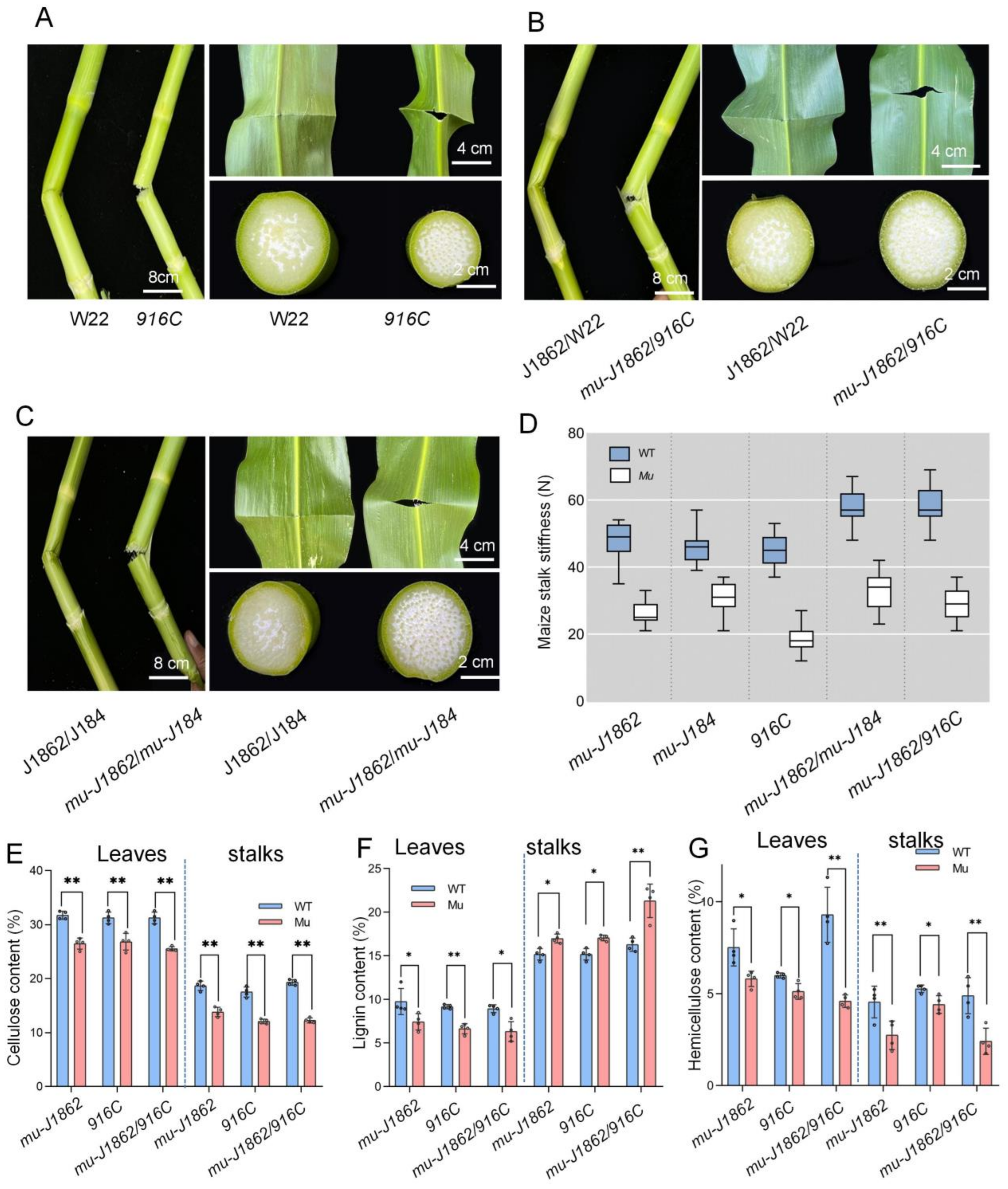
3.3. Allelic Tests
3.4. Stalk Stiffness Test and Composition Measurements of Stalks and Leaves
3.5. Brittle Mutant Exhibits Abnormalities in Sclerenchyma Cells
3.6. Constitutive Expression, Subcellular Localization and Phylogeny of ZmBK2
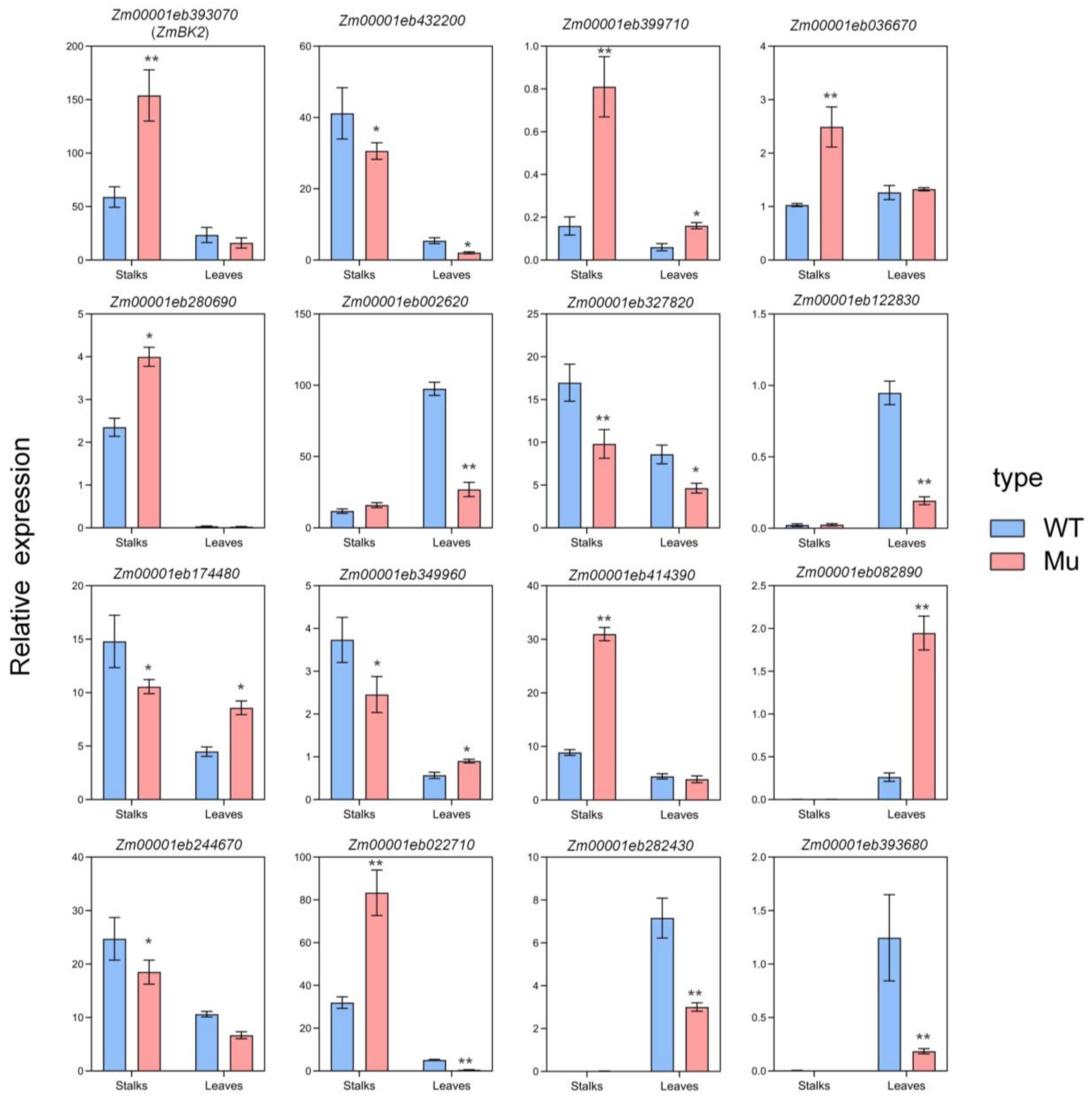
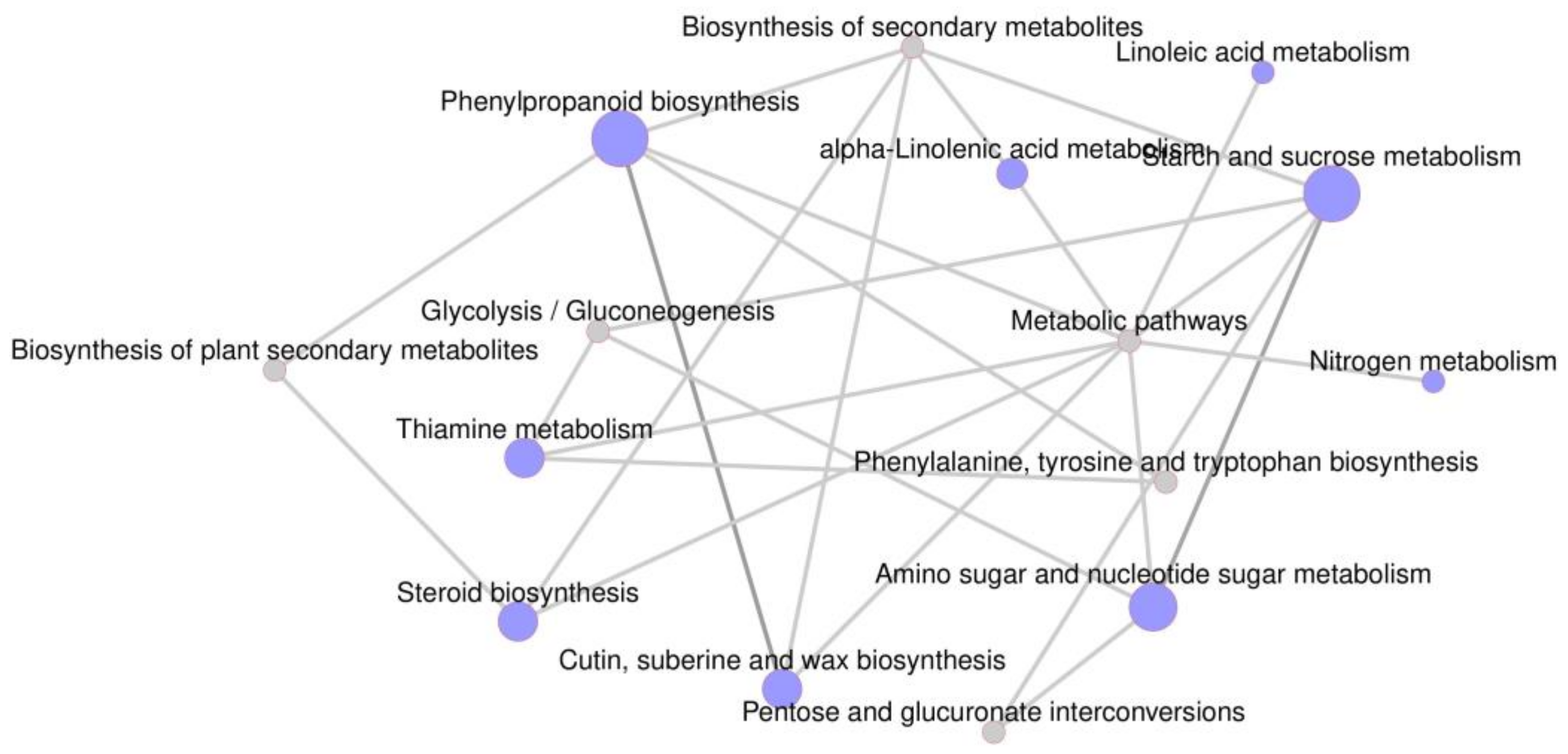
3.7. Transcriptome Alterations in mu-J1862 Stalks and Leaves
3.8. Validation of RNA-seq
4. Discussion
4.1. Abnormal Cellulose Synthesis Affects Plant Brittleness
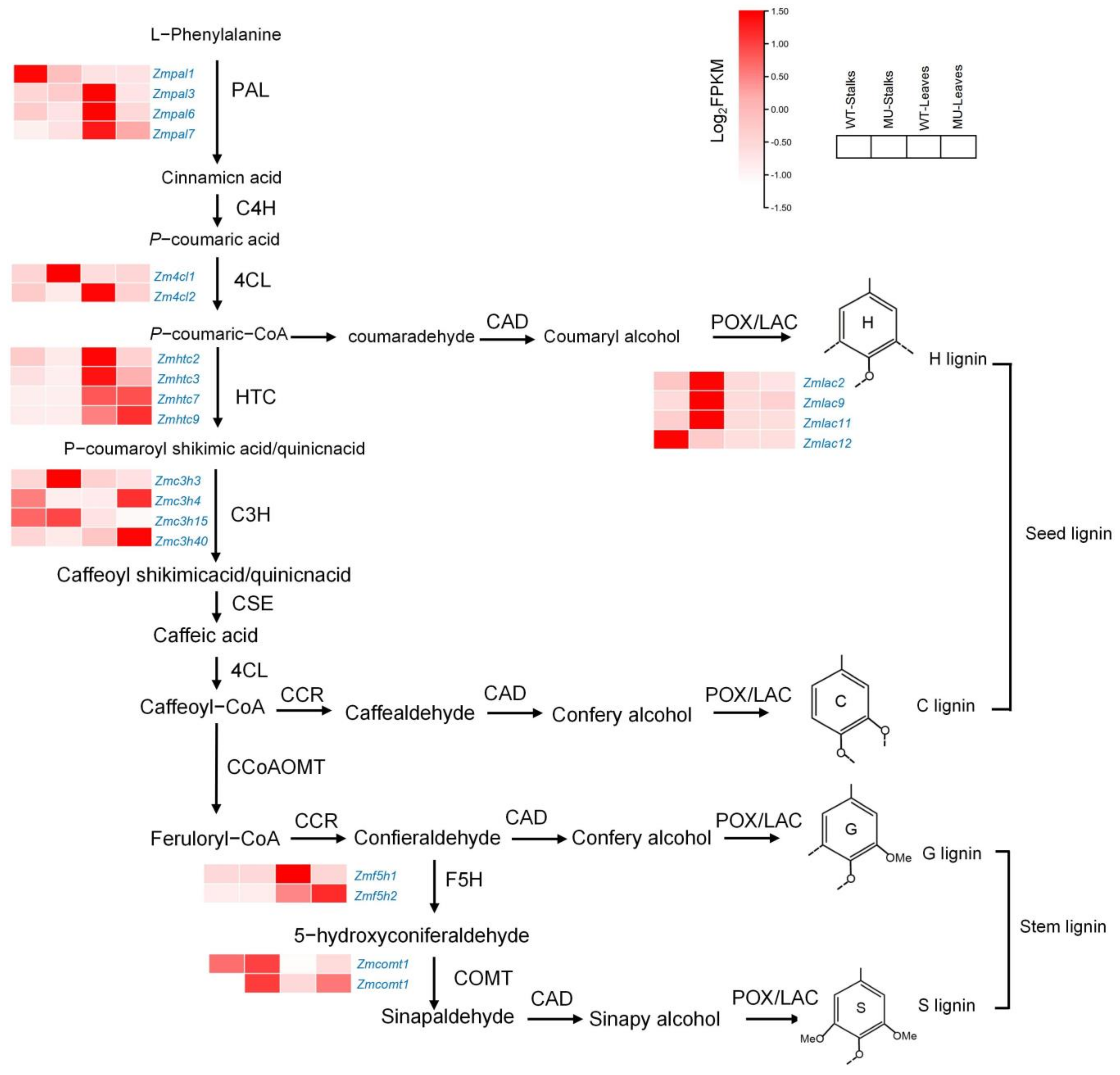
4.2. Lignin Synthesis Compensates for Cellulose Deficiency in Mutants
4.3. ZmBK2 Is a Member of the COBRA Family
4.4. Regulatory Network for Cell Wall Synthesis
4.5. ZmBK2 Can Potentially Reduce Residue Rate in Fresh Maize
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flint-Garcia, S.A.; Jampatong, C.; Darrah, L.L.; McMullen, M.D. Quantitative trait locus analysis of stalk strength in four maize populations. Crop Sci. 2003, 43, 13–22. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Q.; Zhou, Y.; Yan, M.; Sun, L.; Zhang, M.; Fu, Z.; Wang, Y.; Han, B.; Pang, X.; et al. BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell 2003, 15, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Shao, C.; Han, Y.; He, Y.; Yin, Z. Genetic Architecture of Maize Stalk Diameter and Rind Penetrometer Resistance in a Recombinant Inbred Line Population. Genes 2022, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Keegstra, K. Plant cell walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef]
- Desprez, T.; Juraniec, M.; Crowell, E.F.; Jouy, H.; Pochylova, Z.; Parcy, F.; Höfte, H.; Gonneau, M.; Vernhettes, S. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 15572–15577. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Zeef, L.A.H.; Ellis, J.; Goodacre, R.; Turner, S.R. Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 2005, 17, 2281–2295. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Murata, K.; Yamazaki, M.; Onosato, K.; Miyao, A.; Hirochika, H. Three distinct rice cellulose synthase catalytic subunit genes required for cellulose synthesis in the secondary wall. Plant Physiol. 2003, 133, 73–83. [Google Scholar] [CrossRef]
- Atanassov, I.I.; Pittman, J.K.; Turner, S.R. Elucidating the Mechanisms of Assembly and Subunit Interaction of the Cellulose Synthase Complex of Arabidopsis Secondary Cell Walls. J. Biol. Chem. 2009, 284, 3833–3841. [Google Scholar] [CrossRef]
- Appenzeller, L.; Doblin, M.; Barreiro, R.; Wang, H.; Niu, X.; Kollipara, K.; Carrigan, L.; Tomes, D.; Chapman, M.; Dhugga, K.S. Cellulose synthesis in maize: Isolation and expression analysis of the cellulose synthase (CesA) gene family. Cellulose 2004, 11, 287–299. [Google Scholar] [CrossRef]
- Taylor, N.G.; Laurie, S.; Turner, S.R. Multiple cellulose synthase catalytic subunits are required for cellulose synthesis in Arabidopsis. Plant Cell 2000, 12, 2529–2539. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Feng, S.; Wang, Y.; Wang, Y.; Fan, C.; Li, Y.; Liu, Z.; Schneider, R.; Xia, T.; et al. Three AtCesA6-like members enhance biomass production by distinctively promoting cell growth in Arabidopsis. Plant Biotechnol. J. 2018, 16, 976–988. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The Plant Cell Wall: Biosynthesis, construction, and functions. Integr. Plant Biol. 2020, 63, 251–272. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, L.; Qian, Q.; Xiong, G.; Zeng, D.; Li, R.; Guo, L.; Li, J.; Zhou, Y. A missense mutation in the transmembrane domain of CESA4 affects protein abundance in the plasma membrane and results in abnormal cell wall biosynthesis in rice. Plant Mol. Biol. 2009, 71, 509–524. [Google Scholar] [CrossRef]
- Wang, L.; Guo, K.; Li, Y.; Tu, Y.; Hu, H.; Wang, B.; Cui, X.; Peng, L. Expression profiling and integrative analysis of the CESA/CSL superfamily in rice. BMC Plant Biol. 2010, 10, 282. [Google Scholar] [CrossRef]
- Holland, N.; Holland, D.; Helentjaris, T.; Dhugga, K.S.; Cazares, B.X.; Delmer, D.P. A comparative analysis of the plant cellulose synthase (CesA) gene family. Plant Physiol. 2000, 123, 1313–1324. [Google Scholar] [CrossRef]
- Roudier, F.; Schindelman, G.; DeSalle, R.; Benfey, P.N. The COBRA family of putative GPI-anchored proteins in Arabidopsis. A new fellowship in expansion. Plant Physiol. 2002, 130, 538–548. [Google Scholar] [CrossRef]
- Schindelman, G.; Morikami, A.; Jung, J.; Baskin, T.I.; Carpita, N.C.; Derbyshire, P.; McCann, M.C.; Benfey, P.N. COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 2001, 15, 1115–1127. [Google Scholar] [CrossRef]
- Pierleoni, A.; Martelli, P.L.; Casadio, R. PredGPI: A GPI-anchor predictor. BMC Bioinform. 2008, 9, 392. [Google Scholar] [CrossRef]
- Sorek, N.; Sorek, H.; Kijac, A.; Szemenyei, H.J.; Bauer, S.; Hématy, K.; Wemmer, D.E.; Somerville, C.R. The Arabidopsis COBRA protein facilitates cellulose crystallization at the plasma membrane. J. Biol. Chem. 2014, 289, 34911–34920. [Google Scholar] [CrossRef]
- Liu, L.; Shang-Guan, K.; Zhang, B.; Liu, X.; Yan, M.; Zhang, L.; Shi, Y.; Zhang, M.; Qian, Q.; Li, J.; et al. Brittle Culm1, a COBRA-like protein, functions in cellulose assembly through binding cellulose microfibrils. PLoS Genet. 2013, 9, e1003704. [Google Scholar] [CrossRef]
- Jiao, S.; Hazebroek, J.P.; Chamberlin, M.A.; Perkins, M.; Sandhu, A.S.; Gupta, R.; Simcox, K.D.; Li, Y.; Prall, A.; Heetland, L.; et al. Chitinase-like1 Plays a Role in Stalk Tensile Strength in Maize. Plant Physiol. 2019, 181, 1127–1147. [Google Scholar] [CrossRef] [PubMed]
- Ching, A.; Dhugga, K.S.; Appenzeller, L.; Meeley, R.; Bourett, T.M.; Howard, R.J.; Rafalski, A. Brittle stalk 2 encodes a putative glycosylphosphatidylinositol-anchored protein that affects mechanical strength of maize tissues by altering the composition and structure of secondary cell walls. Planta 2006, 224, 1174–1184. [Google Scholar] [CrossRef]
- Li, Q.; Nie, S.; Li, G.; Du, R.; Yang, X.; Liu, B.; Gao, X.; Liu, T.; Zhang, Z.; Zhao, X.; et al. Identification and Fine Mapping of the Recessive Gene BK-5, Which Affects Cell Wall Biosynthesis and Plant Brittleness in Maize. Int. J. Mol. Sci. 2022, 23, 814. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, A.; Langewisch, T.; Olek, A.; Multani, D.S.; McCann, M.C.; Vermerris, W.; Carpita, N.C.; Johal, G. Maize Brittle stalk2 encodes a COBRA-like protein expressed in early organ development but required for tissue flexibility at maturity. Plant Physiol. 2007, 145, 1444–1459. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. NREL Laboratory Analytical Procedure, Issue Date: April 2008, Revision Date: 08-3-2012.
- Jin, X.; Chen, X.; Shi, C.; Li, M.; Guan, Y.; Yu, C.Y.; Yamada, T.; Sacks, E.J.; Peng, J. Determination of hemicellulose, cellulose and lignin content using visible and near infrared spectroscopy in Miscanthus sinensis. Bioresour. Technol. 2017, 241, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with deseq2 moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Ding, S.Y.; Liu, Y.S.; Zeng, Y.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Tao, Z.; Li, X.; Li, Y.; Huang, J.; Li, X.; Han, X.; Feng, S.; Zhang, G.; et al. Cellulose Synthase Mutants Distinctively Affect Cell Growth and Cell Wall Integrity for Plant Biomass Production in Arabidopsis. Plant Cell Physiol. 2018, 59, 1144–1157. [Google Scholar] [CrossRef]
- Watanabe, Y.; Meents, M.J.; Mcdonnell, L.M.; Barkwill, S.; Sampathkumar, A.; Cartwright, H.N.; Demura, T.; Ehrhardt, D.W.; Samuels, A.L.; Mansfield, S.D. Visualization of cellulose synthases in Arabidopsis secondary cell walls. Science 2015, 350, 198–203. [Google Scholar] [CrossRef]
- Desprez, T.; Vernhettes, S.; Fagard, M.; Refrégier, G.; Desnos, T.; Aletti, E.; Pelletier, N.P.S.; Höfte, H. Resistance against Herbicide Isoxaben and Cellulose Deficiency Caused by Distinct Mutations in Same Cellulose Synthase Isoform CESA6. Plant Physiol. 2002, 128, 482–490. [Google Scholar] [CrossRef]
- Fagard, M.; Desnos, T.; Desprez, T.; Goubet, F.; Refregier, G.; Mouille, G.; McCann, M.; Rayon, C.; Vernhettes, S.; Höfte, H. PROCUSTE1 Encodes a Cellulose Synthase Required for Normal Cell Elongation Specifically in Roots and Dark-Grown Hypocotyls of Arabidopsis. Plant Cell 2000, 12, 2409–2423. [Google Scholar] [CrossRef]
- Taylor, N.G.; Howells, R.M.; Huttly, A.K.; Vickers, K.; Turner, S.R. Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc. Natl. Acad. Sci. USA 2003, 100, 1450–1455. [Google Scholar] [CrossRef]
- Sato, K.; Suzuki, R.; Nishikubo, N.; Takenouchi, S.; Ito, S.; Nakano, Y.; Nakaba, S.; Sano, Y.; Funada, R.; Kajita, S.; et al. Isolation of a novel cell wall architecture mutant of rice with defective Arabidopsis COBL4 ortholog BC1 required for regulated deposition of secondary cell wall components. Planta 2010, 232, 257–270. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K. Lignin biosynthesis and structure. Plant Biol. 2010, 15, 895–905. [Google Scholar] [CrossRef]
- Doorsselaere, J.V.; Baucher, M.; Chognot, E.; Chabbert, B.; Tollier, M.T.; Petit-Conil, M.; Leplé, J.C.; Pilate, G.; Cornu, D.; Monties, B. A novel lignin in poplar trees with a reduced caffeic acid/5 hydroxyferulic acid O-methyltransferase activity. Plant J. 2003, 8, 855–864. [Google Scholar] [CrossRef]
- Goujon, T.; Sibout, R.; Eudes, A.; MacKay, J.; Joulanin, L. Genes involved in the biosynthesis of lignin precursors in Arabidopsis thaliana. Plant Physiol. Biochem. 2003, 41, 677–687. [Google Scholar] [CrossRef]
- Fang, L.; Xu, X.; Li, J.; Zheng, F.; Li, M.; Yan, J.; Li, Y.; Zhang, X.; Li, L.; Ma, G.; et al. Transcriptome analysis provides insights into the non-methylated lignin synthesis in Paphiopedilum armeniacum seed. BMC Genom. 2020, 21, 524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wei, F.; Guo, K.; Hu, Z.; Li, Y.; Xie, G.; Wang, Y.; Cai, X.; Peng, L.; Wang, L. A novel FC116/BC10 mutation distinctively causes alteration in the expression of the genes for cell wall polymer synthesis in rice. Front. Plant Sci. 2016, 7, 1366. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, Y.; Tan, W.; Chen, J.; Zhu, M.; Lv, Y.; Liu, Y.; Yu, S.; Zhang, W.; Cai, H. Brittle Culm 1 encodes a COBRA-like protein involved in secondary cell wall cellulose biosynthesis in sorghum. Plant Cell Physiol. 2019, 60, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Xie, P.; Chen, Y.; Chen, Y.; Yu, H.; Su, Y.; Gu, M.; Yan, C. Anatomical and chemical characteristics of culm of rice brittle mutant bc7 (t). Funct. Plant Biol. 2011, 38, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Mewalal, R.; Mizrachi, E.; Mansfield, S.D.; Myburg, A.A. Cell wall-related proteins of unknown function: Missing links in plant cell wall development. Plant Cell Physiol. 2014, 55, 1031–1043. [Google Scholar] [CrossRef]
- Aohara, T.; Kotake, T.; Kaneko, Y.; Takatsuji, H.; Tsumuraya, Y.; Kawasaki, S. Rice BRITTLE CULM 5 (BRITTLE NODE) is involved in secondary cell wall formation in the sclerenchyma tissue of nodes. Plant Cell Physiol. 2009, 50, 1886–1897. [Google Scholar] [CrossRef]
- Hirano, K.; Kotake, T.; Kamihara, K.; Tsuna, K.; Aohara, T.; Kaneko, Y.; Takatsuji, H.; Tsumuraya, Y.; Kawasaki, S. Rice BRITTLE CULM 3 (BC3) encodes a classical dynamin OsDRP2B essential for proper secondary cell wall synthesis. Planta 2010, 232, 95–108. [Google Scholar] [CrossRef]
- Niu, E.; Fang, S.; Shang, X.; Guo, W. Ectopic expression of GhCOBL9A, a cotton glycosyl-phosphatidyl inositol-anchored protein encoding gene, promotes cell elongation, thickening and increased plant biomass in transgenic Arabidopsis. Mol. Genet. Genom. 2018, 293, 1191–1204. [Google Scholar] [CrossRef]
- Du, J.; Anderson, C.T.; Xiao, C. Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat. Plants 2022, 8, 332–340. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.L.; Ye, Z.H. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef]
- Kohorn, B.D. WAKs; cell wall associated kinases. Curr. Opin. Cell. Biol. 2001, 13, 529–533. [Google Scholar] [CrossRef]
- Lally, D.; Ingmire, P.; Tong, H.Y.; He, Z.H. Antisense expression of a cell wall-associated protein kinase WAK4, inhibits cell elongation and alters morphology. Plant Cell 2001, 13, 1317–1331. [Google Scholar]
- Park, A.R.; Cho, S.K.; Yun, U.J.; Jin, M.Y.; Lee, S.H.; Sachetto-Martins, G.; Park, O.K. Interaction of the Arabidopsis receptor protein kinase Wak1 with a glycine-rich protein, AtGRP3. J. Biol. Chem. 2001, 276, 26688–26693. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant cell walls: Wall-associated kinases and cell expansion. Curr. Biol. 2001, 11, R558–R559. [Google Scholar] [CrossRef]
- Wagner, T.A.; Kohorn, B.D. Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell 2001, 13, 303–318. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; McCarthy, R.L.; Reeves, C.K.; Jones, E.G.; Ye, Z.H. Transcriptional activation of secondary wall biosynthesis by rice and maize NAC and MYB transcription factors. Plant Cell Physiol. 2011, 52, 1856–1871. [Google Scholar] [CrossRef]
- Zhong, R.; Yuan, Y.; Spiekerman, J.J.; Guley, J.T.; Egbosiuba, J.C.; Ye, Z.H. Functional characterization of NAC and MYB transcription factors involved in regulation of biomass production in switchgrass (Panicum virgatum). PLoS ONE 2015, 10, e0134611. [Google Scholar] [CrossRef]
- Mao, H.; Yu, L.; Han, R.; Li, Z.; Liu, H. ZmNAC55, a maize stress-responsive NAC transcription factor, confers drought resistance in transgenic Arabidopsis. Plant Physiol. Biochem. 2016, 105, 55–66. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, F.; Zhong, Y.; He, J.; Li, L. Modulation of NAC transcription factor NST1 activity by XYLEM NAC DOMAIN1 regulates secondary cell wall formation in Arabidopsis. J. Exp. Bot. 2020, 71, 1449–1458. [Google Scholar] [CrossRef]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Mimura, T.; Fukuda, H.; Demura, T. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. MYB20, MYB42, MYB43, and MYB85 regulate phenylalanine and lignin biosynthesis during secondary cell wall formation. Plant Physiol. 2020, 182, 1272–1283. [Google Scholar] [CrossRef]
- Öhman, D.; Demedts, B.; Kumar, M.; Gerber, L.; Gorzsas, A.; Goeminne, G.; Hedenström, M.; Ellis, B.; Boerjan, W.; Sundberg, B. MYB103 is required for FERULATE-5-HYDROXYLASE expression and syringyl lignin biosynthesis in Arabidopsis stems. Plant J. 2013, 73, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.C.; Ko, J.H.; Kim, J.Y.; Kim, J.; Bae, H.J.; Han, K.H. MYB46 directly regulates the gene expression of secondary wall-associ-ated cellulose synthases in Arabidopsis. Plant J. 2013, 73, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Jeon, H.W.; Kim, W.C.; Kim, J.Y.; Han, K.H. The MYB46/MYB83-mediated transcriptional regulatory programme is a gatekeeper of secondary wall biosynthesis. Ann. Bot. 2014, 114, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Yang, J.; Liu, J. Analysis of Agronomic Traits Combining Ability of Sweetwaxy Double Recessive Corn Inbred Lines. Bangladesh J. Bot. 2022, 51, 923–929. [Google Scholar] [CrossRef]





| Group | Total Numbers | Wild-Type | Mutant Type | χ2(0.05) ≤ 3.84 |
|---|---|---|---|---|
| (B73/mu-J1862) F2 | 672 | 511 | 161 | 0.389 |
| (Huang Zaosi/mu-J1862) F2 | 457 | 338 | 119 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Zhao, Y.; Liu, Q.; Diao, Y.; Wang, Q.; Yu, J.; Jiang, E.; Zhang, Y.; Liu, B. Identification of ZmBK2 Gene Variation Involved in Regulating Maize Brittleness. Genes 2023, 14, 1126. https://doi.org/10.3390/genes14061126
Xu W, Zhao Y, Liu Q, Diao Y, Wang Q, Yu J, Jiang E, Zhang Y, Liu B. Identification of ZmBK2 Gene Variation Involved in Regulating Maize Brittleness. Genes. 2023; 14(6):1126. https://doi.org/10.3390/genes14061126
Chicago/Turabian StyleXu, Wei, Yan Zhao, Qingzhi Liu, Yuqiang Diao, Qingkang Wang, Jiamin Yu, Enjun Jiang, Yongzhong Zhang, and Baoshen Liu. 2023. "Identification of ZmBK2 Gene Variation Involved in Regulating Maize Brittleness" Genes 14, no. 6: 1126. https://doi.org/10.3390/genes14061126




