Genome-Wide Identification, Characterization and Expression Profiling of the CONSTANS-like Genes in Potato (Solanum tuberosum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Identification of COL Gene Family Members in S. tuberosum
2.3. Phylogenetic Tree Reconstruction, Analysis of Gene Structure and Protein Motifs
2.4. Chromosomal Location
2.5. COL Gene Selection Pressure and Duplication Type Analysis
2.6. Tissue Expression Characteristics of the StCOL Gene in S. tuberosum
2.7. Analysis of Cis-Elements in the Promoter Regions of StCOL Genes
3. Results
3.1. Identification and Chromosome Distribution of COL Gene Family Members
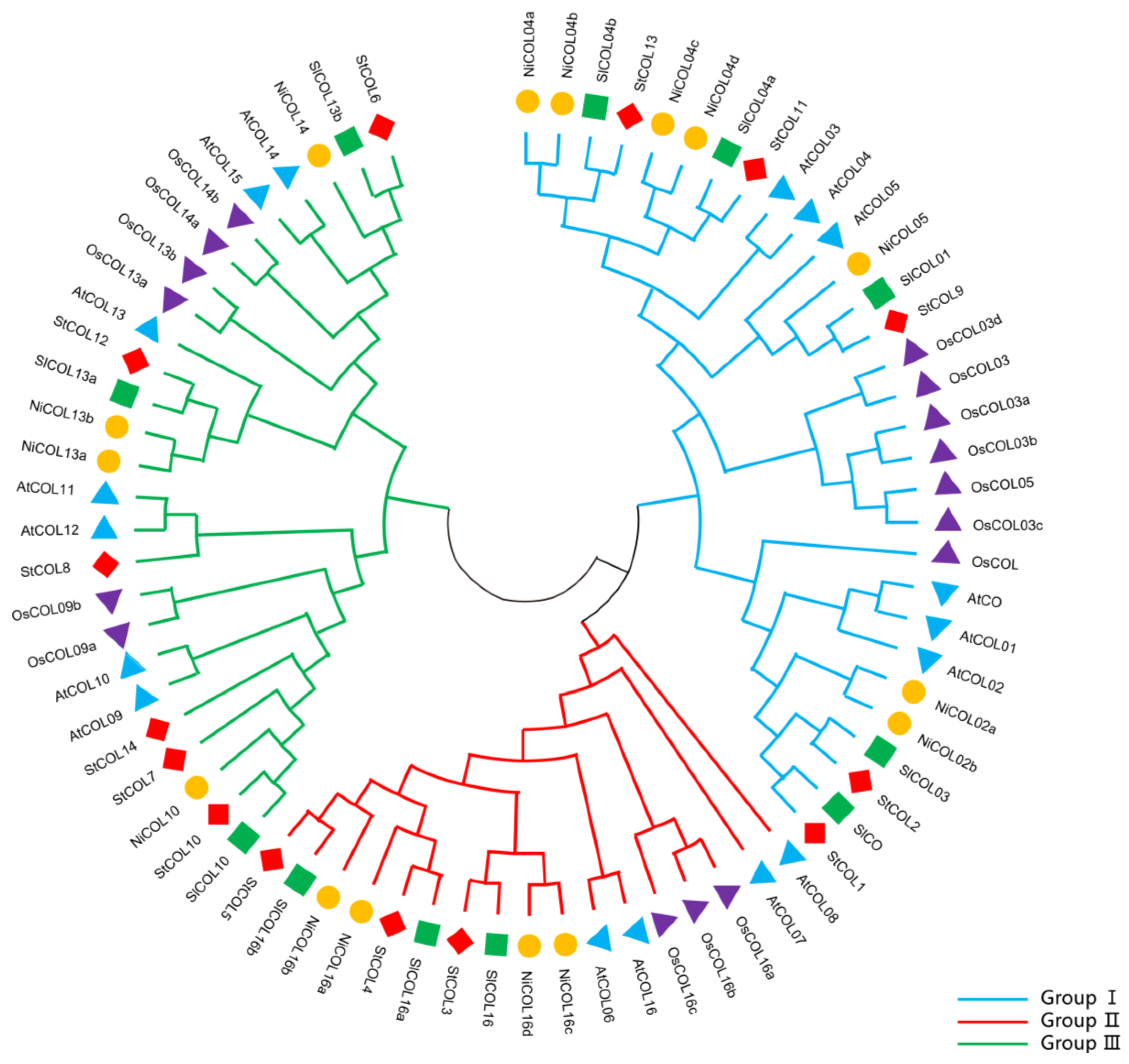
3.2. Phylogenetic Tree Analysis of COL Protein Families in Five Plant Species
3.3. Phylogeny, Gene Structure and Protein Motifs
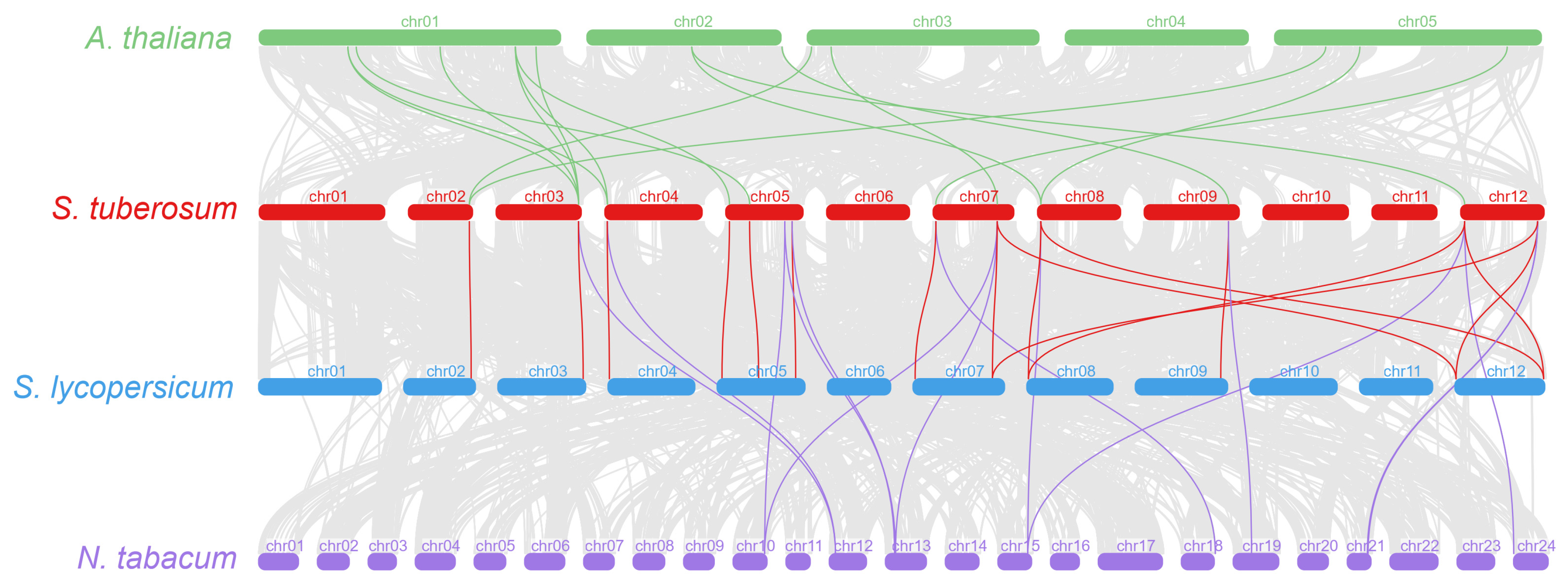
3.4. Chromosome Localization
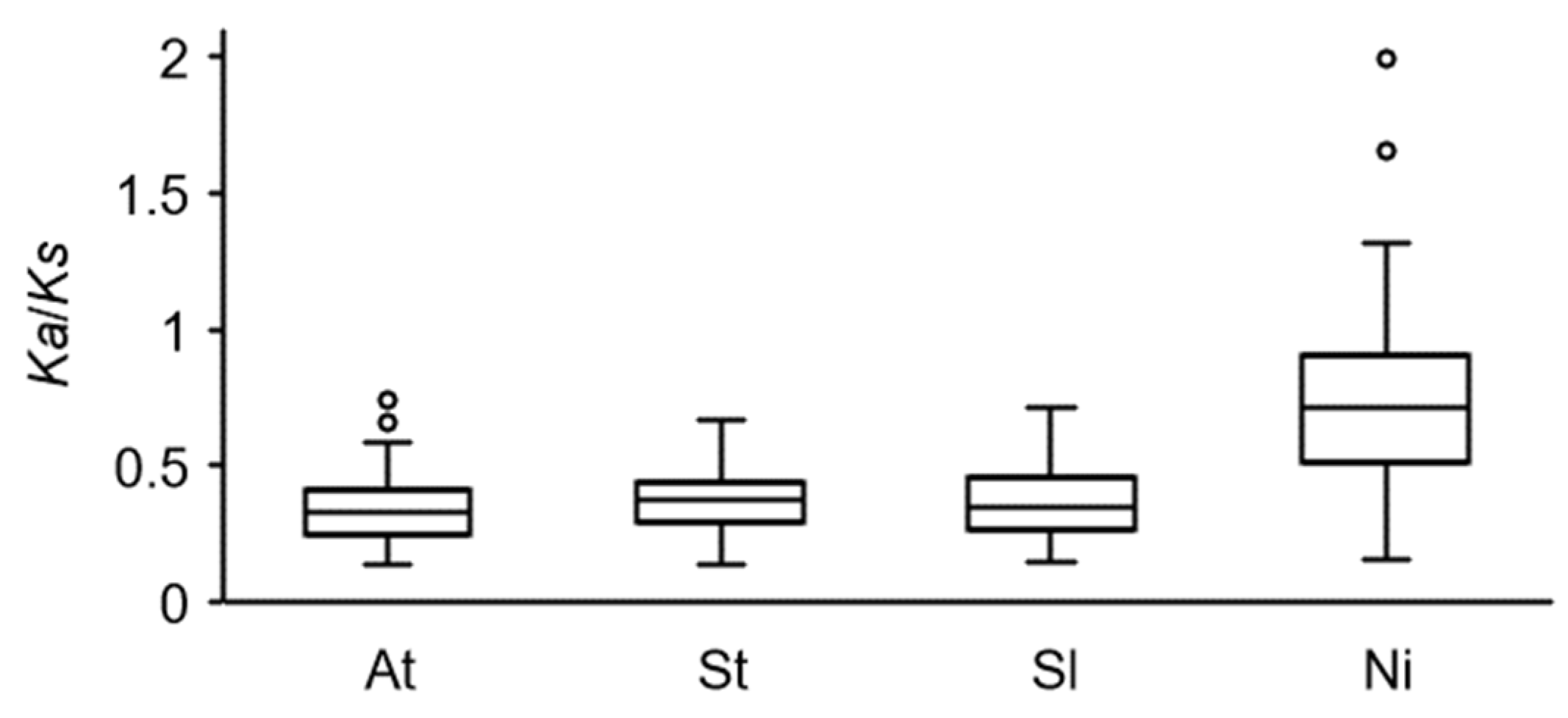
3.5. Selection Pressure on COL Genes and Contraction Versus Expansion
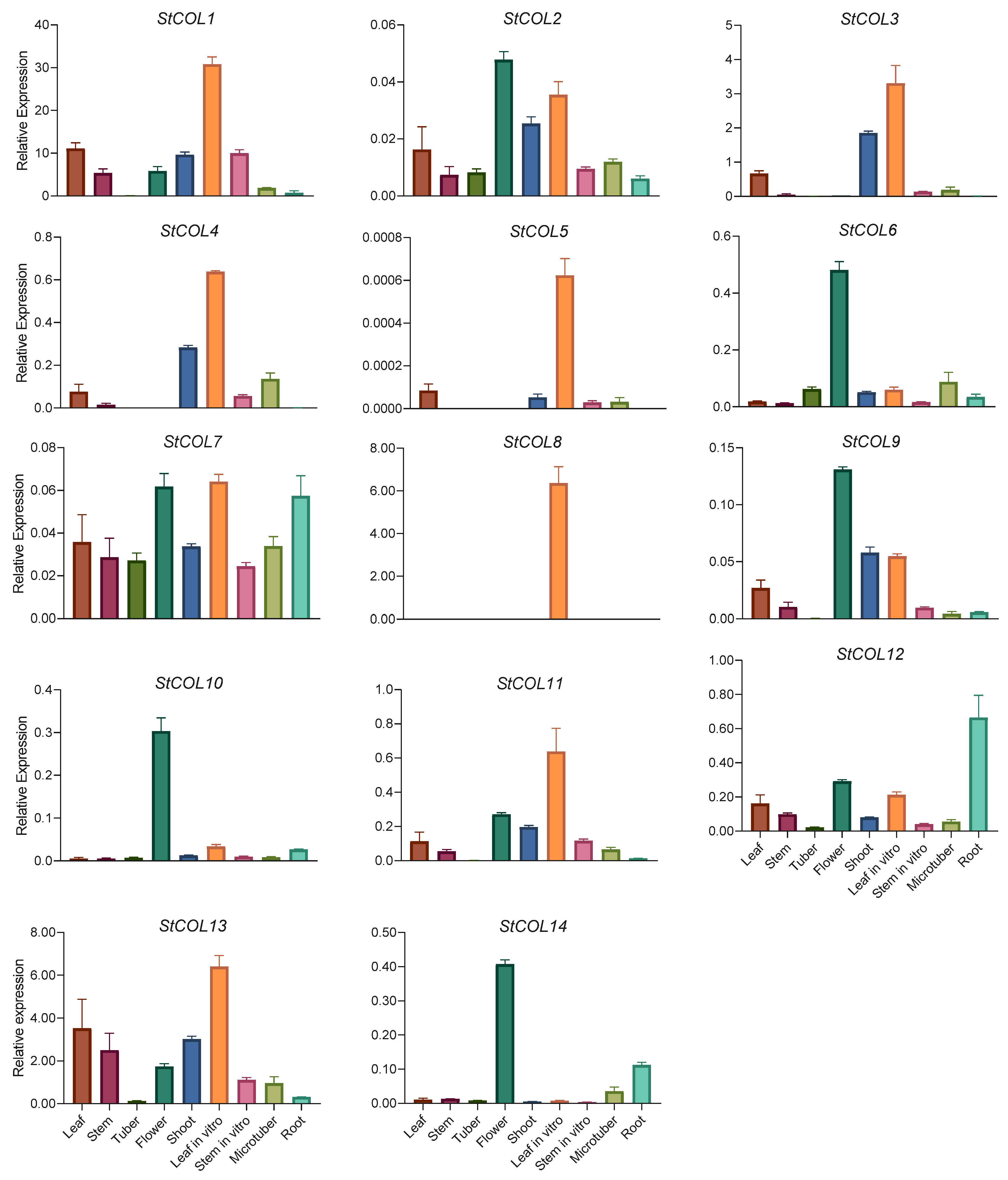
3.6. Tissue Expression Characteristics of StCOL Genes
4. Discussion
4.1. Phylogenetic and Gene Structure Analysis
4.2. Functional Conservation and Differentiation of COL Gene Family Members in S. tuberosum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef]
- Amasino, R. Seasonal and developmental timing of flowering. Plant J. 2010, 61, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Falcón, M.; Bou, J.; Prat, S. Seasonal control of tuberization in potato: Conserved elements with the flowering response. Annu. Rev. Plant Biol. 2006, 57, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Weigel, D. Move on up, it’s time for change-mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2007, 21, 2371–2384. [Google Scholar] [CrossRef] [PubMed]
- Gregory, L.E. Some factors for tuberization in the potato plant. Am. J. Bot. 1956, 43, 281–288. [Google Scholar] [CrossRef]
- Jackson, S.D.; James, P.E.; Carrera, E.; Prat, S.; Thomas, B. Regulation of transcript levels of a potato gibberellin 20-oxidase gene by light and phytochrome B. Plant Physiol. 2000, 124, 423–430. [Google Scholar] [CrossRef]
- Martínez-García, J.F.; Virgós-Soler, A.; Prat, S. Control of photoperiod-regulated tuberization in potato by the Arabidopsis flowering-time gene CONSTANS. Proc. Natl. Acad. Sci. USA 2002, 99, 15211–15216. [Google Scholar] [CrossRef]
- González-Schain, N.D.; Díaz-Mendoza, M.; Żurczak, M.; Suárez-López, P. Potato CONSTANS is involved in photoperiodic tuberization in a graft-transmissible manner. Plant J. 2012, 70, 678–690. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Cruz-Oró, E.; Franco-Zorrilla, J.M.; Prat, S. Potato StCONSTANS-like1 suppresses storage organ formation by directly activating the FT-like StSP5G repressor. Curr. Biol. 2016, 26, 872–881. [Google Scholar] [CrossRef]
- Sheng, P.; Wu, F.; Tan, J.; Zhang, H.; Ma, W.; Chen, L.; Wang, J.; Wang, J.; Zhu, S.; Guo, X.; et al. A CONSTANS-like transcriptional activator, OsCOL13, functions as a negative regulator of flowering downstream of OsphyB and upstream of Ehd1 in rice. Plant Mol. Biol. 2016, 92, 209–222. [Google Scholar] [CrossRef]
- Liu, L.D.; Ding, Q.Y.; Liu, J.; Yang, C.L.; Chen, H.; Zhang, S.F.; Zhu, J.C.; Wang, D.J. Brassica napus COL transcription factor BnCOL2 negatively affects the tolerance of transgenic Arabidopsis to drought stress. Environ. Exp. Bot. 2020, 178, 104171. [Google Scholar] [CrossRef]
- Khatun, K.; Debnath, S.; Robin AH, K.; Wai, A.H.; Nath, U.K.; Lee, D.J.; Kim, C.K.; Chung, M.Y. Genome-wide identification, genomic organization, and expression profiling of the CONSTANS-like (COL) gene family in petunia under multiple stresses. BMC Genom. 2021, 22, 727. [Google Scholar] [CrossRef]
- Griffiths, S.; Dunford, R.P.; Coupland, G.; Laurie, D.A. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol. 2003, 131, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Botto, J.F. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, S.; Tang, Q.; Tang, S. Identification of a CONSTANS homologous gene with distinct diurnal expression patterns in varied photoperiods in ramie (Boehmeria nivea L. gaud). Gene 2015, 560, 63–70. [Google Scholar] [CrossRef]
- Borden, K.L.B. RING domains: Master builders of molecular scaffolds? J. Mol. Biol. 2000, 295, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Torok, M.; Etkin, L.D. Two B or not two B? Overview of the rapidly expanding B-box family of proteins. Differentiation 2001, 67, 63–71. [Google Scholar] [CrossRef]
- Lagercrantz, U.; Axelsson, T. Rapid evolution of the family of CONSTANS LIKE genes in plants. Mol. Biol. Evol. 2000, 17, 1499–1507. [Google Scholar] [CrossRef]
- Valverde, F. CONSTANS and the evolutionary origin of photoperiodic timing of flowering. J. Exp. Bot. 2011, 62, 2453–2463. [Google Scholar] [CrossRef]
- De los Reyes, P.; Romero-Campero, F.J.; Gao, H.; Serrano-Bueno, G.; Romero, J.M.; Valverde, F. CONSTANS alters the circadian clock in Arabidopsis thaliana. bioRxiv 2023, 19, 524697. [Google Scholar] [CrossRef]
- Kardailsky, I.; Shukla, V.K.; Ahn, J.H.; Dagenais, N.; Christensen, S.K.; Nguyen, J.T.; Chory, J.; Harrison, M.J.; Weigel, D. Activation tagging of the floral inducer FT. Science 1999, 286, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Lopez, P.; Wheatley, K.; Robson, F.; Onouchi, H.; Valverde, F.; Coupland, G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 2001, 410, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Roussot, C.; Suarez-Lopez, P.; Corbesier, L.; Vincent, C.; Pineiro, M.; Hepworth, S.; Mouradov, A.; Justin, S.; Turnbull, C.; et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 2004, 131, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Hecht, V.F.; Picard, K.; Diwadkar, P.; Laurie, R.E.; Wen, J.; Mysore, K.; Macknight, R.C.; Weller, J.L. Isolation and functional analysis of CONSTANS-LIKE genes suggests that a central role for CONSTANS in flowering time control is not evolutionarily conserved in Medicago truncatula. Front. Plant Sci. 2014, 5, 486. [Google Scholar] [CrossRef]
- Izawa, T.; Oikawa, T.; Sugiyama, N.; Tanisaka, T.; Yano, M.; Shimamoto, K. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 2002, 16, 2006–2020. [Google Scholar] [CrossRef]
- Kojima, S.; Takahashi, Y.; Kobayashi, Y.; Monna, L.; Sasaki, T.; Araki, T.; Yano, M. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002, 43, 1096–1105. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kobayashi, Y.; Goto, K.; Abe, M.; Araki, T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 2005, 46, 1175–1189. [Google Scholar] [CrossRef]
- Sawa, M.; Nusinow, D.A.; Kay, S.A.; Imaizumi, T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 2007, 318, 261–265. [Google Scholar] [CrossRef]
- Hayama, R.; Coupland, G. The molecular basis of diversity in the photoperiodic flowering responses of Arabidopsis and rice. Plant Physiol. 2004, 135, 677–684. [Google Scholar] [CrossRef]
- Böhlenius, H.; Huang, T.; Charbonnel-Campaa, L.; Brunner, A.M.; Jansson, S.; Strauss, S.H.; Nilsson, O. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 2006, 312, 1040–1043. [Google Scholar] [CrossRef]
- Ballerini, E.S.; Kramer, E.M. In the light of evolution: A reevaluation of conservation in the CO-FT regulon and its role in photoperiodic regulation of flowering time. Front. Plant Sci. 2011, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Hassidim, M.; Harir, Y.; Yakir, E.; Kron, I.; Green, R.M. Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis. Planta 2009, 230, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Hettiarachchi, G.; Deng, X.W.; Holm, M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 2006, 18, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Ingkasuwan, P.; Netrphan, S.; Prasitwattanaseree, S.; Tanticharoen, M.; Bhumiratana, S.; Meechai, A.; Chaijaruwanich, J.; Takahashi, H.; Cheevadhanarak, S. Inferring transcriptional gene regulation network of starch metabolism in Arabidopsis thaliana leaves using graphical Gaussian model. BMC Syst. Biol. 2012, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.F.; Wang, Z.Y. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005, 43, 758–768. [Google Scholar] [CrossRef]
- Yano, M.; Katayose, Y.; Ashikari, M.; Yamanouchi, U.; Monna, L.; Fuse, T.; Baba, T.; Yamamoto, K.; Umehara, Y.; Nagamura, Y.; et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 2000, 12, 2473–2484. [Google Scholar] [CrossRef]
- Tan, J.; Jin, M.; Wang, J.; Wu, F.; Sheng, P.; Cheng, Z.; Wang, J.; Zheng, X.; Chen, L.; Wang, M.; et al. OsCOL10, a CONSTANS-Like gene, functions as a flowering time repressor downstream of Ghd7 in rice. Plant Cell Physiol. 2016, 57, 798–812. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, X.M.; Chen, D.; Zhang, Y.; Ma, W.; Zhang, H.; Sun, L.; Yang, Z.; Zhao, C.; Zhan, X.; et al. OsCOL16, encoding a CONSTANS-like protein, represses flowering by up-regulating Ghd7 expression in rice. Plant Sci. 2017, 260, 60–69. [Google Scholar] [CrossRef]
- González-Schain, N.D.; Suárez-López, P. CONSTANS delays flowering and affects tuber yield in potato. Biol. Plant. 2008, 52, 251–258. [Google Scholar] [CrossRef]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef]
- Plantenga FD, M.; Heuvelink, E.; Rienstra, J.A.; Visser RG, F.; Bachem CW, B.; Marcelis LF, M. Coincidence of potato CONSTANS (StCOL1) expression and light cannot explain night-break repression of tuberization. Physiol. Plant 2019, 167, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Pham, G.M.; Hamilton, J.P.; Wood, J.C.; Burke, J.T.; Zhao, H.N.; Vaillancourt, B.; Ou, S.J.; Jiang, J.M.; Buell, C.R. Construction of a chromosome-scale long-read reference genome assembly for potato. GigaScience 2020, 9, giaa100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tang, D.; Huang, W.; Yang, Z.M.; Zhang, Y.; Hamilton, J.P.; Visser RG, F.; Bachem CW, B.; Buell, C.R.; Zhang, Z.H.; et al. Haplotype-resolved genome analyses of a heterozygous diploid potato. Nat. Genet. 2020, 52, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, Z.; Tang, D.; Zhu, Y.; Wang, P.; Li, D.; Zhu, G.; Xiong, X.; Shang, Y.; Li, C.; et al. Genome design of hybrid potato. Cell 2021, 184, 3873–3883. [Google Scholar] [CrossRef]
- Tang, D.; Jia, Y.; Zhang, J.; Li, H.; Cheng, L.; Wang, P.; Bao, Z.; Liu, Z.; Feng, S.; Zhu, X.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, 427–432. [Google Scholar] [CrossRef]
- Krejci, A.; Hupp, T.R.; Lexa, M.; Vojtesek, B.; Muller, P. Hammock: A hidden Markov model-based peptide clustering algorithm to identify protein-interaction consensus motifs in large datasets. Bioinformatics 2016, 32, 9–16. [Google Scholar] [CrossRef]
- Robsonet, F.; Costa, M.M.; Hepworth, S.R.; Vizir, I.; Pin˜eiro, M.; Reeves, P.H.; Putterill, J.; Coupland, G. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001, 28, 619–631. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. In Current Protocols Bioinformatics; John Wiley & Sons Inc.: New York, NY, USA, 2002; Chapter 2, Unit 2.3. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGAX: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.C.; Gao, G. Gsds 2.0: An upgraded gene feature visualization server. Bioinformatics 2014, 31, 1296. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Yadav, C.B.; Bonthala, V.S.; Muthamilarasan, M.; Pandey, G.; Khan, Y.; Prasad, M. Genome-wide development of transposable elements-based markers in foxtail millet and construction of an integrated database. DNA Res. 2015, 22, 79–90. [Google Scholar] [CrossRef]
- Huang, X.; Yang, L.; Jin, Y.; Lin, J.; Liu, F. Generation, annotation, and analysis of a large-scale expressed sequence tag library from Arabidopsis pumila to explore salt-responsive genes. Front. Plant Sci. 2017, 8, 955. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, F.; Huang, W.; Sun, Q.; Huang, X. Identification of reliable reference genes for qRT-PCR in the ephemeral plant Arabidopsis pumila based on full-length transcriptome data. Sci. Rep. 2019, 9, 8408. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, N.; Si, H.; Calderón-Urrea, A. Selection and validation of reference genes for rt-qpcr analysis in potato under abiotic stress. Plant Methods 2017, 13, 85. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Khanna, R.; Kronmiller, B.; Maszle, D.R.; Coupland, G.; Holm, M.; Mizuno, T.; Wu, S.H. The Arabidopsis B-box zinc finger family. Plant Cell 2009, 21, 3416–3420. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Yu, F.H.; Guo, Q.; Wang, Y.; Zhang, Z.H.; Liu, Y.X. Genome-wide identification, characterization, and expression profile analysis of CONSTANS-like genes in Woodland Strawberry (Fragaria vesca). Front. Plant Sci. 2022, 13, 931721. [Google Scholar] [CrossRef]
- Huang, Z.N.; Bai, X.Y.; Duan, W.K.; Chen, B.Q.; Chen, G.D.; Xu, B.H.; Cheng, R.; Wang, J.Z. Genome-wide identification and expression profiling of CONSTANS-Like genes in Pepper (Capsicum annuum): Gaining an insight to their phylogenetic evolution and stress-specific roles. Front. Plant Sci. 2022, 13, 828209. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Li, Z.; Yin, M.; Huang, S.Q.; Tao, J.; Chen, A.G.; Li, J.J.; Tang, H.J.; Chang, L.; Deng, Y.; et al. Genome-wide identification, expression, and sequence analysis of CONSTANS-like gene family in cannabis reveals a potential role in plant flowering time regulation. BMC Plant Biol. 2021, 21, 142. [Google Scholar] [CrossRef]
- Song, X.; Duan, W.; Huang, Z.; Liu, G.; Wu, P.; Liu, T.; Li, Y.; Hou, X. Comprehensive analysis of the flowering genes in Chinese cabbage and examination of evolutionary pattern of CO-like genes in plant kingdom. Sci. Rep. 2015, 5, 14631. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wei, Q.; Wang, W.; Hu, H.; Ma, W.; Zhu, Q.; Bao, C. Genome-wide identification and characterization of CONSTANS-like gene family in radish (Raphanus sativus). PLoS ONE 2018, 13, e0204137. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.Y.; Wang, J.N.; Kuang, J.F.; Shan, W.; Lu, W.J. Molecular characterization and expression profiles of MaCOL1, a CONSTANS-like gene in banana fruit. Gene 2012, 496, 110–117. [Google Scholar] [CrossRef]
- Wang, H.G.; Zhang, Z.L.; Li, H.Y.; Zhao, X.Y.; Liu, X.M.; Ortiz, M.; Lin, C.T.; Liu, B. CONSTANS-LIKE 7 regulates branching and shade avoidance response in Arabidopsis. J. Exp. Bot. 2013, 64, 1017–1024. [Google Scholar] [CrossRef]
- Yang, T.W.; He, Y.; Niu, S.B.; Yan, S.W.; Zhang, Y. Identification and characterization of the CONSTANS (CO)/CONSTANS-like (COL) genes related to photoperiodic signaling and flowering in tomato. Plant Sci. 2020, 301, 110653. [Google Scholar] [CrossRef]
- Cao, D.; Lin, Z.; Huang, L.; Damaris, R.N.; Li, M.; Yang, P. A CONSTANS-LIKE gene of Nelumbo nucifera could promote potato tuberization. Planta 2021, 253, 65. [Google Scholar] [CrossRef]
- Ordoñez-Herrera, N.; Trimborn, L.; Menje, M.; Henschel, M.; Robers, L.; Kaufholdt, D.; Hänsch, R.; Adrian, J.; Ponnu, J.; Hoecker, U. The transcription factor COL12 is a substrate of the COP1/SPA E3 ligase and regulates flowering time and plant architecture. Plant Physiol. 2018, 176, 1327–1340. [Google Scholar] [CrossRef]
- Cui, L.; Zheng, F.; Wang, J.; Zhang, C.; Zhang, D.; Gao, S.; Zhang, C.; Ye, J.; Zhang, Y.; Ouyang, B.; et al. The tomato CONSTANS-LIKE protein SlCOL1 regulates fruit yield by repressing SFT gene expression. BMC Plant Biol. 2022, 22, 429. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Chung, J.S.; Lee, K.H.; Kim, C.S. The CONSTANS like 4 transcription factor, AtCOL4, positively regulates abiotic stress tolerance through an abscisic acid-dependent manner in Arabidopsis. J. Integr. Plant Biol. 2014, 57, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, S.Y.; Sun, D.Y.; Liu, W.; Gu, F.W.; Liu, Y.Z.; Guo, T.; Wang, H.; Wang, J.F.; Chen, Z.Q. CONSTANS-Like 9 (OsCOL9) interacts with receptor for activated C-kinase 1 (OsRACK1) to regulate blast resistance through salicylic acid and ethylene signaling pathways. PLoS ONE 2016, 11, e0166249. [Google Scholar] [CrossRef]
- Liu, J.H.; Shen, J.Q.; Xu, Y.; Li, X.H.; Xiao, J.H.; Xiong, L.Z. Ghd2, a CONSTANS like gene, confers drought sensitivity through regulation of senescence in rice. J. Exp. Bot. 2016, 67, 5785–5798. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Seo, P.J.; Park, C.M. The E3 ubiquitin ligase HOS1 regulates Arabidopsis flowering by mediating CONSTANS degradation under cold stress. J. Biol. Chem. 2012, 287, 43277–43287. [Google Scholar] [CrossRef]

| Gene | Gene ID | Amino Acid Number/aa | Molecular Weight/Da | Theoretical Isoelectric Point | Subcellular Localization |
|---|---|---|---|---|---|
| StCOL1 | Soltu.DM.02G030260.1 | 413 | 45,873.9 | 5.82 | Nucleus |
| StCOL2 | Soltu.DM.02G030280.1 | 405 | 44,940.9 | 5.57 | Nucleus |
| StCOL3 | Soltu.DM.03G034030.1 | 401 | 45,829.1 | 5.61 | Nucleus |
| StCOL4 | Soltu.DM.04G002000.1 | 428 | 48,809.4 | 5.44 | Nucleus |
| StCOL5 | Soltu.DM.05G003170.1 | 447 | 51,218.4 | 5.62 | Nucleus |
| StCOL6 | Soltu.DM.05G012520.1 | 453 | 49,919.0 | 6.09 | Nucleus |
| StCOL7 | Soltu.DM.05G017200.1 | 413 | 45,047.1 | 5.61 | Nucleus |
| StCOL8 | Soltu.DM.05G018860.1 | 420 | 46,241.8 | 5.20 | Nucleus |
| StCOL9 | Soltu.DM.07G001900.1 | 385 | 42,403.6 | 6.05 | Nucleus |
| StCOL10 | Soltu.DM.07G014960.1 | 411 | 45,673.9 | 5.41 | Nucleus |
| StCOL11 | Soltu.DM.08G002010.1 | 347 | 38,651.0 | 5.30 | Nucleus |
| StCOL12 | Soltu.DM.09G022940.1 | 379 | 43,059.9 | 5.78 | Nucleus |
| StCOL13 | Soltu.DM.12G003910.1 | 357 | 39,093.3 | 5.30 | Nucleus |
| StCOL14 | Soltu.DM.12G023800.1 | 411 | 45,673.9 | 5.41 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Li, T.; Wu, X.; Yao, X.; Ai, H.; Zhang, Y.; Gan, Z.; Huang, X. Genome-Wide Identification, Characterization and Expression Profiling of the CONSTANS-like Genes in Potato (Solanum tuberosum L.). Genes 2023, 14, 1174. https://doi.org/10.3390/genes14061174
Li R, Li T, Wu X, Yao X, Ai H, Zhang Y, Gan Z, Huang X. Genome-Wide Identification, Characterization and Expression Profiling of the CONSTANS-like Genes in Potato (Solanum tuberosum L.). Genes. 2023; 14(6):1174. https://doi.org/10.3390/genes14061174
Chicago/Turabian StyleLi, Ruining, Ting Li, Xiang Wu, Xuyang Yao, Hao Ai, Yingjie Zhang, Zhicheng Gan, and Xianzhong Huang. 2023. "Genome-Wide Identification, Characterization and Expression Profiling of the CONSTANS-like Genes in Potato (Solanum tuberosum L.)" Genes 14, no. 6: 1174. https://doi.org/10.3390/genes14061174






