miR-497 Regulates LATS1 through the PPARG Pathway to Participate in Fatty Acid Synthesis in Bovine Mammary Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and RNA Extraction
2.2. Bioinformatics Analysis of Sequencing Results
2.3. Small RNA Length Distribution
2.4. Determination of Milk Composition
2.5. Cell Culture and Transfection
2.6. Triglyceride Assay and Cholesterol Testing
2.7. Determination of Intracellular Fatty Acid Content
2.8. RT-qPCR and Western Blotting
2.9. Luciferase Reporter Assay
2.10. Oil Red O Staining
2.11. Statistical Analysis
3. Results
3.1. Sequencing Quality Analysis and Length Distribution
3.2. Small RNA Analysis with Typical and Unique Sequences
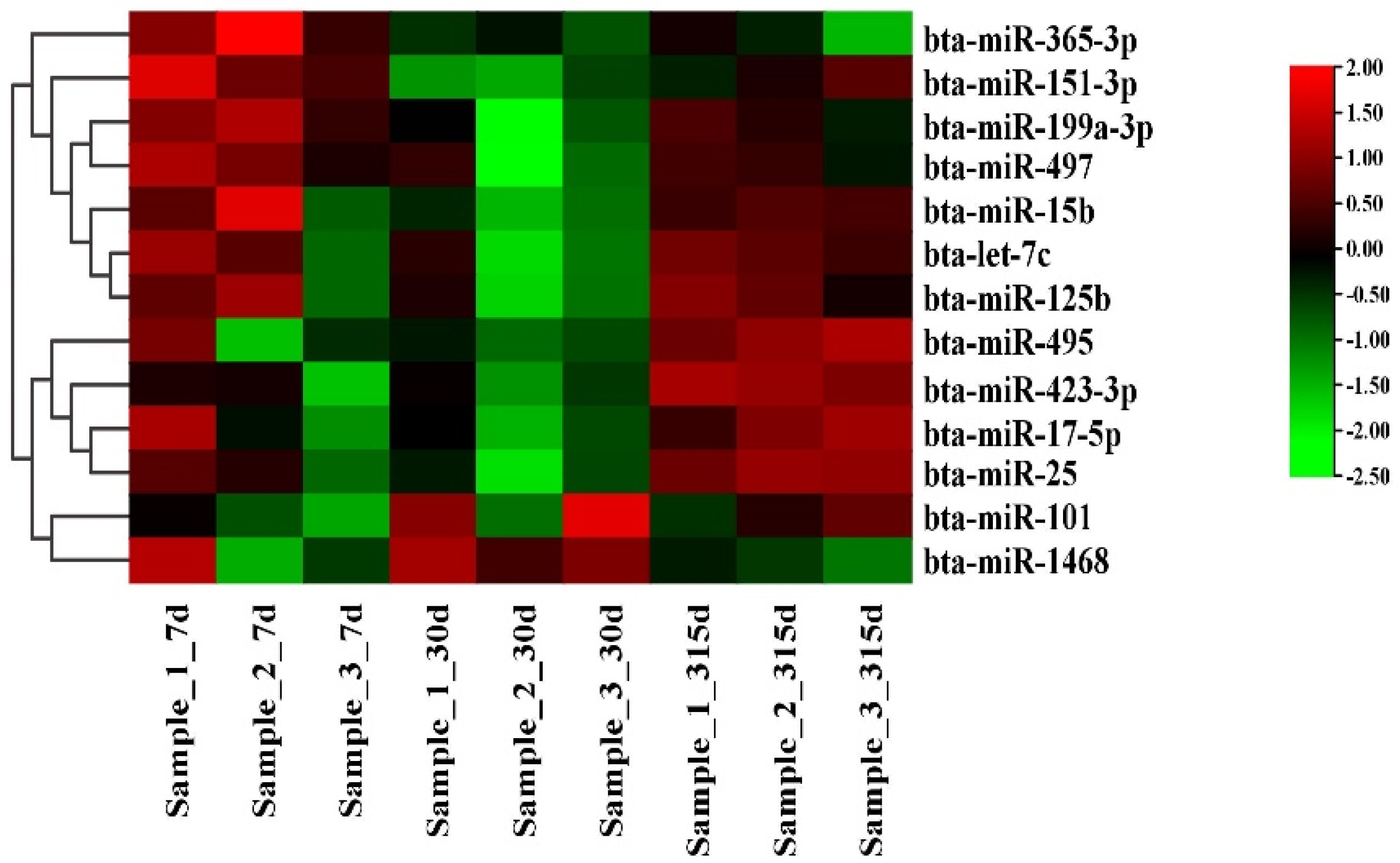
3.3. Differential Expression Analysis of miRNA
3.4. MiRNA Differential Expression Analysis
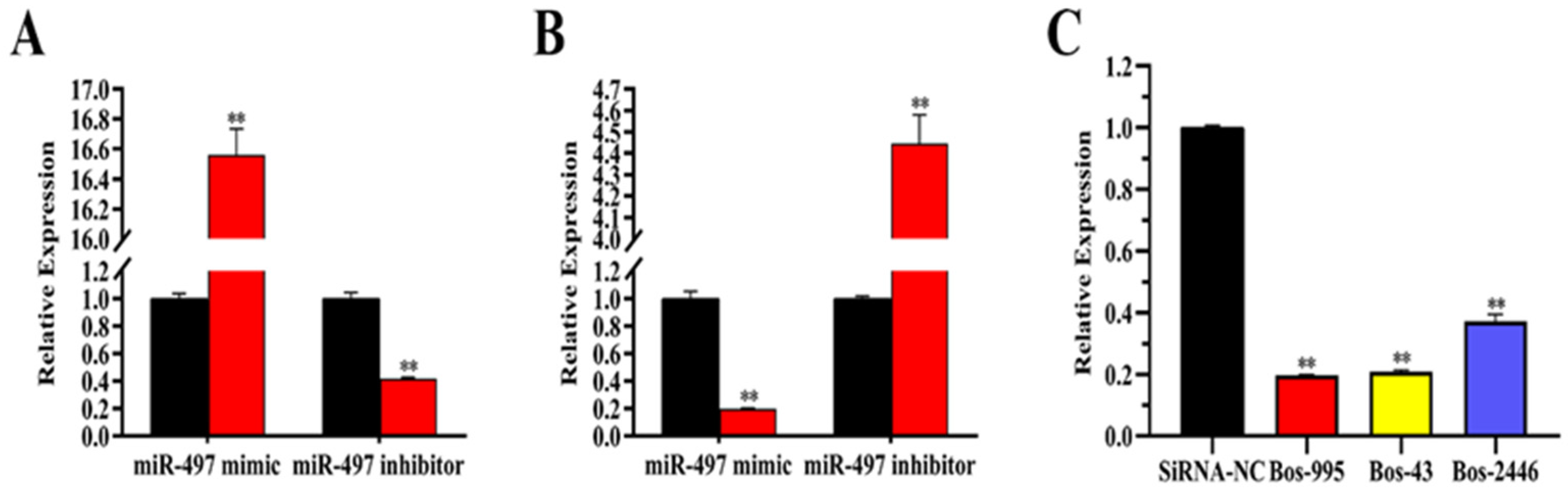
3.5. Transfection Efficiency of miRNA and siRNA
3.6. miR-497 Specifically Targets LATS1 in BEMCs
3.7. Functional Evaluation of miR-497 and LATS1 in BEMCs
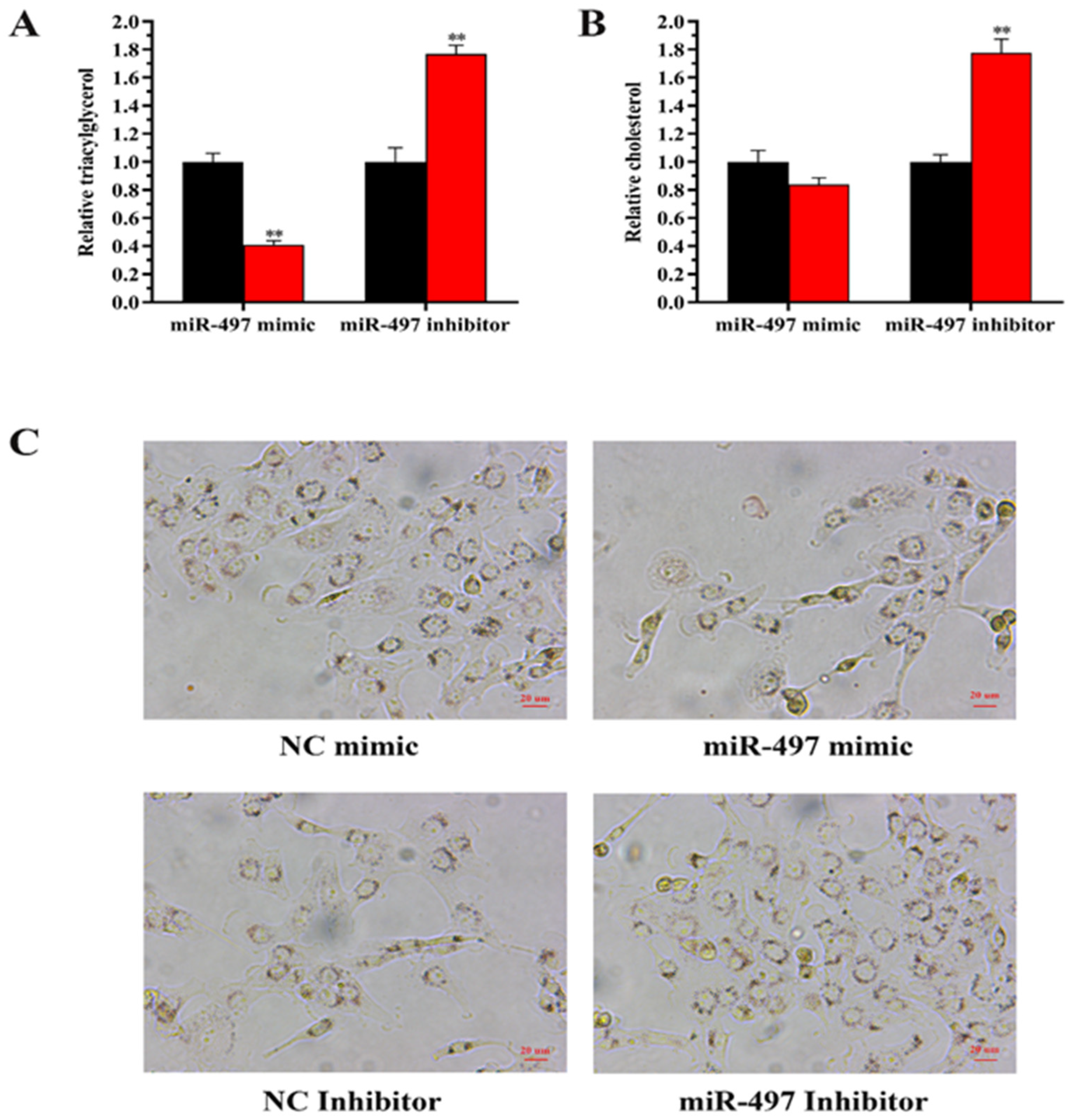
3.7.1. The Expression of miR-497 Reduces the Content in BEMCs
3.7.2. Expression of LATS1 Increases the Level of TAG in BEMCs
3.7.3. Effects of miR-497 and siRNA LATS1 on Fatty Acid Composition of Cells
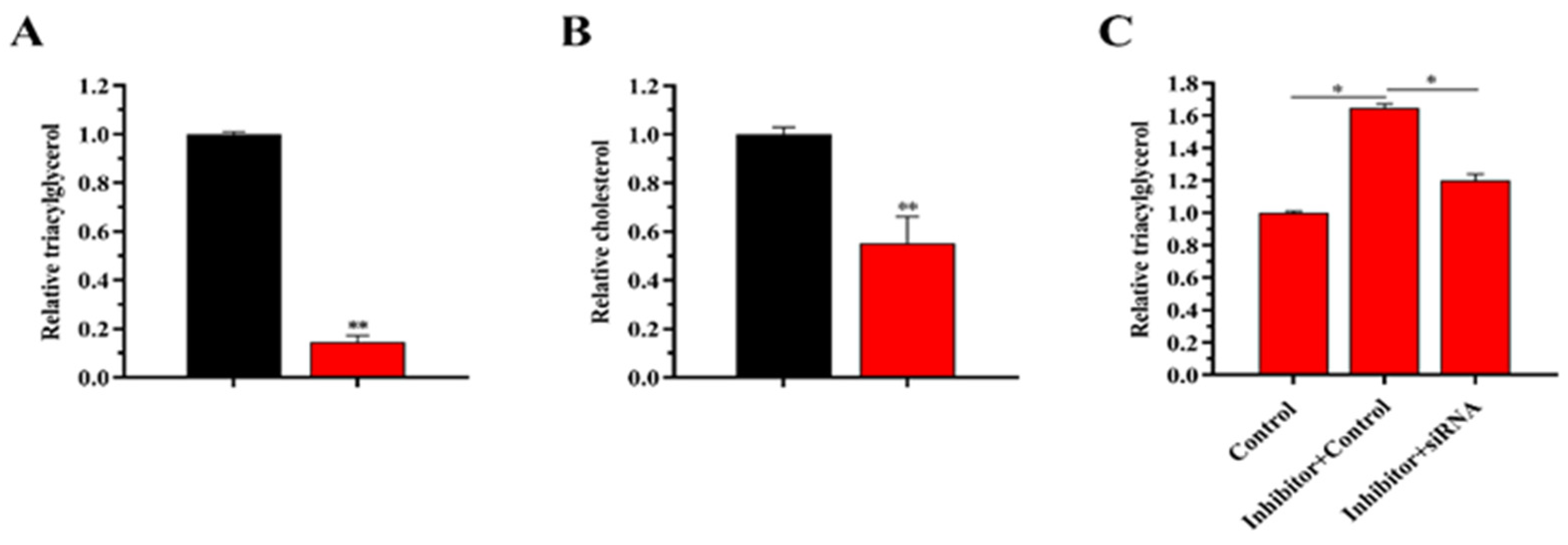
3.7.4. siRNA-LATS1 Partially Abrogates (“Rescues”) the Reduction in TAG Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altomonte, I.; Salari, F.; Licitra, R.; Martini, M. Use of microalgae in ruminant nutrition and implications on milk quality—A review. Livest. Sci. 2018, 214, 25–35. [Google Scholar] [CrossRef]
- Amaral, Y.; Marano, D.; Oliveira, E.; Moreira, M.E. Impact of pre-pregnancy excessive body weight on the composition of polyunsaturated fatty acids in breast milk: A systematic review. Int. J. Food Sci. Nutr. 2020, 71, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Luo, J.; Zhu, J.; Li, J.; Sun, Y.; Lin, X.; Zhang, L.; Yao, D.; Shi, H. PPARγ Regulates Genes Involved in Triacylglycerol Synthesis and Secretion in Mammary Gland Epithelial Cells of Dairy Goats. PPAR Res. 2013, 2013, 310948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Li, Q.; Gao, X. Expression and function of leptin and its receptor in dairy goat mammary gland. J. Dairy Res. 2010, 77, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.O.; Jakobsen, K. Changes in mammary glucose and protein uptake in relation to milk synthesis during lactation in high- and low-yielding goats. Comp. Biochem. Physiol. Part A Physiol. 1993, 106, 359–365. [Google Scholar] [CrossRef]
- Ma, L.; Qiu, H.; Chen, Z.; Li, L.; Zeng, Y.; Luo, J.; Gou, D. miR-25 modulates triacylglycerol and lipid accumulation in goat mammary epithelial cells by repressing PGC-1beta. J. Anim. Sci. Biotechnol. 2018, 9, 48. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, S.; Wang, X.; Fan, Y.; Zhan, T.; Arbab, A.A.I.; Li, M.; Zhang, H.; Mao, Y.; Loor, J.J.; et al. MicroRNA-106b Regulates Milk Fat Metabolism via ATP Binding Cassette Subfamily A Member 1 (ABCA1) in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2019, 67, 3981–3990. [Google Scholar] [CrossRef]
- Horie, T.; Ono, K.; Horiguchi, M.; Nishi, H.; Nakamura, T.; Nagao, K.; Kinoshita, M.; Kuwabara, Y.; Marusawa, H.; Iwanaga, Y.; et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 17321–17326. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.-H.; Zeng, M.-Y.; Yu, X.-H.; Zeng, G.-F.; He, L.-H.; Zheng, X.-L.; Zhang, D.-W.; Ouyang, X.-P.; Tang, C.-K. Visceral adipose tissue-derived serine protease inhibitor accelerates cholesterol efflux by up-regulating ABCA1 expression via the NF-κB/miR-33a pathway in THP-1 macropahge-derived foam cells. Biochem. Biophys. Res. Commun. 2018, 500, 318–324. [Google Scholar] [CrossRef]
- Ono, K.; Horie, T.; Nishino, T.; Baba, O.; Kuwabara, Y.; Yokode, M.; Kita, T.; Kimura, T. MicroRNA-33a/b in Lipid Metabolism. Circ. J. 2015, 79, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Wang, G.; Xie, Z.; Zhang, C.; Wang, J. Identification and characterization of microRNA in the dairy goat (Capra hircus) mammary gland by Solexa deep-sequencing technology. Mol. Biol. Rep. 2012, 39, 9361–9371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Q.; Gao, J.-L.; Liao, X.-D.; Huang, T.-H.; Zhang, M.-N.; Wang, M.-Q.; Tian, Y.; Bai, J.; Zhou, C.-H. miR-454 regulates triglyceride synthesis in bovine mammary epithelial cells by targeting PPAR-γ. Gene 2019, 691, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, J.; Zhang, C.; Ma, Y.; Sun, S.; Zhang, T.; Loor, J.J. Mechanism of prolactin inhibition of miR-135b via methylation in goat mammary epithelial cells. J. Cell. Physiol. 2018, 233, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Genevet, A.; Wehr, M.C.; Brain, R.; Thompson, B.J.; Tapon, N. Kibra Is a Regulator of the Salvador/Warts/Hippo Signaling Network. Dev. Cell 2010, 18, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Chu, S.; Liang, Y.; Xu, T.; Sun, Y.; Li, M.; Zhang, H.; Wang, X.; Mao, Y.; Loor, J.J.; et al. miR-497 regulates fatty acid synthesis via LATS2 in bovine mammary epithelial cells. Food Funct. 2020, 11, 8625–8636. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, S.; Wang, X.; Sun, Y.; Xu, T.; Mao, Y.; Loor, J.J.; Yang, Z. MiR-16a Regulates Milk Fat Metabolism by Targeting Large Tumor Suppressor Kinase 1 (LATS1) in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2019, 67, 11167–11178. [Google Scholar] [CrossRef]
- Dils, R.R. Comparative Aspects of Milk Fat Synthesis. J. Dairy Sci. 1986, 69, 904–910. [Google Scholar] [CrossRef]
- Shen, P.; Kershaw, J.C.; Yue, Y.; Wang, O.; Kim, K.-H.; McClements, D.J.; Park, Y. Effects of conjugated linoleic acid (CLA) on fat accumulation, activity, and proteomics analysis in Caenorhabditis elegans. Food Chem. 2018, 249, 193–201. [Google Scholar] [CrossRef]
- Sun, S.-X.; Hua, X.-M.; Deng, Y.-Y.; Zhang, Y.-N.; Li, J.-M.; Wu, Z.; Limbu, S.M.; Lu, D.-S.; Yin, H.-W.; Wang, G.-Q.; et al. Tracking pollutants in dietary fish oil: From ocean to table. Environ. Pollut. 2018, 240, 733–744. [Google Scholar] [CrossRef]
- Nasrollahi, S.M.; Zali, A.; Ghorbani, G.R.; Khani, M.; Maktabi, H.; Beauchemin, K.A. Effects of increasing diet fermentability on intake, digestion, rumen fermentation, blood metabolites and milk production of heat-stressed dairy cows. Animal 2019, 13, 2527–2535. [Google Scholar] [CrossRef]
- Billa, P.A.; Faulconnier, Y.; Larsen, T.; Leroux, C.; Pires, J. Milk metabolites as noninvasive indicators of nutritional status of mid-lactation Holstein and Montbéliarde cows. J. Dairy Sci. 2020, 103, 3133–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Arbab, A.A.I.; Zhang, H.; Yang, Y.; Lu, X.; Han, Z.; Yang, Z. MicroRNA-193a-5p Regulates the Synthesis of Polyunsaturated Fatty Acids by Targeting Fatty Acid Desaturase 1 (FADS1) in Bovine Mammary Epithelial Cells. Biomolecules 2021, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, C.; Li, H.; Huang, L.; Sun, Q.; Dong, Y.; Tian, C.; Gao, S.; Dong, H.; Guan, D.; et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010, 20, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Munch, E.M.; Harris, R.; Mohammad, M.; Benham, A.L.; Pejerrey, S.M.; Showalter, L.; Hu, M.; Shope, C.D.; Maningat, P.D.; Gunaratne, P.H.; et al. Transcriptome Profiling of microRNA by Next-Gen Deep Sequencing Reveals Known and Novel miRNA Species in the Lipid Fraction of Human Breast Milk. PLoS ONE 2013, 8, e50564. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Zhang, L.; Lian, C.; Lu, C.; Zhang, Y.; Pan, Q.; Yang, R.; Zhao, Z. Deep Sequencing and Screening of Differentially Expressed MicroRNAs Related to Milk Fat Metabolism in Bovine Primary Mammary Epithelial Cells. Int. J. Mol. Sci. 2016, 17, 200. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Guo, W.; Zhao, Y.; Wang, Y.; Zha, R.; Ding, J.; Liang, L.; Yang, G.; Chen, Z.; Ma, B.; et al. MicroRNA-135a acts as a putative tumor suppressor by directly targeting very low density lipoprotein receptor in human gallbladder cancer. Cancer Sci. 2014, 105, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Guo, W.; Tang, K.; Wang, Y.; Zan, L.; Yang, W. Bta-miR-34b regulates milk fat biosynthesis by targeting mRNA decapping enzyme 1A (DCP1A) in cultured bovine mammary epithelial cells1. J. Anim. Sci. 2019, 97, 3823–3831. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.; He, Q.; Loor, J.J.; Luo, J. Association between the expression of miR-26 and goat milk fatty acids. Reprod. Domest. Anim. 2018, 53, 1478–1482. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Jin, X.; Lo, L.; Liu, J. Expression profiles of microRNAs from lactating and non-lactating bovine mammary glands and identification of miRNA related to lactation. BMC Genom. 2012, 13, 731. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Luo, J.; Sun, S.; Cao, D.; Shi, H.; Loor, J.J. miR-148a and miR-17–5p synergistically regulate milk TAG synthesis via PPARGC1A and PPARA in goat mammary epithelial cells. RNA Biol. 2017, 14, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, X.; Wang, D.; Ning, C.; Wang, H.; Zhang, Q.; Jiang, L. Functional validation of GPIHBP1 and identification of a functional mutation in GPIHBP1 for milk fat traits in dairy cattle. Sci. Rep. 2017, 7, 8546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Liu, X.; Bai, X.; Xiao, C.; Dong, Y. A Study of the Regulatory Mechanism of the CB1/PPARγ2/PLIN1/HSL Pathway for Fat Metabolism in Cattle. Front. Genet. 2021, 12, 631187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ke, H.; Xu, H.; Wang, G.-D.; Zhang, H.; Zou, L.; Xiang, S.; Li, M.; Peng, L.; Zhou, M.; et al. TDP-43 facilitates milk lipid secretion by post-transcriptional regulation of Btn1a1 and Xdh. Nat. Commun. 2020, 11, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Liu, J.; Huang, G.; Zhao, Y.; Yue, X.; Wu, H.; Li, J.; Zhu, J.; Shen, Z.; Haffty, B.G.; et al. Cullin3–KLHL25 ubiquitin ligase targets ACLY for degradation to inhibit lipid synthesis and tumor progression. Genes Dev. 2016, 30, 1956–1970. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhao, Y.; Iqbal, A.; Xia, L.; Bai, Z.; Sun, H.; Fang, X.; Yang, R.; Zhao, Z. Effects of polymorphism of the GPAM gene on milk quality traits and its relation to triglyceride metabolism in bovine mammary epithelial cells of dairy cattle. Arch. Anim. Breed. 2021, 64, 35–44. [Google Scholar] [CrossRef]
- Lee, Y.K.; Sohn, J.H.; Han, J.S.; Park, Y.J.; Jeon, Y.G.; Ji, Y.; Dalen, K.T.; Sztalryd, C.; Kimmel, A.R.; Kim, J.B. Perilipin 3 Deficiency Stimulates Thermogenic Beige Adipocytes through PPARα Activation. Diabetes 2018, 67, 791–804. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.O.; Lemaitre, R.N.; Fahrenbruch, C.E.; Hesselson, S.; Sotoodehnia, N.; McKnight, B.; Rice, K.M.; Kwok, P.-Y.; Siscovick, D.S.; Rea, T.D. Common Variation in Fatty Acid Genes and Resuscitation from Sudden Cardiac Arrest. Circ. Cardiovasc. Genet. 2012, 5, 422–429. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, W.; Zhang, C.; Shahzad, K.; Luo, J.; Loor, J.J. Transcriptome-Wide Analysis Reveals the Role of PPARγ Controlling the Lipid Metabolism in Goat Mammary Epithelial Cells. PPAR Res. 2016, 2016, 9195680. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Gautier, M.; Hayes, H.; Laurent, P.; Zaragoza, P.; Eggen, A.; Rodellar, C. Assignment of mitochondrial glycerol-3-phosphate acyltransferase (GPAM) gene to bovine chromosome 26 (26q22) by in situ hybridization and confirmation by somatic cell hybrid mapping. Cytogenet. Genome Res. 2002, 97, 276F. [Google Scholar] [CrossRef]
- Roy, R.; Ordovas, L.; Taourit, S.; Zaragoza, P.; Eggen, A.; Rodellar, C. Genomic structure and an alternative transcript of bovine mitochondrial glycerol-3-phosphate acyltransferase gene (GPAM). Cytogenet. Genome Res. 2006, 112, 82–89. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Z.; Yu, X.; Li, J.; Lu, C.; Yang, R. Bovine lipid metabolism related gene GPAM: Molecular characterization, function identification, and association analysis with fat deposition traits. Gene 2017, 609, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Periasamy, K.; Rodriguez-Zas, S.L.; Hurley, W.L.; Loor, J.J. A Novel Dynamic Impact Approach (DIA) for Functional Analysis of Time-Course Omics Studies: Validation Using the Bovine Mammary Transcriptome. PLoS ONE 2012, 7, e32455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.F.; Luo, J.; Zhao, W.; Yang, Y.; Tian, H.; Shi, H.; Bionaz, M. Overexpression of SREBP1 (sterol regulatory element binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J. Dairy Sci. 2016, 99, 783–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Zhou, B.; Yang, Q.; Pan, Y.; Yang, W.; Freedland, S.J.; Ding, L.-W.; Freeman, M.R.; Breunig, J.J.; Bhowmick, N.A.; et al. A Transcriptional Regulatory Loop of Master Regulator Transcription Factors, PPARG, and Fatty Acid Synthesis Promotes Esophageal Adenocarcinoma. Cancer Res. 2021, 81, 1216–1229. [Google Scholar] [CrossRef]
- Furth, N.; Aylon, Y. The LATS1 and LATS2 tumor suppressors: Beyond the Hippo pathway. Cell Death Differ. 2017, 24, 1488–1501. [Google Scholar] [CrossRef] [Green Version]


| Items | 5′-3′ | 3′-5′ |
|---|---|---|
| MiR-497 Mimic | CAGCAGCACACUGUGGUUUGUA | CAAACCACAGUGUGCUGCUGUU |
| MiR-497 Inhibitor | UACAAACCACAGUGUGCUGCUG | |
| Negative Control | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| Inhibitor NC | CAGUACUUUUGUGUAGUACAA | |
| LATS1-bos-43 | CCAGAAGGAUAUAGACAAATT | UUUGUCUAUAUCCUUCUGGTT |
| LATS1-bos-995 | GCAGACAGCCAAUCAUCAUTT | AUGAUGAUUGGCUGUCUGCTT |
| LATS1-bos-2446 | GCAGUCGAAAGUGUUCAUATT | UAUGAACACUUUCGACUGCTT |
| Items | Forward Primer | Reverse Primer |
|---|---|---|
| miR-497 | 5′-GTGCAGGGTCCGAGGT-3′ | 5′-TAGCCTGCAGCACACTGTGGT-3′ |
| U6 | 5′-GCTTCGGCAGCACATATACTAAAAT-3′ | 5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| GAPDH | 5′-GTCGATGGCTAGTCGTAGCATCGAT-3′ | 5′-TGCTAGCTGGCATGCCCGATCGATC-3′ |
| LATS1 | 5′-TCTTTGGTTGGGACTCCTAAT-3′ | 5′-TTCTTGCCTAAGCGATCTTCT-3′ |
| GPAM | 5′-ATTGACCCTTGGCACGATAG-3′ | 5′-AACAGCACCTTCCCACAAAG-3′ |
| HSL | 5′-GGGAGCACTACAAACGCAACG-3′ | 5′-TGAATGATCCGCTCAAACTCG-3′ |
| CD36 | 5′-GTACAGATGCAGCCTCATTTCC-3′ | 5′-TGGACCTGCAAATATCAGAGGA-3′ |
| ELOVL6 | 5′-GGAAGCCTTTAGTGCTCTGGTC-3′ | 5′-ATTGTATCTCCTAGTTCGGGTGC-3′ |
| PLIN3 | 5′-GGTGGAGGGTCAGGAGAAA-3′ | 5′-TCACGGAACATGGCGAGT-3′ |
| PPARG | 5′-CCTTCACCACCGTTGACTTCT-3′ | 5′-GATACAGGCTCCACTTTGATTGC-3′ |
| SLC27A6 | 5′-CAACTTGCTCATAAACTTTTTCCAAG-3′ | 5′-TGGTGTGGTTGTGCCAGGT-3′ |
| XDH | 5′-GATCATCCACTTTTCTGCCAATG-3′ | 5′-CCTCGTCTTGGTGCTTCCAA-3′ |
| ACLY | 5′-TTACCCAGAGGAAGCCTAC-3′ | 5′-AGGATCTTGCCATCTGGGTGC-3′ |
| Fatty Acid (%) | Treatment | p Value | |
|---|---|---|---|
| miR-497 NC-Mimic | miR-497 Mimic | ||
| C9:0 | 7.38 ± 0.25 | 3.45 ± 0.03 | <0.01 |
| C12:0 | 0.65 ± 0.04 | 0.97 ± 0.02 | <0.01 |
| C14:0 | 6.07 ± 0.06 | 8.89 ± 0.06 | <0.01 |
| C15:0 | 0.93 ± 0.03 | 0.60 ± 0.02 | <0.01 |
| C16:0 | 34.88 ± 0.07 | 40.67 ± 0.26 | <0.01 |
| C16:1 | 2.73 ± 0.11 | 1.90 ± 0.04 | <0.01 |
| C17:0 | 1.42 ± 0.08 | 1.07 ± 0.13 | 0.03 |
| C17:1 | 0.39 ± 0.02 | 0.25 ± 0.02 | <0.01 |
| C18:0 | 23.54 ± 0.10 | 26.65 ± 0.18 | <0.01 |
| 9Z C18:1 | 12.26 ± 0.04 | 8.21 ± 0.25 | <0.01 |
| 9E C18:1 | 5.59 ± 0.14 | 3.56 ± 0.10 | <0.01 |
| 9,12(Z,Z)C18:2 | 1.92 ± 0.05 | 0.95 ± 0.04 | <0.01 |
| C20:0 | 0.35 ± 0.03 | 0.67 ± 0.02 | <0.01 |
| cis-11C20:1 | 0.39 ± 0.01 | 0.30 ± 0.02 | <0.01 |
| 8,11,14-C20:3 | 0.33 ± 0.03 | 0.22 ± 0.03 | 0.02 |
| 5,8,11,14-C20:4(all Z) | 0.58 ± 0.04 | 0.75 ± 0.04 | 0.01 |
| 5,8,11,14,17C20:5(EPA) | 0.30 ± 0.00 | 0.26 ± 0.00 | <0.01 |
| 4,7,10,13,16,19-C22:6(DHA) | 0.28 ± 0.03 | 0.64 ± 0.04 | <0.01 |
| Fatty Acid (%) | Treatment | p Value | |
|---|---|---|---|
| NC-Inhibitor | MiR-497 Inhibitor | ||
| C9:0 | 2.86 ± 0.05 | 3.87 ± 0.05 | <0.01 |
| C12:0 | 1.67 ± 0.04 | 0.49 ± 0.03 | <0.01 |
| C14:0 | 9.09 ± 0.09 | 6.08 ± 0.06 | <0.01 |
| C15:0 | 0.86 ± 0.03 | 1.57 ± 0.03 | <0.01 |
| C16:0 | 36.42 ± 0.41 | 31.58 ± 0.30 | <0.01 |
| C16:1 | 3.62 ± 0.06 | 5.03 ± 0.03 | <0.01 |
| C17:0 | 0.74 ± 0.02 | 1.89 ± 0.04 | <0.01 |
| C17:1 | 0.33 ± 0.02 | 0.40 ± 0.02 | 0.02 |
| C18:0 | 27.37 ± 0.33 | 24.11 ± 0.23 | <0.01 |
| 9Z C18:1 | 6.92 ± 0.04 | 11.15 ± 0.26 | <0.01 |
| 9E C18:1 | 4.00 ± 0.14 | 6.62 ± 0.08 | <0.01 |
| 9,12(Z,Z)C18:2 | 1.86 ± 0.04 | 3.57 ± 0.20 | <0.01 |
| C20:0 | 0.61 ± 0.02 | 0.42 ± 0.01 | <0.01 |
| cis-11C20:1 | 0.33 ± 0.01 | 0.40 ± 0.02 | <0.01 |
| 8,11,14-C20:3 | 0.30 ± 0.04 | 0.43 ± 0.02 | 0.02 |
| 5,8,11,14-C20:4(all Z) | 1.84 ± 0.05 | 1.26 ± 0.11 | <0.01 |
| 5,8,11,14,17C20:5(EPA) | 0.33 ± 0.04 | 0.44 ± 0.03 | 0.04 |
| 4,7,10,13,16,19-C22:6(DHA) | 0.83 ± 0.02 | 0.69 ± 0.02 | <0.01 |
| Fatty Acid (%) | Treatment | p Value | |
|---|---|---|---|
| SiRNA-NC | SiRNA-LATS1 | ||
| C9:0 | 8.38 ± 0.25 | 2.44 ± 0.04 | <0.01 |
| C12:0 | 0.55 ± 0.06 | 1.57 ± 0.07 | <0.01 |
| C14:0 | 6.65 ± 0.11 | 6.89 ± 0.06 | <0.05 |
| C15:0 | 0.79 ± 0.03 | 0.64 ± 0.02 | <0.01 |
| C16:0 | 22.79 ± 0.07 | 30.27 ± 0.16 | <0.01 |
| C16:1 | 7.22 ± 0.14 | 3.47 ± 0.06 | <0.01 |
| C17:0 | 1.85 ± 0.10 | 0.95 ± 0.07 | <0.01 |
| C17:1 | 0.37 ± 0.02 | 0.24 ± 0.01 | <0.01 |
| C18:0 | 21.42 ± 0.46 | 32.59 ± 0.16 | <0.01 |
| 9Z C18:1 | 16.77 ± 0.07 | 8.30 ± 0.12 | <0.01 |
| 9E C18:1 | 7.26 ± 0.18 | 5.06 ± 0.02 | <0.01 |
| 9,12(Z,Z)C18:2 | 3.25 ± 0.12 | 1.88 ± 0.06 | <0.01 |
| C20:0 | 0.79 ± 0.06 | 2.48 ± 0.07 | <0.01 |
| cis-11C20:1 | 0.38 ± 0.02 | 0.31 ± 0.02 | 0.03 |
| 8,11,14-C20:3 | 0.35 ± 0.01 | 0.29 ± 0.01 | <0.01 |
| 5,8,11,14-C20:4(all Z) | 0.61 ± 0.07 | 0.95 ± 0.03 | <0.01 |
| 5,8,11,14,17C20:5(EPA) | 0.32 ± 0.02 | 0.26 ± 0.02 | 0.03 |
| 4,7,10,13,16,19-C22:6(DHA) | 0.25 ± 0.03 | 1.42 ± 0.02 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, S.; Yang, Y.; Nazar, M.; Chen, Z.; Yang, Z. miR-497 Regulates LATS1 through the PPARG Pathway to Participate in Fatty Acid Synthesis in Bovine Mammary Epithelial Cells. Genes 2023, 14, 1224. https://doi.org/10.3390/genes14061224
Chu S, Yang Y, Nazar M, Chen Z, Yang Z. miR-497 Regulates LATS1 through the PPARG Pathway to Participate in Fatty Acid Synthesis in Bovine Mammary Epithelial Cells. Genes. 2023; 14(6):1224. https://doi.org/10.3390/genes14061224
Chicago/Turabian StyleChu, Shuangfeng, Yi Yang, Mudasir Nazar, Zhi Chen, and Zhangping Yang. 2023. "miR-497 Regulates LATS1 through the PPARG Pathway to Participate in Fatty Acid Synthesis in Bovine Mammary Epithelial Cells" Genes 14, no. 6: 1224. https://doi.org/10.3390/genes14061224






