Genome-Wide Identification, Characterization and Expression Analysis of Plant Nuclear Factor (NF-Y) Gene Family Transcription Factors in Saccharum spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of NF-Y Gene Members in Sugarcane
2.2. Physiochemical Properties and Subcellular Localization Prediction
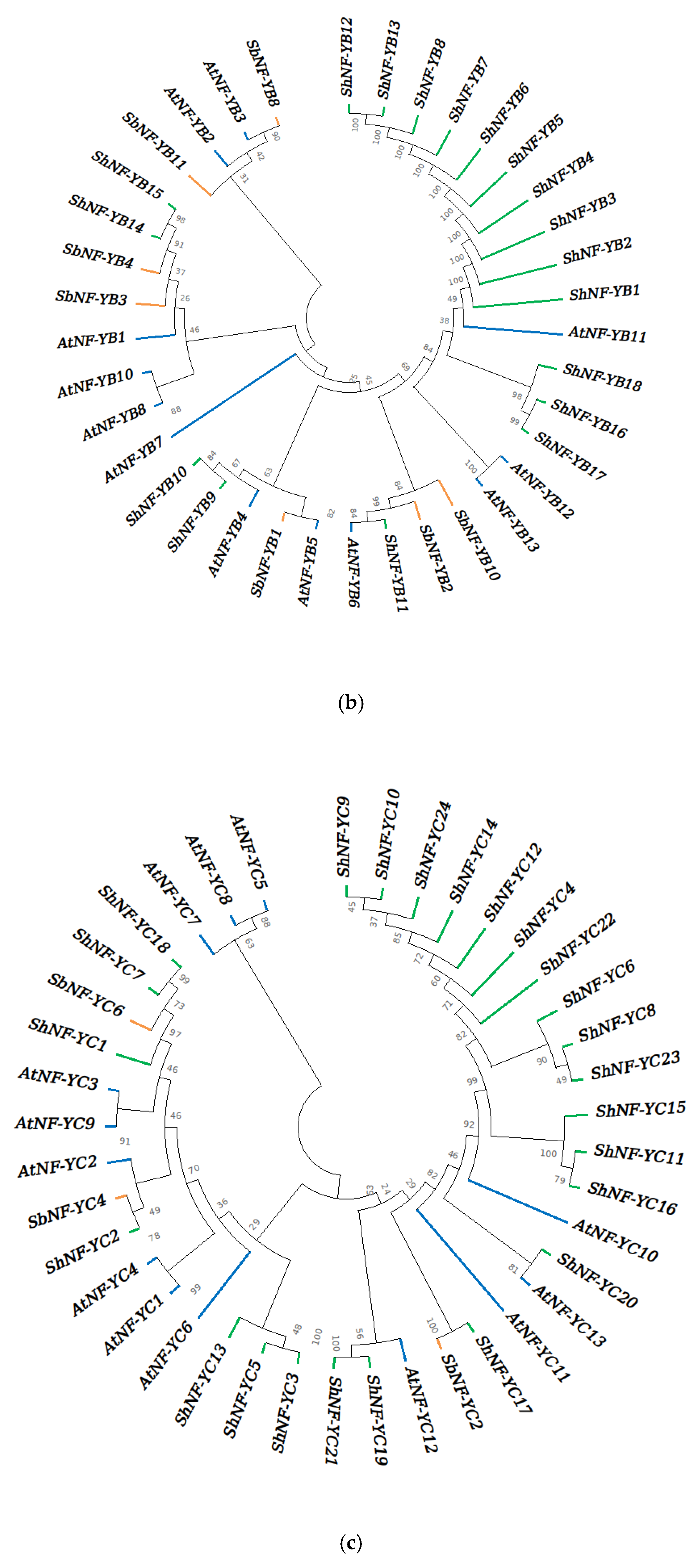
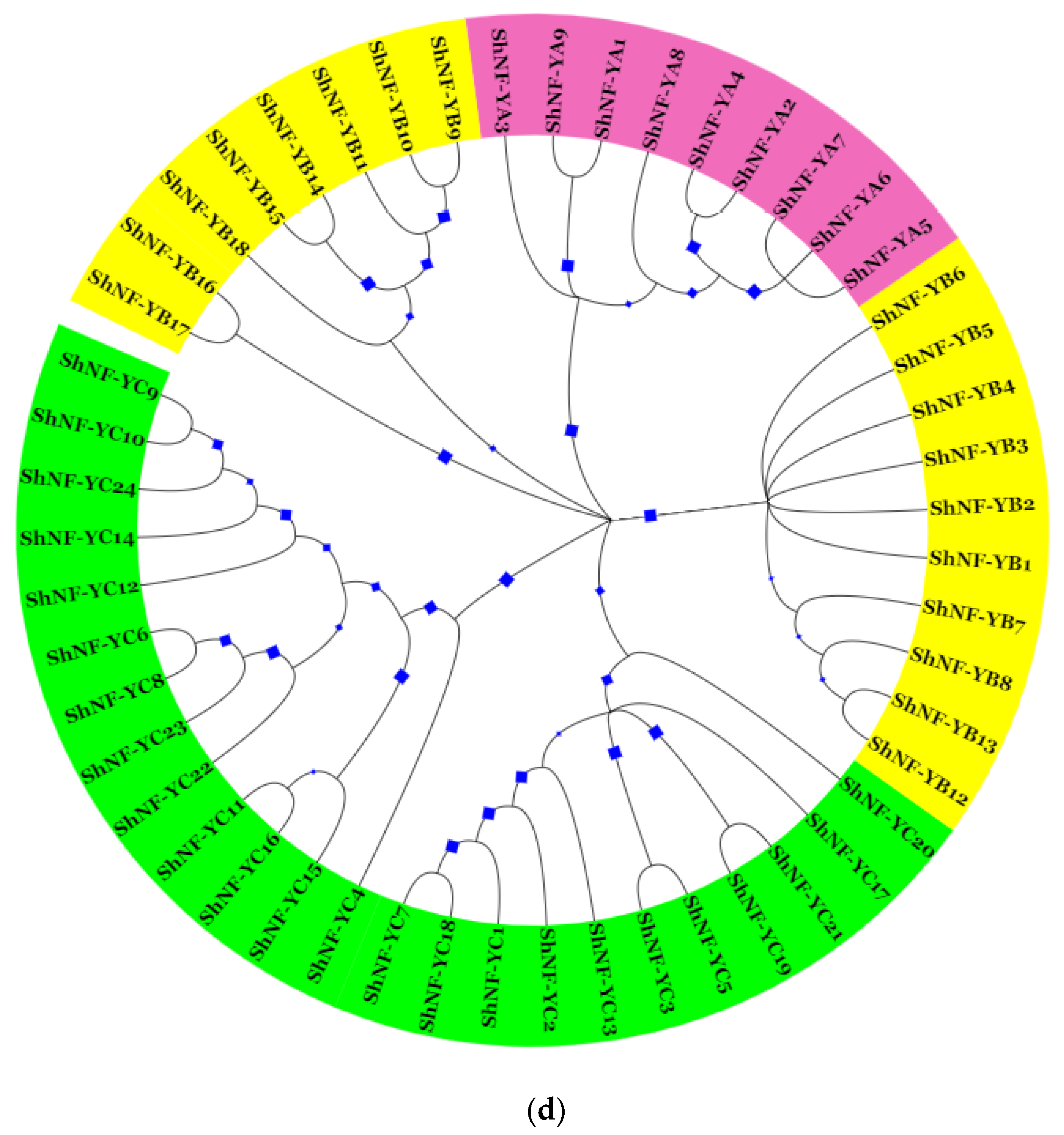
2.3. Multiple Sequence Alignment and Phylogenetic Analysis
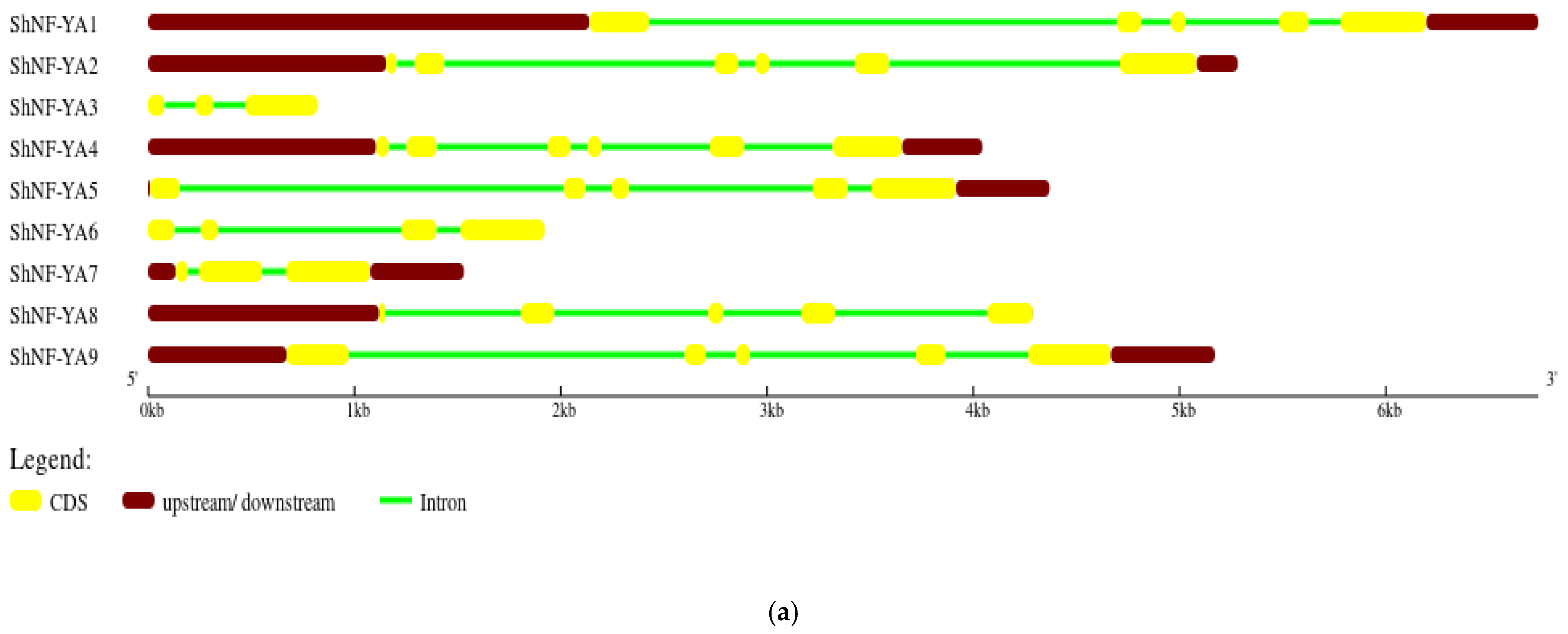
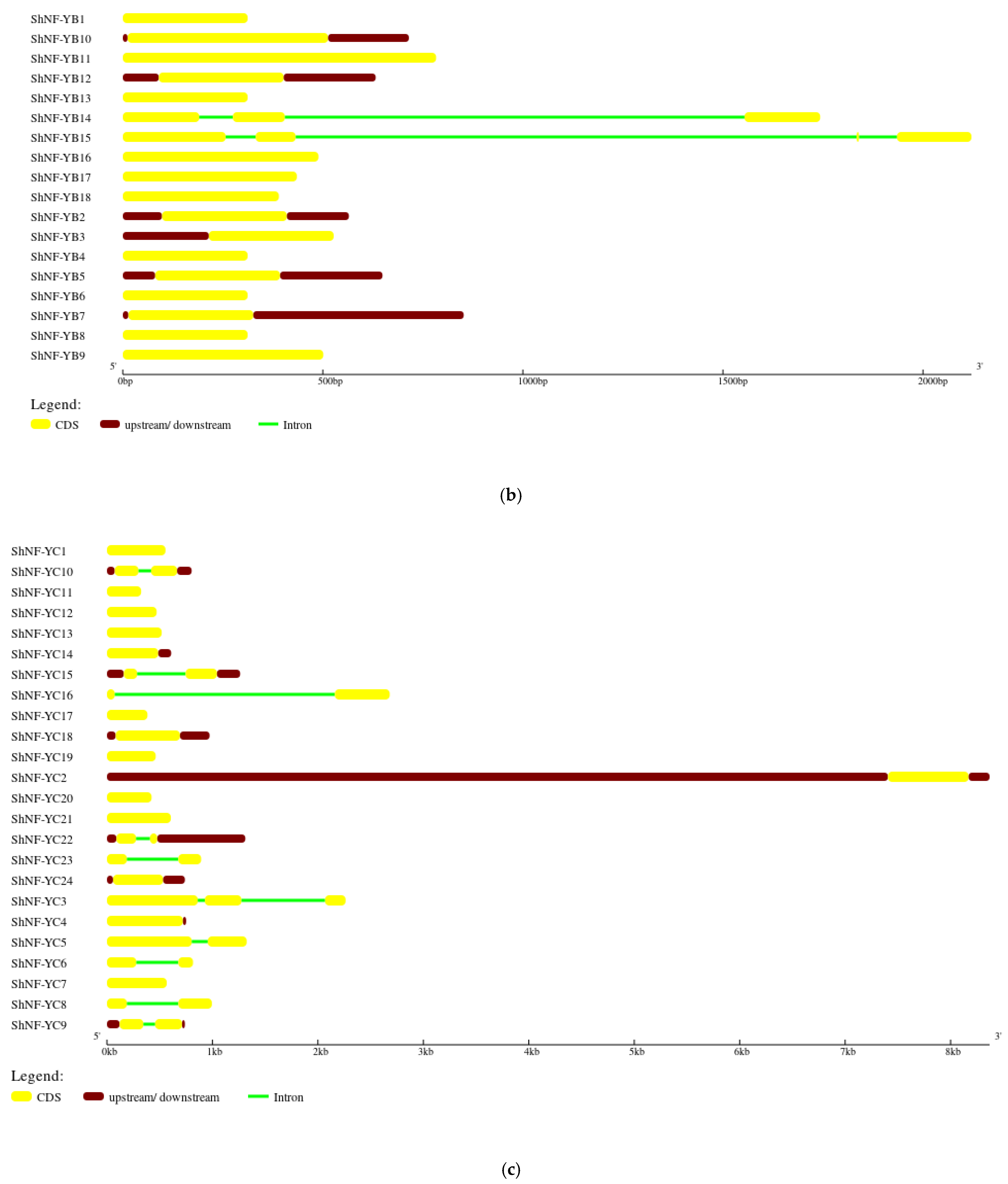
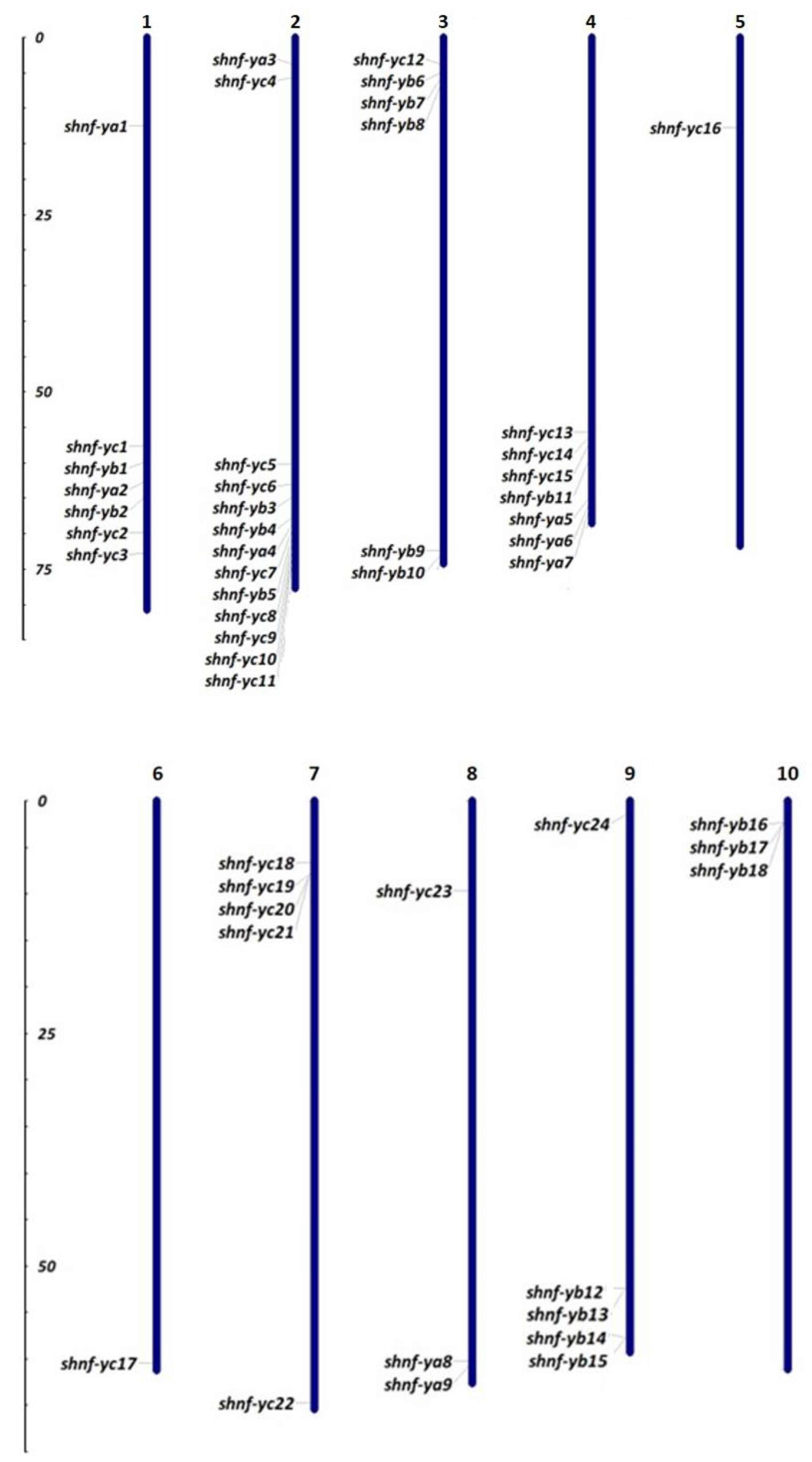
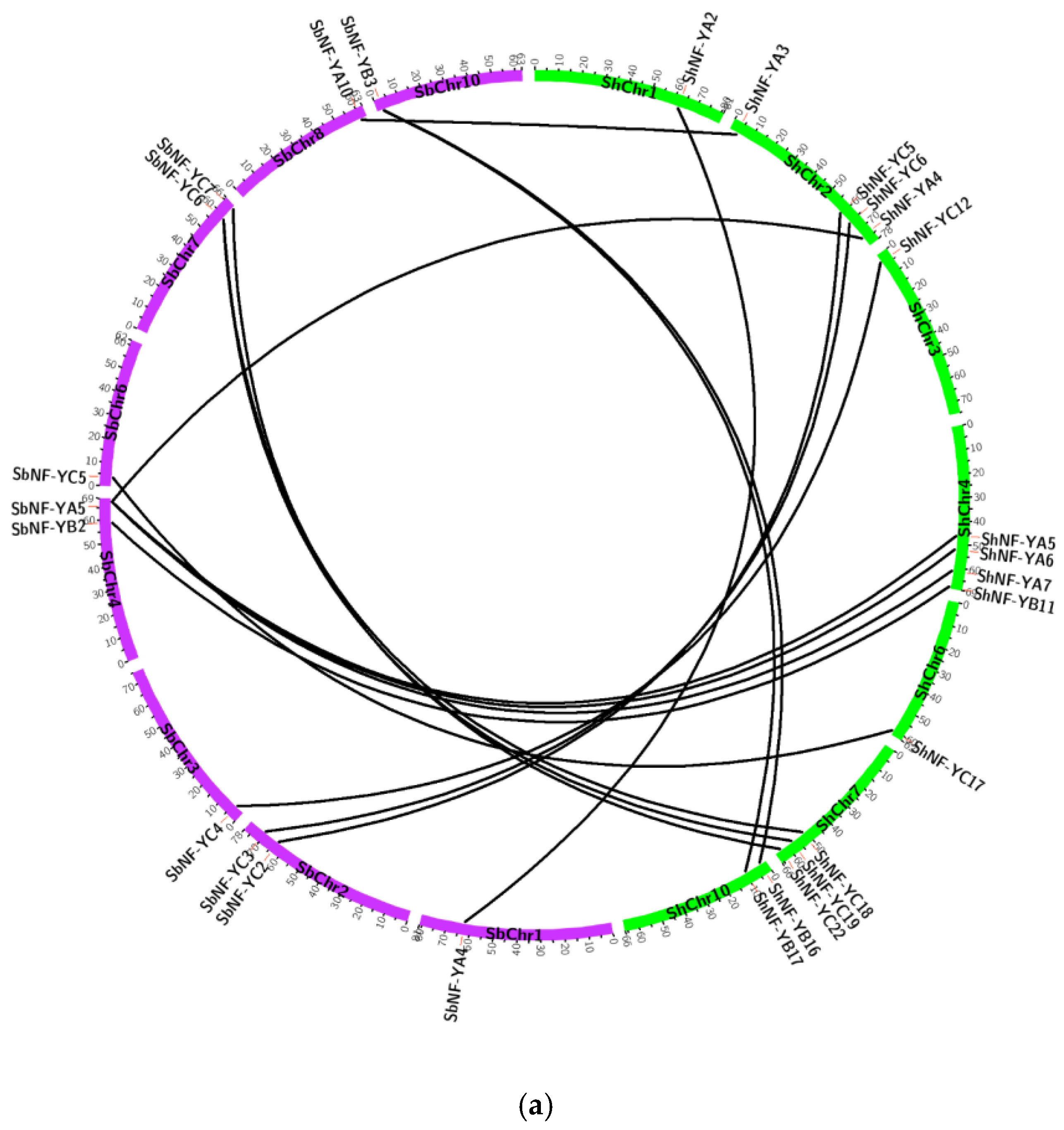
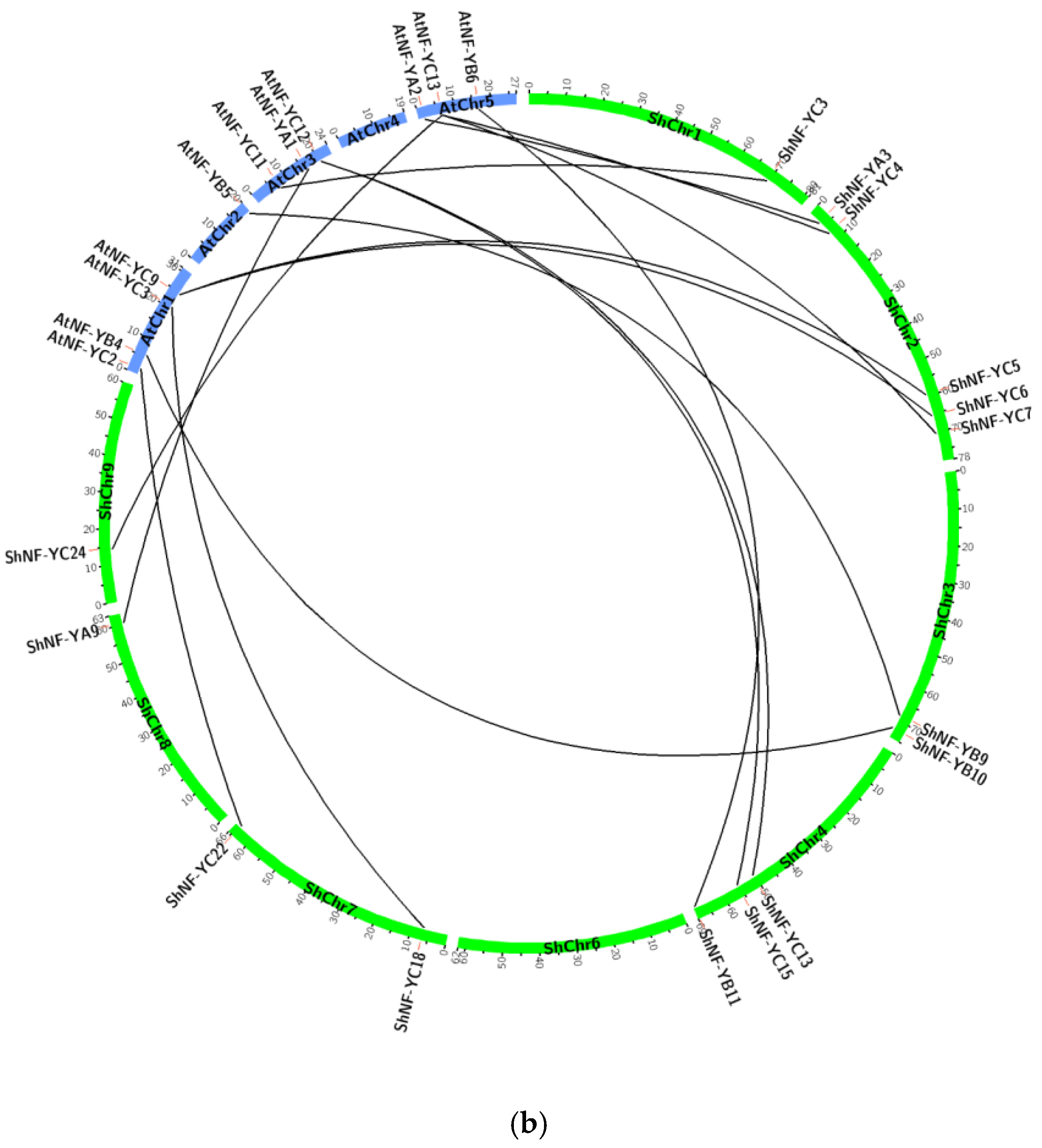
2.4. Chromosomal Distribution, Gene Structure, and Synteny Analysis of ShNF-Y Genes
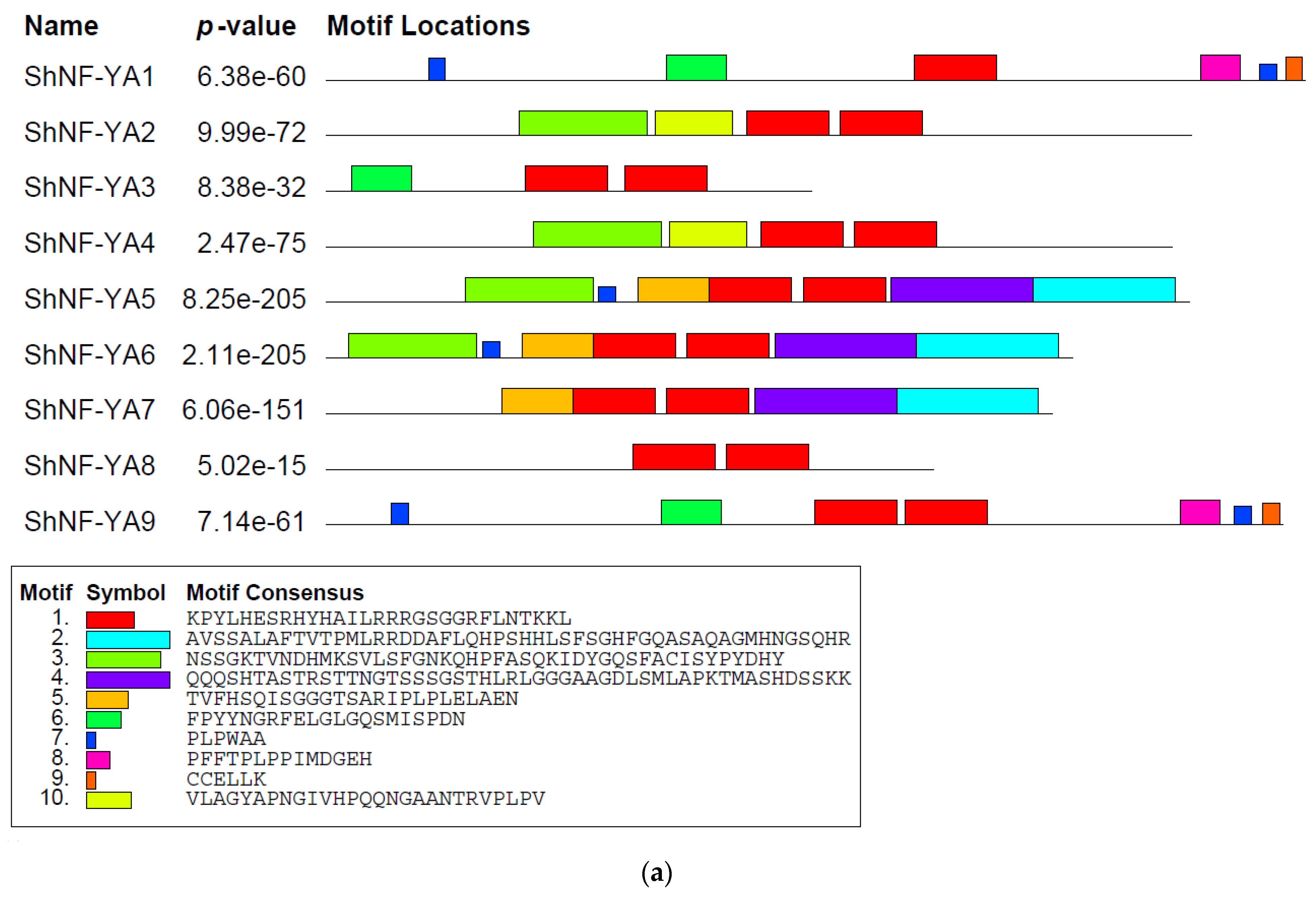
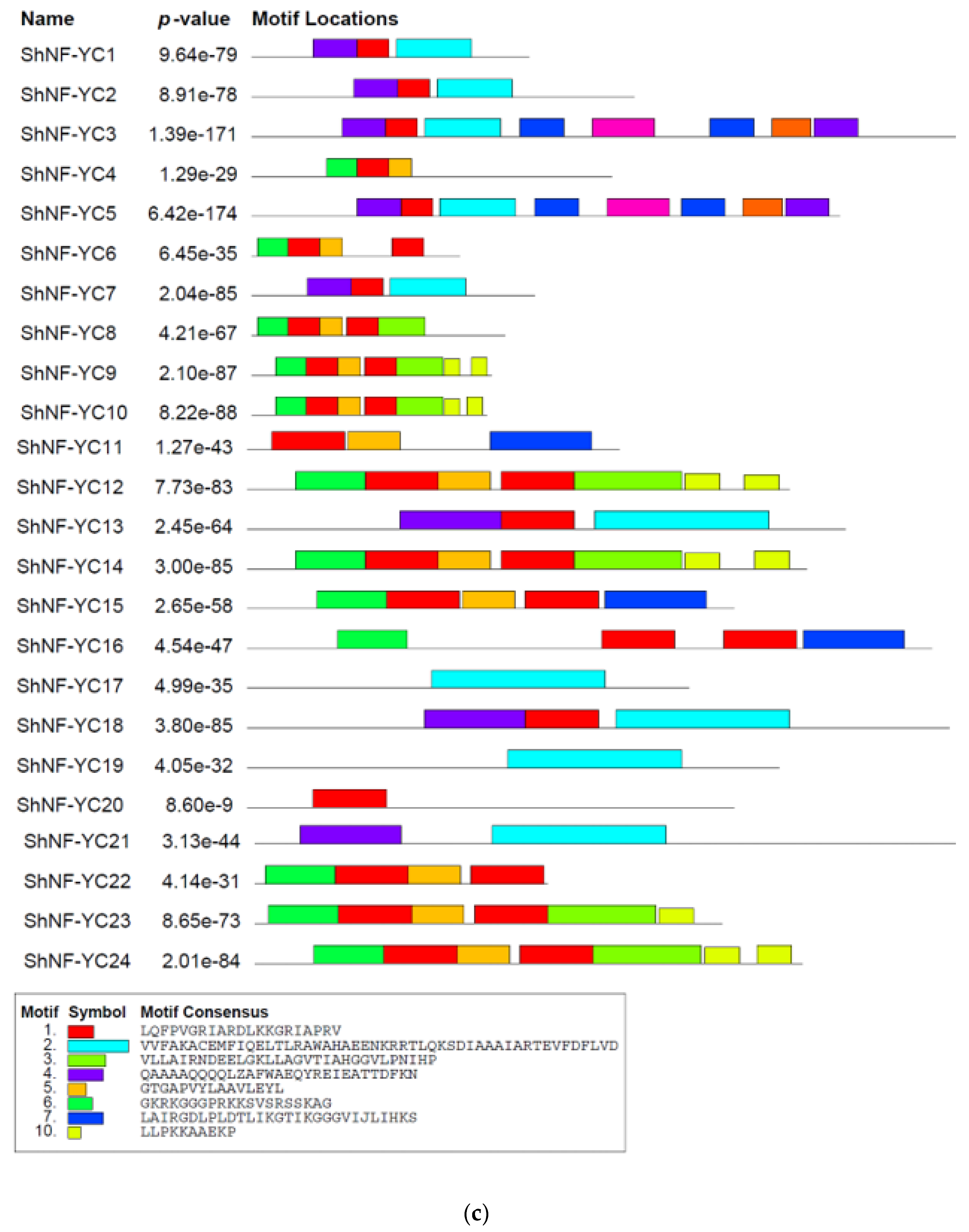
2.5. Identification of Conserved Motifs in ShNF-Y Genes and Proteins
2.6. Three-Dimensional Structure Prediction of ShNF-Y Proteins
2.7. RNA Extraction and Real-Time Quantitative PCR
3. Results
3.1. Identification, Characterization of ShNF-Y Transcription Factors, and Conserved Domain/Motif Analysis
3.2. Physiochemical Properties and Subcellular Localization Analysis
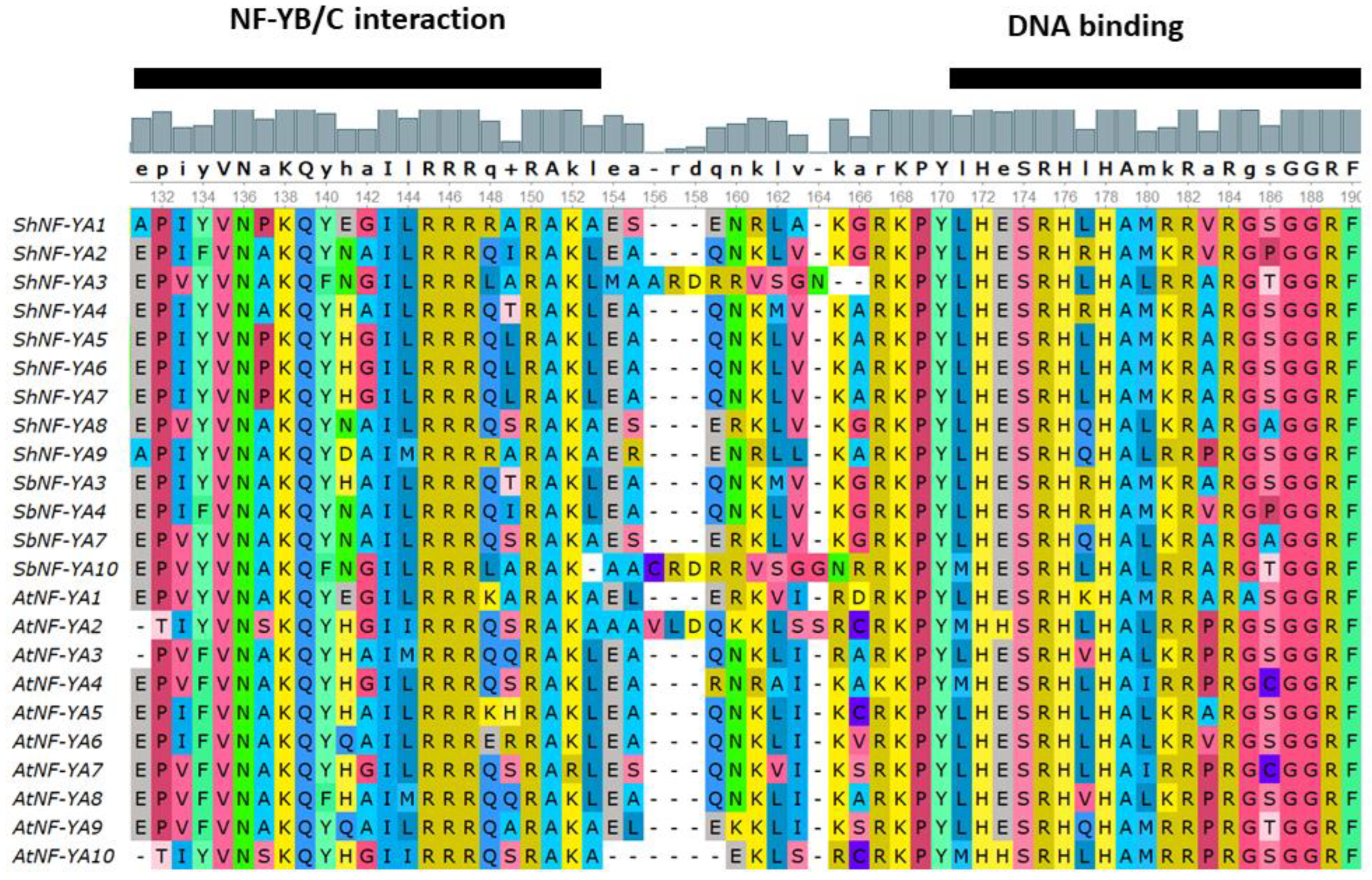
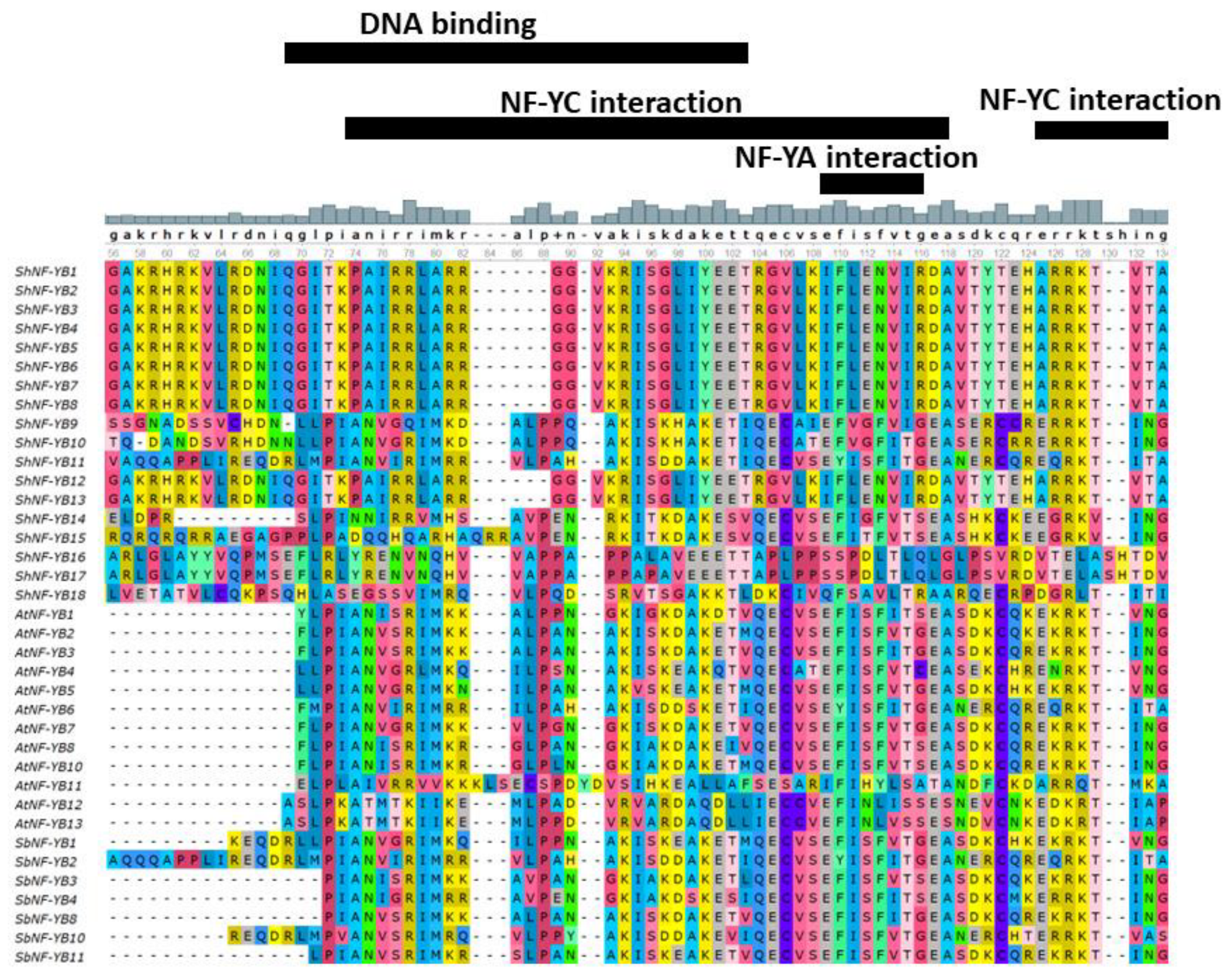
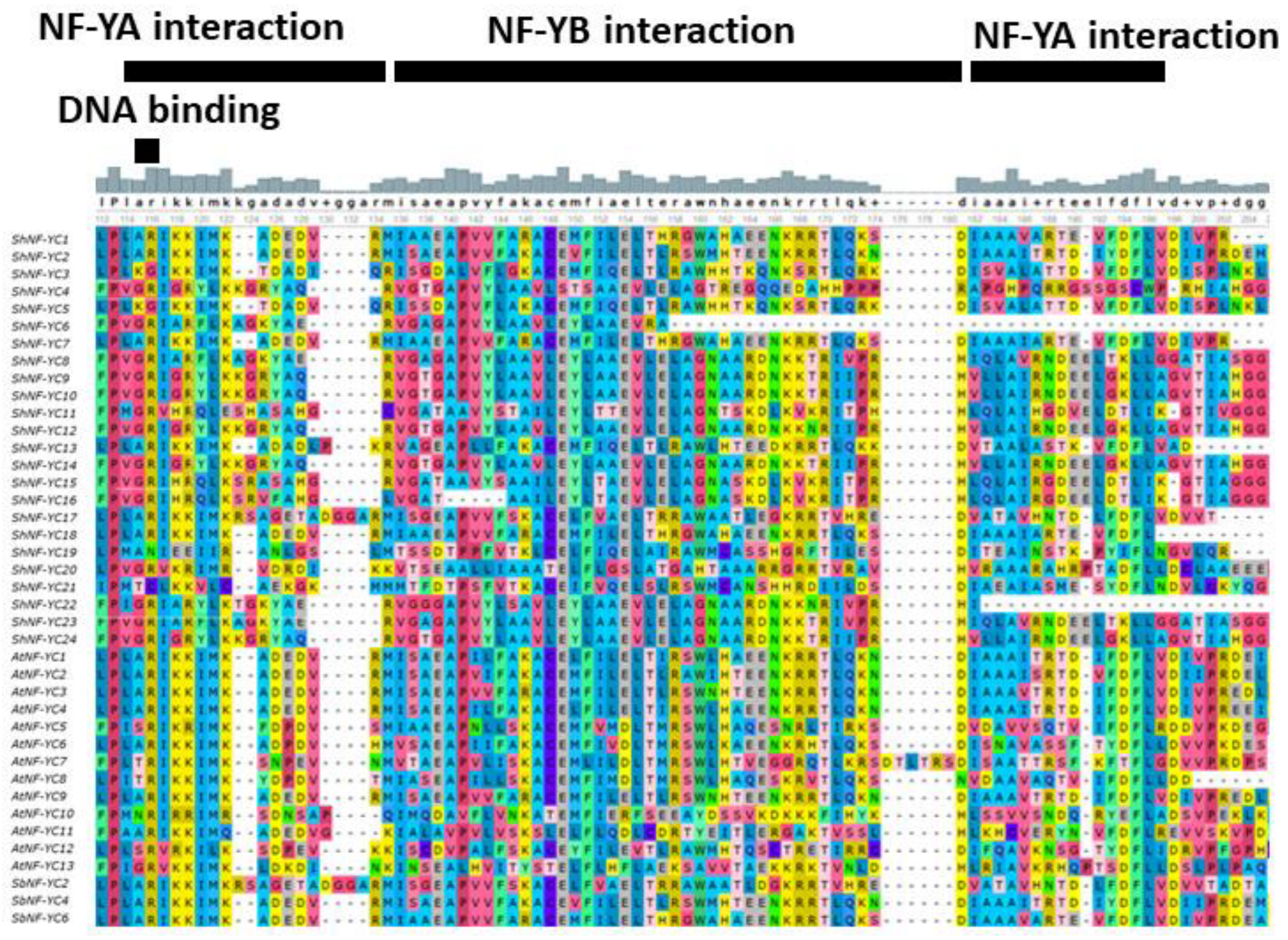
3.3. Multiple Sequence Alignment and Phylogenetic Analysis of ShNF-Y Proteins
3.4. Gene Structure, Chromosomal Distribution, and Synteny Analysis of ShNF-Y Genes
3.5. Three-Dimensional Structure Prediction of ShNF-Y Proteins
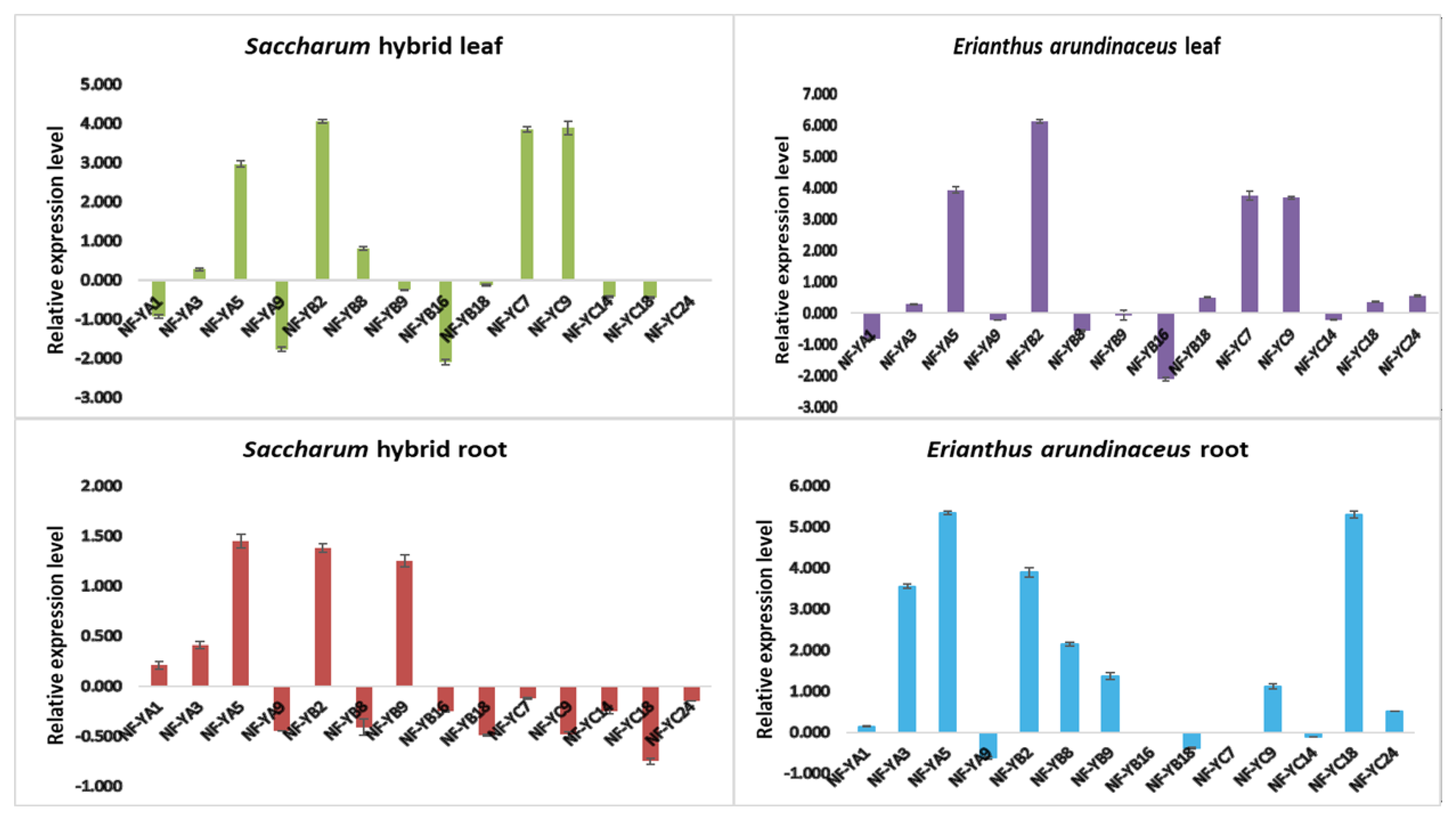
3.6. Expression Analysis of NF-Y Genes in the Saccharum Complex under Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakashima, K.; Tran, L.S.P.; Van Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef]
- Chaves-Sanjuan, A.; Gnesutta, N.; Gobbini, A.; Martignago, D.; Bernardini, A.; Fornara, F.; Mantovani, R.; Nardini, M. Structural determinants for NF-Y subunit organization and NF-Y/DNA association in plants. Plant J. 2021, 105, 49–61. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Ganie, S.A.; Wani, S.H.; Guddimalli, R.; Karumanchi, A.R.; Edupuganti, S.; Naravula, J.; Kumar, V.; Polavarapu, R.; Suravajhala, P. Nuclear Factor-Y (NF-Y): Developmental and Stress-Responsive Roles in the Plant Lineage. J. Plant Growth Regul. 2022, 42, 2711–2735. [Google Scholar] [CrossRef]
- Liu, J.-X.; Howell, S.H. bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 2010, 22, 782–796. [Google Scholar] [CrossRef]
- Li, C.; Distelfeld, A.; Comis, A.; Dubcovsky, J. Wheat flowering repressor VRN2 and promoter CO2 compete for interactions with NUCLEAR FACTOR-Y complexes. Plant J. 2011, 67, 763–773. [Google Scholar] [CrossRef]
- Leyva-González, M.A.; Ibarra-Laclette, E.; Cruz-Ramírez, A.; Herrera-Estrella, L. Functional and transcriptome analysis reveals an acclimatization strategy for abiotic stress tolerance mediated by Arabidopsis NF-YA family members. PLoS ONE 2012, 7, e48138. [Google Scholar] [CrossRef]
- Hou, X.; Zhou, J.; Liu, C.; Liu, L.; Shen, L.; Yu, H. Nuclear factor Y-mediated H3K27me3 demethylation of the SOC1 locus orchestrates flowering responses of Arabidopsis. Nat. Commun. 2014, 5, 4601. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Y.; Lai, W.; Hu, L.; Jiang, L.; Liu, S. In silico identification and expression analysis of nuclear factor Y (NF-Y) transcription factors in cucumber. Agronomy 2020, 10, 236. [Google Scholar] [CrossRef]
- Liu, R.; Wu, M.; Liu, H.L.; Gao, Y.M.; Chen, J.; Yan, H.W.; Xiang, Y. Genome-wide identification and expression analysis of the NF-Y transcription factor family in Populus. Physiol. Plant. 2021, 171, 309–327. [Google Scholar] [CrossRef]
- Maheshwari, P.; Kummari, D.; Palakolanu, S.R.; Nagasai Tejaswi, U.; Nagaraju, M.; Rajasheker, G.; Jawahar, G.; Jalaja, N.; Rathnagiri, P.; Kavi Kishor, P. Genome-wide identification and expression profile analysis of nuclear factor Y family genes in Sorghum bicolor L.(Moench). PLoS ONE 2019, 14, e0222203. [Google Scholar] [CrossRef]
- Li, W.-X.; Oono, Y.; Zhu, J.; He, X.-J.; Wu, J.-M.; Iida, K.; Lu, X.-Y.; Cui, X.; Jin, H.; Zhu, J.-K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 2008, 20, 2238–2251. [Google Scholar] [CrossRef]
- Kwong, R.W.; Bui, A.Q.; Lee, H.; Kwong, L.W.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. Leafy Cotyledon1-LIKE defines a class of regulators essential for embryo development. Plant Cell 2003, 15, 5–18. [Google Scholar] [CrossRef]
- Warpeha, K.M.; Upadhyay, S.; Yeh, J.; Adamiak, J.; Hawkins, S.I.; Lapik, Y.R.; Anderson, M.B.; Kaufman, L.S. The GCR1, GPA1, PRN1, NF-Y signal chain mediates both blue light and abscisic acid responses in Arabidopsis. Plant Physiol. 2007, 143, 1590–1600. [Google Scholar] [CrossRef]
- Kumimoto, R.W.; Adam, L.; Hymus, G.J.; Repetti, P.P.; Reuber, T.L.; Marion, C.M.; Hempel, F.D.; Ratcliffe, O.J. The Nuclear Factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta 2008, 228, 709–723. [Google Scholar] [CrossRef]
- Ballif, J.; Endo, S.; Kotani, M.; MacAdam, J.; Wu, Y. Over-expression of HAP3b enhances primary root elongation in Arabidopsis. Plant Physiol. Biochem. 2011, 49, 579–583. [Google Scholar] [CrossRef]
- Stephenson, P.G.; Moore, C.M.; Terry, M.J.; Zubkov, M.V.; Bibby, T.S. Improving photosynthesis for algal biofuels: Toward a green revolution. Trends Biotechnol. 2011, 29, 615–623. [Google Scholar] [CrossRef]
- Sun, X.; Ling, S.; Lu, Z.; Ouyang, Y.-d.; Liu, S.; Yao, J. OsNF-YB1, a rice endosperm-specific gene, is essential for cell proliferation in endosperm development. Gene 2014, 551, 214–221. [Google Scholar] [CrossRef]
- Myers, Z.A.; Kumimoto, R.W.; Siriwardana, C.L.; Gayler, K.K.; Risinger, J.R.; Pezzetta, D.; Holt III, B.F. NUCLEAR FACTOR Y, subunit C (NF-YC) transcription factors are positive regulators of photomorphogenesis in Arabidopsis thaliana. PLoS Genet. 2016, 12, e1006333. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Ran, Q.; Li, P.; Peng, Z.; Zhang, J. ZmNF-YB16 overexpression improves drought resistance and yield by enhancing photosynthesis and the antioxidant capacity of maize plants. Front. Plant Sci. 2018, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, N.; Zhu, X.; Ma, R.; Liu, S.; Wang, X.; Yang, J.; Si, H. Genome-wide analysis of NF-Y genes in potato and functional identification of StNF-YC9 in drought tolerance. Front. Plant Sci. 2021, 12, 749688. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shi, H.; Guo, Z. Overexpression of a NF-YC gene results in enhanced drought and salt tolerance in transgenic seashore paspalum. Front. Plant Sci. 2018, 9, 1355. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-J.; He, G.-H.; Zheng, W.-J.; Lu, P.-P.; Chen, M.; Gong, Y.-M.; Ma, Y.-Z.; Xu, Z.-S. Foxtail millet NF-Y families: Genome-wide survey and evolution analyses identified two functional genes important in abiotic stresses. Front. Plant Sci. 2015, 6, 1142. [Google Scholar] [CrossRef] [PubMed]
- Surya Krishna, S.; Harish Chandar, S.; Ravi, M.; Valarmathi, R.; Lakshmi, K.; Prathima, P.T.; Manimekalai, R.; Viswanathan, R.; Hemaprabha, G.; Appunu, C. Transgene-Free Genome Editing for Biotic and Abiotic Stress Resistance in Sugarcane: Prospects and Challenges. Agronomy 2023, 13, 1000. [Google Scholar] [CrossRef]
- Surya Krishna, S.; Viswanathan, R.; Valarmathi, R.; Lakshmi, K.; Appunu, C. CRISPR/Cas-Mediated Genome Editing Approach for Improving Virus Resistance in Sugarcane. Sugar Tech 2023, 1–16. [Google Scholar] [CrossRef]
- Mohanan, M.V.; Pushpanathan, A.; Sasikumar, S.P.T.; Selvarajan, D.; Jayanarayanan, A.N.; Ramalingam, S.; Karuppasamy, S.N.; Subbiah, R.; Ram, B.; Chinnaswamy, A. Ectopic expression of DJ-1/PfpI domain containing Erianthus arundinaceus Glyoxalase III (EaGly III) enhances drought tolerance in sugarcane. Plant Cell Rep. 2020, 39, 1581–1594. [Google Scholar] [CrossRef]
- Narayan, J.A.; Chakravarthi, M.; Nerkar, G.; Manoj, V.; Dharshini, S.; Subramonian, N.; Premachandran, M.; Kumar, R.A.; Surendar, K.K.; Hemaprabha, G. Overexpression of expansin EaEXPA1, a cell wall loosening protein enhances drought tolerance in sugarcane. Ind. Crops Prod. 2021, 159, 113035. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xuanyuan, G.; Lu, C.; Zhang, R.; Jiang, J. Overexpression of StNF-YB3. 1 reduces photosynthetic capacity and tuber production, and promotes ABA-mediated stomatal closure in potato (Solanum tuberosum L.). Plant Sci. 2017, 261, 50–59. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, D.; Liu, Y.; Luo, C.; Zhou, Y.; Zhang, L. Overexpression of a NF-YB3 transcription factor from Picea wilsonii confers tolerance to salinity and drought stress in transformed Arabidopsis thaliana. Plant Physiol. Biochem. 2015, 94, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Palmeros-Suárez, P.A.; Massange-Sánchez, J.A.; Martínez-Gallardo, N.A.; Montero-Vargas, J.M.; Gómez-Leyva, J.F.; Délano-Frier, J.P. The overexpression of an Amaranthus hypochondriacus NF-YC gene modifies growth and confers water deficit stress resistance in Arabidopsis. Plant Sci. 2015, 240, 25–40. [Google Scholar] [CrossRef]
- Qu, B.; He, X.; Wang, J.; Zhao, Y.; Teng, W.; Shao, A.; Zhao, X.; Ma, W.; Wang, J.; Li, B. A wheat CCAAT box-binding transcription factor increases the grain yield of wheat with less fertilizer input. Plant Physiol. 2015, 167, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, R. The molecular biology of the CCAAT-binding factor NF-Y. Gene 1999, 239, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wenkel, S.; Turck, F.; Singer, K.; Gissot, L.; Le Gourrierec, J.; Samach, A.; Coupland, G. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 2006, 18, 2971–2984. [Google Scholar] [CrossRef]
- Yamamoto, A.; Kagaya, Y.; Toyoshima, R.; Kagaya, M.; Takeda, S.; Hattori, T. Arabidopsis NF-YB subunits LEC1 and LEC1-LIKE activate transcription by interacting with seed-specific ABRE-binding factors. Plant J. 2009, 58, 843–856. [Google Scholar] [CrossRef]
- Quach, T.N.; Nguyen, H.T.; Valliyodan, B.; Joshi, T.; Xu, D.; Nguyen, H.T. Genome-wide expression analysis of soybean NF-Y genes reveals potential function in development and drought response. Mol. Genet. Genom. 2015, 290, 1095–1115. [Google Scholar] [CrossRef]
- Stephenson, T.J.; McIntyre, C.L.; Collet, C.; Xue, G.-P. Genome-wide identification and expression analysis of the NF-Y family of transcription factors in Triticum aestivum. Plant Mol. Biol. 2007, 65, 77–92. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, Z.; Wang, Y.; Li, S.; Liang, Z. Genome-wide identification and characterization of the NF-Y gene family in grape (Vitis vinifera L.). BMC Genom. 2016, 17, 605. [Google Scholar] [CrossRef]
- Olesen, J.T.; Guarente, L. The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: Model for the HAP2/3/4 complex. Genes Dev. 1990, 4, 1714–1729. [Google Scholar] [CrossRef]
- Peter, S.C.; Murugan, N.; Mohanan, M.V.; Sasikumar, S.P.T.; Selvarajan, D.; Jayanarayanan, A.N.; Shivalingamurthy, S.G.; Chennappa, M.; Ramanathan, V.; Govindakurup, H. Isolation, characterization and expression analysis of stress responsive plant nuclear transcriptional factor subunit (NF-YB2) from commercial Saccharum hybrid and wild relative Erianthus arundinaceus. 3 Biotech 2020, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tang, S.; An, Y.; Zheng, D.-C.; Xia, X.-L.; Yin, W.-L. Overexpression of the poplar NF-YB7 transcription factor confers drought tolerance and improves water-use efficiency in Arabidopsis. J. Exp. Bot. 2013, 64, 4589–4601. [Google Scholar] [CrossRef]
- Cai, X.; Ballif, J.; Endo, S.; Davis, E.; Liang, M.; Chen, D.; DeWald, D.; Kreps, J.; Zhu, T.; Wu, Y. A putative CCAAT-binding transcription factor is a regulator of flowering timing in Arabidopsis. Plant Physiol. 2007, 145, 98–105. [Google Scholar] [CrossRef]
- Wei, X.; Xu, J.; Guo, H.; Jiang, L.; Chen, S.; Yu, C.; Zhou, Z.; Hu, P.; Zhai, H.; Wan, J. DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol. 2010, 153, 1747–1758. [Google Scholar] [CrossRef]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, X.; Gao, Y.; Yang, S. Genome-wide identification and characterization of GRAS genes in soybean (Glycine max). BMC Plant Biol. 2020, 20, 490. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L.; Martins, C.P.; Sousa, A.O.; Camillo, L.R.; Araújo, C.P.; Alcantara, G.M.; Camargo, D.S.; Cidade, L.C.; de Almeida, A.-A.F.; Costa, M.G. Genome-wide characterization and expression analysis of citrus nuclear factor-y (NF-Y) transcription factors identified a novel NF-YA gene involved in drought-stress response and tolerance. PLoS ONE 2018, 13, e0199187. [Google Scholar] [CrossRef]
- Siefers, N.; Dang, K.K.; Kumimoto, R.W.; Bynum IV, W.E.; Tayrose, G.; Holt III, B.F. Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 2009, 149, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-K.; Kim, H.I.; Jang, G.; Chung, P.J.; Jeong, J.S.; Kim, Y.S.; Bang, S.W.; Jung, H.; Do Choi, Y.; Kim, J.-K. The NF-YA transcription factor OsNF-YA7 confers drought stress tolerance of rice in an abscisic acid independent manner. Plant Sci. 2015, 241, 199–210. [Google Scholar] [CrossRef]
- Augustine, S.M.; Syamaladevi, D.P.; Premachandran, M.; Ravichandran, V.; Subramonian, N. Physiological and molecular insights to drought responsiveness in Erianthus spp. Sugar Tech 2015, 17, 121–129. [Google Scholar] [CrossRef]



| Gene Name | Cds Length | Gene Length | Protein Length | Molecular Weight (kDA) | Protein Theoretical pI | GRAVY |
|---|---|---|---|---|---|---|
| NF-YA Subunit | ||||||
| ShNF-YA1 | 1038 | 6739 | 345 | 36.542 | 9.61 | −0.533 |
| ShNF-YA2 | 918 | 5282 | 305 | 33.192 | 9.73 | −0.650 |
| ShNF-YA3 | 516 | 821 | 171 | 19.334 | 11.77 | −0.743 |
| ShNF-YA4 | 897 | 4044 | 298 | 32.588 | 8.21 | −0.848 |
| ShNF-YA5 | 915 | 4370 | 304 | 32.894 | 11.18 | −0.730 |
| ShNF-YA6 | 792 | 1924 | 263 | 28.528 | 10.37 | −0.580 |
| ShNF-YA7 | 771 | 1531 | 256 | 28.552 | 10.58 | −0.388 |
| ShNF-YA8 | 645 | 4290 | 214 | 23.355 | 7.97 | −1.101 |
| ShNF-YA9 | 1014 | 5171 | 337 | 36.532 | 9.24 | −0.500 |
| NF-YB Subunit | ||||||
| ShNF-YB1 | 312 | 312 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB2 | 312 | 565 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB3 | 312 | 527 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB4 | 312 | 312 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB5 | 312 | 649 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB6 | 312 | 312 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB7 | 312 | 852 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB8 | 312 | 312 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB9 | 501 | 501 | 166 | 18.324 | 5.95 | −0.480 |
| ShNF-YB10 | 501 | 715 | 166 | 18.363 | 6.52 | −0.821 |
| ShNF-YB11 | 783 | 783 | 260 | 27.691 | 6.40 | −0.579 |
| ShNF-YB12 | 312 | 632 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB13 | 312 | 312 | 103 | 11.409 | 11.48 | −0.524 |
| ShNF-YB14 | 510 | 1743 | 169 | 18.693 | 5.82 | −0.435 |
| ShNF-YB15 | 549 | 2121 | 182 | 20.447 | 9.83 | −0.804 |
| ShNF-YB16 | 489 | 489 | 162 | 17.652 | 4.52 | −0.141 |
| ShNF-YB17 | 435 | 435 | 144 | 15.681 | 4.40 | −0.143 |
| ShNF-YB18 | 390 | 390 | 129 | 13.982 | 9.35 | 0.018 |
| NF-YC Subunit | ||||||
| ShNF-YC1 | 555 | 555 | 184 | 19.855 | 5.50 | −0.280 |
| ShNF-YC2 | 765 | 8370 | 254 | 28.516 | 5.04 | −0.668 |
| ShNF-YC3 | 1404 | 2263 | 467 | 52.251 | 5.19 | −0.262 |
| ShNF-YC4 | 720 | 751 | 239 | 25.574 | 10.74 | −0.478 |
| ShNF-YC5 | 1173 | 1325 | 390 | 43.027 | 5.66 | 10.681 |
| ShNF-YC6 | 417 | 816 | 138 | 14.617 | 10.76 | −0.094 |
| ShNF-YC7 | 567 | 567 | 188 | 20.032 | 5.36 | −0.065 |
| ShNF-YC8 | 507 | 995 | 168 | 17.849 | 11.63 | −0.136 |
| ShNF-YC9 | 480 | 739 | 159 | 16.482 | 10.68 | −0.397 |
| ShNF-YC10 | 471 | 803 | 156 | 16.294 | 10.68 | −0.388 |
| ShNF-YC11 | 324 | 324 | 107 | 11.491 | 8.67 | 0.110 |
| ShNF-YC12 | 471 | 471 | 156 | 16.299 | 10.68 | −0.312 |
| ShNF-YC13 | 519 | 519 | 172 | 18.911 | 5.72 | −0.641 |
| ShNF-YC14 | 486 | 609 | 161 | 16.795 | 11.14 | −0.346 |
| ShNF-YC15 | 423 | 1263 | 140 | 14.813 | 10.25 | −0.386 |
| ShNF-YC16 | 594 | 2681 | 197 | 22.139 | 10.34 | −0.120 |
| ShNF-YC17 | 384 | 384 | 127 | 13.454 | 6.05 | −0.207 |
| ShNF-YC18 | 609 | 973 | 202 | 21.449 | 5.37 | −0.130 |
| ShNF-YC19 | 462 | 462 | 153 | 17.293 | 5.13 | −0.448 |
| ShNF-YC20 | 423 | 423 | 140 | 14.765 | 10.54 | −0.246 |
| ShNF-YC21 | 606 | 600 | 201 | 23.414 | 8.14 | −0.636 |
| ShNF-YC22 | 255 | 1312 | 84 | 8.882 | 10.62 | −0.360 |
| ShNF-YC23 | 405 | 893 | 134 | 13.957 | 10.05 | −0.181 |
| ShNF-YC24 | 474 | 739 | 157 | 16.288 | 10.68 | −0.314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swathik Clarancia, P.; Naveenarani, M.; Ashwin Narayan, J.; Krishna, S.S.; Thirugnanasambandam, P.P.; Valarmathi, R.; Suresha, G.S.; Gomathi, R.; Kumar, R.A.; Manickavasagam, M.; et al. Genome-Wide Identification, Characterization and Expression Analysis of Plant Nuclear Factor (NF-Y) Gene Family Transcription Factors in Saccharum spp. Genes 2023, 14, 1147. https://doi.org/10.3390/genes14061147
Swathik Clarancia P, Naveenarani M, Ashwin Narayan J, Krishna SS, Thirugnanasambandam PP, Valarmathi R, Suresha GS, Gomathi R, Kumar RA, Manickavasagam M, et al. Genome-Wide Identification, Characterization and Expression Analysis of Plant Nuclear Factor (NF-Y) Gene Family Transcription Factors in Saccharum spp. Genes. 2023; 14(6):1147. https://doi.org/10.3390/genes14061147
Chicago/Turabian StyleSwathik Clarancia, Peter, Murugan Naveenarani, Jayanarayanan Ashwin Narayan, Sakthivel Surya Krishna, Prathima Perumal Thirugnanasambandam, Ramanathan Valarmathi, Giriyapur Shivalingamurthy Suresha, Raju Gomathi, Raja Arun Kumar, Markandan Manickavasagam, and et al. 2023. "Genome-Wide Identification, Characterization and Expression Analysis of Plant Nuclear Factor (NF-Y) Gene Family Transcription Factors in Saccharum spp." Genes 14, no. 6: 1147. https://doi.org/10.3390/genes14061147