CCND1 Overexpression in Idiopathic Dilated Cardiomyopathy: A Promising Biomarker?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Preprocessing
2.3. DEG Identification
2.4. Enrichment and Functional Analysis
2.5. PPI Network Construction and HUB Detection
2.6. Sampling
2.7. Total RNA Isolation, cDNA Synthesis, and RT-PCR Validation
2.8. Over-Representation ANALYSIS
3. Results
3.1. Data Preprocessing and Identification of DEGs
3.2. Differentially Expressed Gene Enrichment Analysis
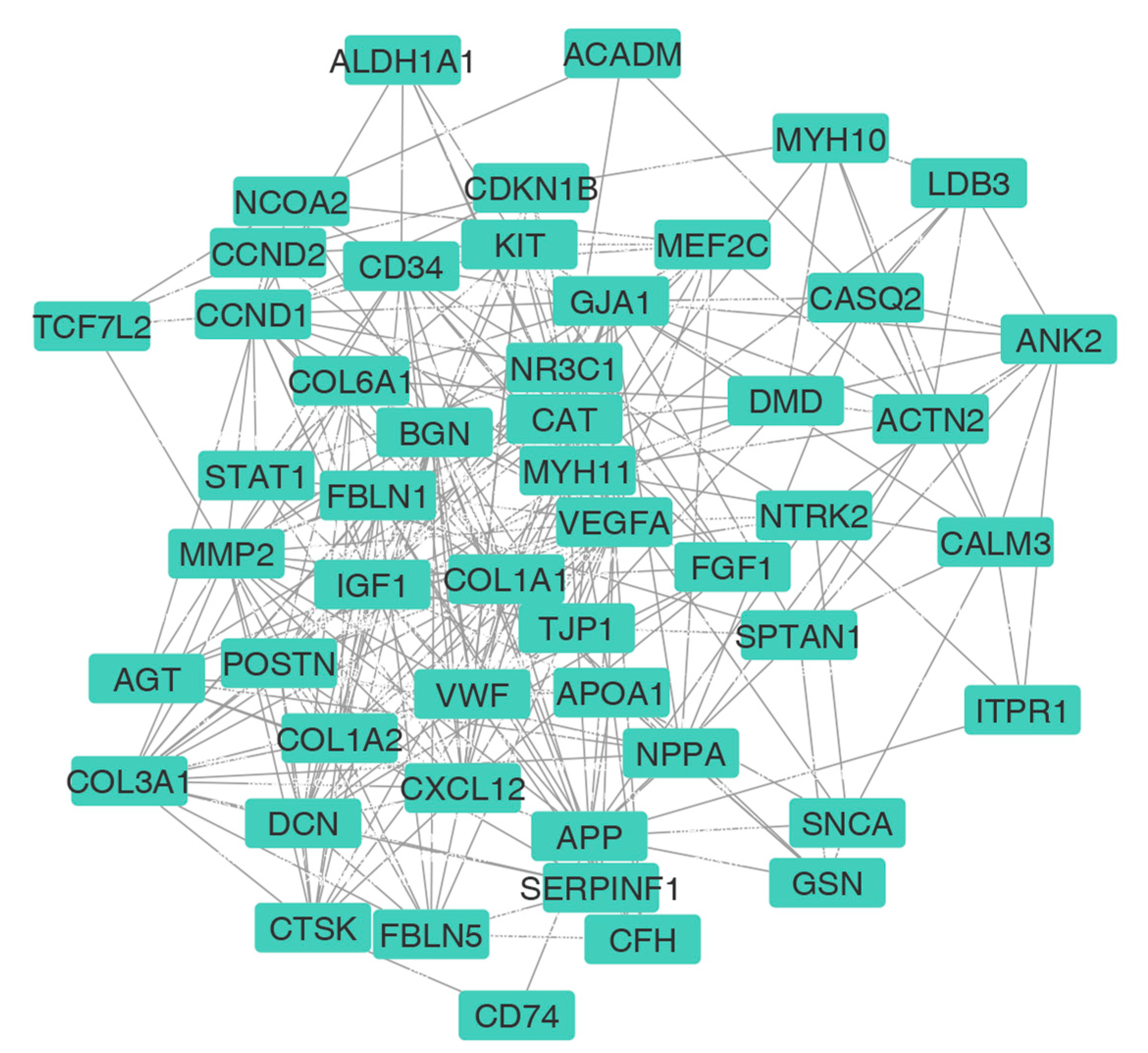
3.3. PPI Network Construction and HUB Selection
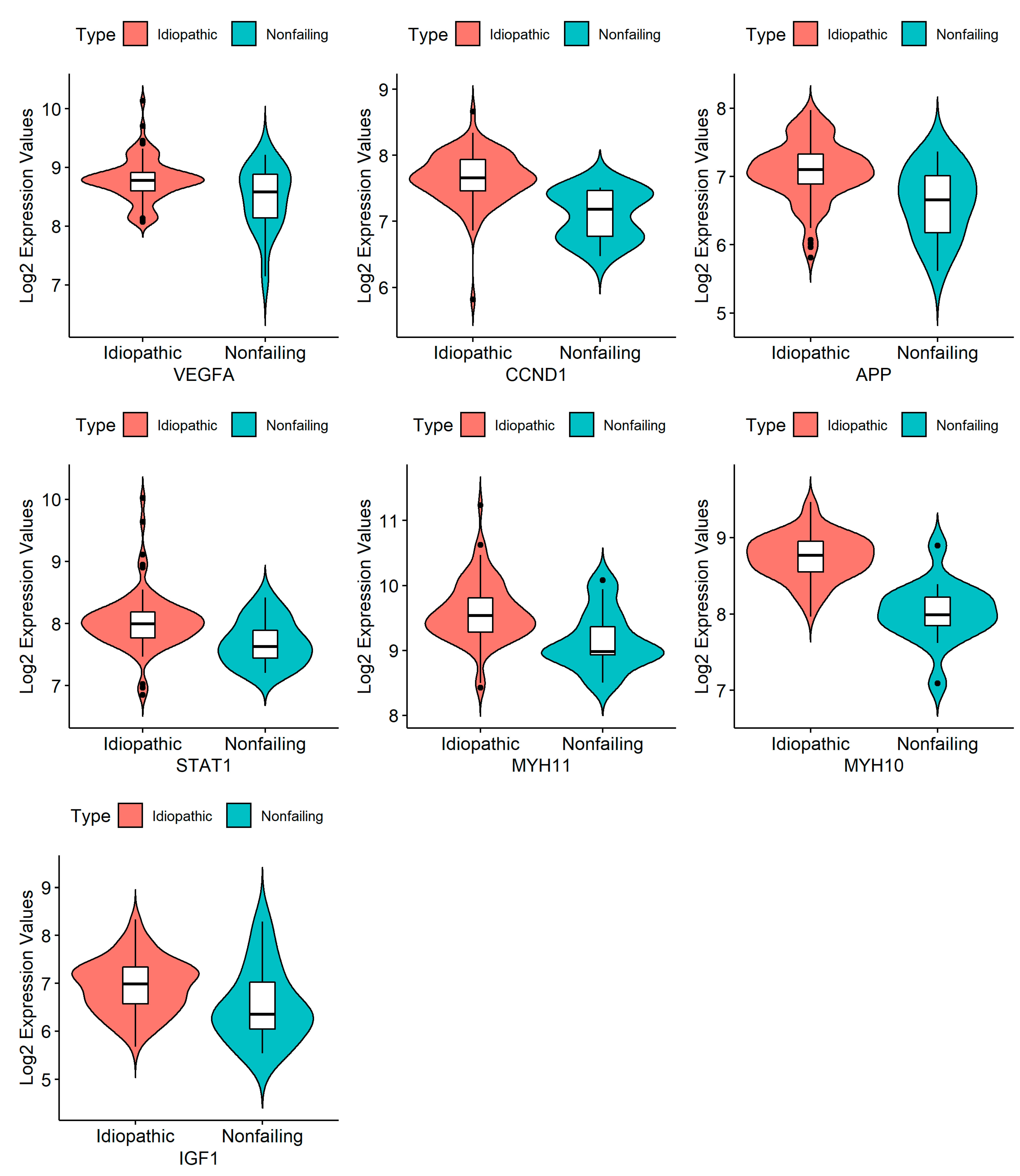
3.4. RNA Isolation and Quantification, cDNA Synthesis, and RT-PCR
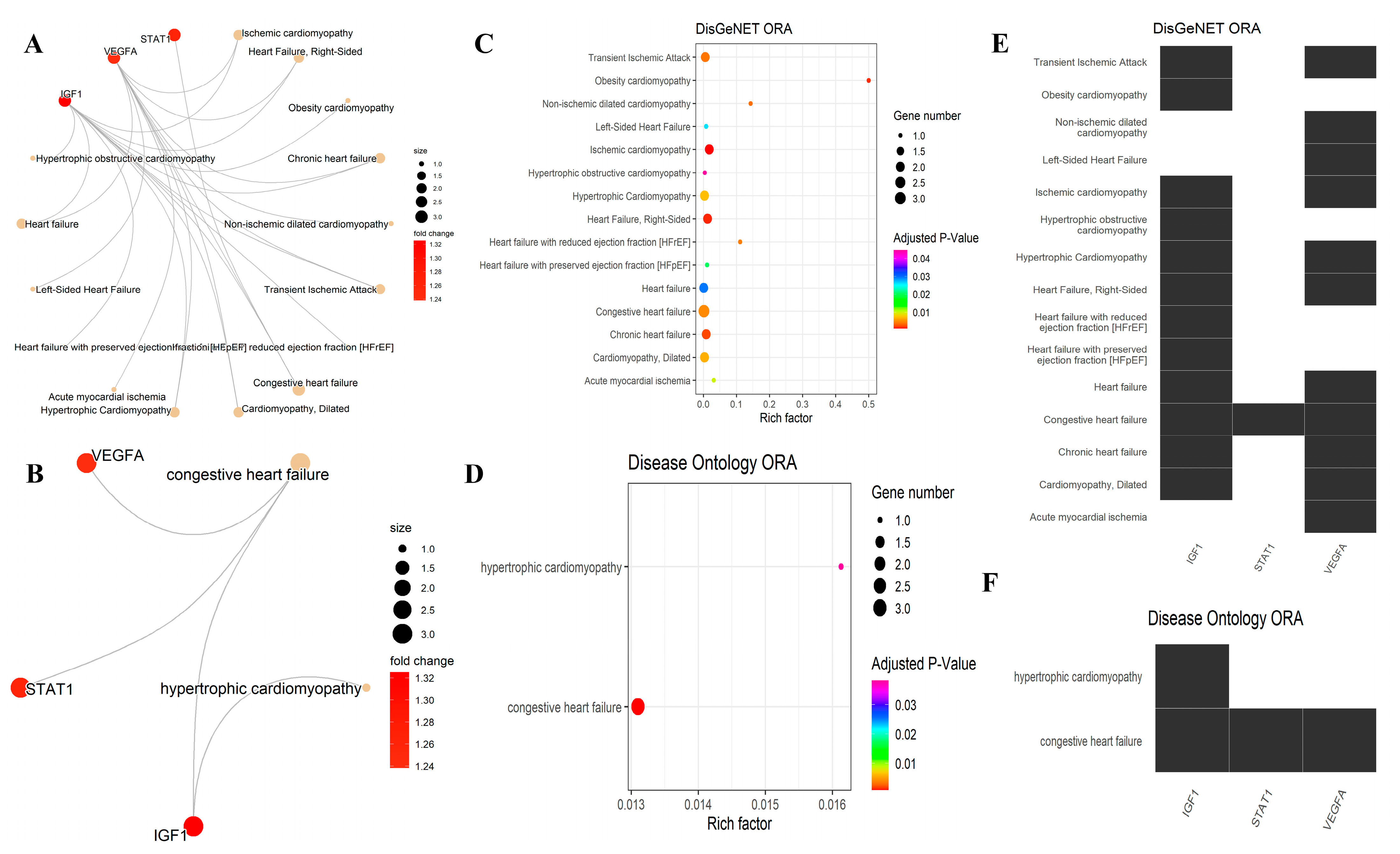
3.5. Over-Representation Analysis for the DisGeNET and DO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, J.L.; Towbin, J.A. Dilated cardiomyopathy. Lancet 2010, 375, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Phelan, D.; Wilson, G.; James, P.; Lockhart, P. The genetics of cardiomyopathy, new technologies and the path to personalised medicine. OA Genet. 2013, 1, 1–10. [Google Scholar]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef]
- Bailly, C.; Henriques, S.; Tsabedze, N.; Krause, A. Role of family history and clinical screening in the identification of families with idiopathic dilated cardiomyopathy in Johannesburg, South Africa. S. Afr. Med. J. 2019, 109, 673–678. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Dorn, I.I.G.W.; Shetty, A.; Parihar, A.; Dave, T.; Robinson, S.W.; Gottlieb, S.S.; Donahue, M.P.; Tomaselli, G.F.; Kraus, W.E. A genome-wide association study of idiopathic dilated cardiomyopathy in African Americans. J. Pers. Med. 2018, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Woulfe, K.C.; Siomos, A.K.; Nguyen, H.; SooHoo, M.; Galambos, C.; Stauffer, B.L.; Sucharov, C.; Miyamoto, S. Fibrosis and fibrotic gene expression in pediatric and adult patients with idiopathic dilated cardiomyopathy. J. Card. Fail. 2017, 23, 314–324. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.M.; Hawkins, N.M.; Jhund, P.S.; MacDonald, M.R.; Solomon, S.D.; Granger, C.B.; Yusuf, S.; Pfeffer, M.A.; Swedberg, K.; Petrie, M.C. Clinical characteristics and outcomes of young and very young adults with heart failure: The CHARM programme (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity). J. Am. Coll. Cardiol. 2013, 62, 1845–1854. [Google Scholar] [CrossRef] [Green Version]
- Merlo, M.; Pyxaras, S.A.; Pinamonti, B.; Barbati, G.; Di Lenarda, A.; Sinagra, G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J. Am. Coll. Cardiol. 2011, 57, 1468–1476. [Google Scholar] [CrossRef] [Green Version]
- Aziz, H.; Zaas, A.; Ginsburg, G.S. Peripheral blood gene expression profiling for cardiovascular disease assessment. Genom. Med. 2007, 1, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Liew, C.-C.; Ma, J.; Tang, H.-C.; Zheng, R.; Dempsey, A.A. The peripheral blood transcriptome dynamically reflects system wide biology: A potential diagnostic tool. J. Lab. Clin. Med. 2006, 147, 126–132. [Google Scholar] [CrossRef]
- Basu, M.; Wang, K.; Ruppin, E.; Hannenhalli, S. Predicting tissue-specific gene expression from whole blood transcriptome. Sci. Adv. 2021, 7, eabd6991. [Google Scholar] [CrossRef] [PubMed]
- Cappuzzello, C.; Napolitano, M.; Arcelli, D.; Melillo, G.; Melchionna, R.; Di Vito, L.; Carlini, D.; Silvestri, L.; Brugaletta, S.; Liuzzo, G. Gene expression profiles in peripheral blood mononuclear cells of chronic heart failure patients. Physiol. Genom. 2009, 38, 233–240. [Google Scholar] [CrossRef]
- Kontaraki, J.E.; Marketou, M.E.; Zacharis, E.A.; Parthenakis, F.I.; Vardas, P.E. Early cardiac gene transcript levels in peripheral blood mononuclear cells in patients with untreated essential hypertension. J. Hypertens. 2011, 29, 791–797. [Google Scholar] [CrossRef]
- Kurano, M.; Darestani, S.G.; Shinnakasu, A.; Yamamoto, K.; Dochi, Y.; Uemura, K.; Ikeda, Y.; Kikuchi, A.; Hashiguchi, H.; Deguchi, T. mRNA expression of platelet activating factor receptor (PAFR) in peripheral blood mononuclear cells is associated with albuminuria and vascular dysfunction in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 136, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Kontaraki, J.E.; Parthenakis, F.I.; Nyktari, E.G.; Patrianakos, A.P.; Vardas, P.E. Myocardial gene expression alterations in peripheral blood mononuclear cells of patients with idiopathic dilated cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 541–548. [Google Scholar] [CrossRef]
- Marketou, M.E.; Kontaraki, J.; Patrianakos, A.; Kanoupakis, E.; Plevritaki, A.; Mavrakis, H.; Kallergis, E.; Koutalas, E.; Anastasiou, I.; Chlouverakis, G. Long-term prognostic value of myocardin expression levels in non-ischemic dilated cardiomyopathy. Heart Vessel. 2021, 36, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xie, X.; Li, J.; Zhang, Y.; Xu, C.; Yang, J. Early right ventricular apical pacing-induced gene expression alterations are associated with deterioration of left ventricular systolic function. Dis. Markers 2017, 2017, 8405196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simantirakis, Ε.; Arkolaki, E.; Kontaraki, J.; Chlouverakis, G.; Mavrakis, H.; Kallergis, E.; Parthenakis, F.; Vardas, P. The impact of paced QRS duration on the expression of genes related to contractile function of the left ventricle in chronically paced patients from the right ventricular apex. Hell. J. Cardiol. 2020, 61, 274–278. [Google Scholar] [CrossRef]
- Arkolaki, E.G.; Simantirakis, E.N.; Kontaraki, J.E.; Chrysostomakis, S.I.; Patrianakos, A.P.; Chlouverakis, G.I.; Nakou, E.S.; Vardas, P.E. Alterations in the expression of genes related to contractile function and hypertrophy of the left ventricle in chronically paced patients from the right ventricular apex. Ep Eur. 2015, 17, 1563–1570. [Google Scholar] [CrossRef] [Green Version]
- Martin-Lorenzo, M.; Gonzalez-Calero, L.; Maroto, A.S.; Martinez, P.J.; Zubiri, I.; de la Cuesta, F.; Mourino-Alvarez, L.; Barderas, M.G.; Heredero, A.; Aldamiz-Echevarría, G. Cytoskeleton deregulation and impairment in amino acids and energy metabolism in early atherosclerosis at aortic tissue with reflection in plasma. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 725–732. [Google Scholar] [CrossRef]
- Anand, S.S.; Yusuf, S.; Vuksan, V.; Devanesen, S.; Teo, K.K.; Montague, P.A.; Kelemen, L.; Yi, C.; Lonn, E.; Gerstein, H. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: The Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet 2000, 356, 279–284. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, S.; Ma, Y.; Ma, J.; Hassan, W.; Shang, J. Peripheral-blood gene expression profiling studies for coronary artery disease and its severity in Xinjiang population in China. Lipids Health Dis. 2018, 17, 154. [Google Scholar] [CrossRef] [Green Version]
- Sinnaeve, P.R.; Donahue, M.P.; Grass, P.; Seo, D.; Vonderscher, J.; Chibout, S.-D.; Kraus, W.E.; Sketch, M., Jr.; Nelson, C.; Ginsburg, G.S. Gene expression patterns in peripheral blood correlate with the extent of coronary artery disease. PLoS ONE 2009, 4, e7037. [Google Scholar] [CrossRef]
- Huang, H.; Luo, B.; Wang, B.; Wu, Q.; Liang, Y.; He, Y. Identification of Potential Gene Interactions in Heart Failure Caused by Idiopathic Dilated Cardiomyopathy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7697–7709. [Google Scholar] [CrossRef]
- Laguna, J.C.; Alegret, M. Regulation of gene expression in atherosclerosis: Insights from microarray studies in monocytes/macrophages. Pharmacogenomics 2012, 13, 477–495. [Google Scholar] [CrossRef]
- Nührenberg, T.G.; Langwieser, N.; Binder, H.; Kurz, T.; Stratz, C.; Kienzle, R.-P.; Trenk, D.; Zohlnhöfer-Momm, D.; Neumann, F.-J. Transcriptome analysis in patients with progressive coronary artery disease: Identification of differential gene expression in peripheral blood. J. Cardiovasc. Transl. Res. 2013, 6, 81–93. [Google Scholar] [CrossRef]
- Hannenhalli, S.; Putt, M.E.; Gilmore, J.M.; Wang, J.; Parmacek, M.S.; Epstein, J.A.; Morrisey, E.E.; Margulies, K.B.; Cappola, T.P. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation 2006, 114, 1269–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.A.; Stewart, P.A.; Kuenzi, B.M.; Eschrich, J.A. Escape Excel: A tool for preventing gene symbol and accession conversion errors. PLoS ONE 2017, 12, e0185207. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.-H.; Wang, S.; Zhang, Y.; Huang, T.; Cai, Y.-D. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS ONE 2017, 12, e0184129. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Yan, G.-R.; He, Q.-Y. DOSE: An R/Bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics 2015, 31, 608–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schriml, L.M.; Arze, C.; Nadendla, S.; Chang, Y.-W.W.; Mazaitis, M.; Felix, V.; Feng, G.; Kibbe, W.A. Disease Ontology: A backbone for disease semantic integration. Nucleic Acids Res. 2012, 40, D940–D946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñero, J.; Queralt-Rosinach, N.; Bravo, À.; Deu-Pons, J.; Bauer-Mehren, A.; Baron, M.; Sanz, F.; Furlong, L.I. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database 2015, 2015, bav028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourke, L.T.; Knight, R.A.; Latchman, D.S.; Stephanou, A.; Mccormick, J. Signal transducer and activator of transcription-1 localizes to the mitochondria and modulates mitophagy. Jak-Stat 2013, 2, e25666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Lemaire, H.-G.; Unterbeck, A.; Salbaum, J.M.; Masters, C.L.; Grzeschik, K.-H.; Multhaup, G.; Beyreuther, K.; Müller-Hill, B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 1987, 325, 733–736. [Google Scholar] [CrossRef]
- Troncone, L.; Luciani, M.; Coggins, M.; Wilker, E.H.; Ho, C.-Y.; Codispoti, K.E.; Frosch, M.P.; Kayed, R.; del Monte, F. Aβ Amyloid Pathology Affects the Hearts of Patients With Alzheimer’s Disease: Mind the Heart. J. Am. Coll. Cardiol. 2016, 68, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Lombardi, R.; Karmouch, J.; Tsai, J.-Y.; Czernuszewicz, G.; Taylor, M.R.; Mestroni, L.; Coarfa, C.; Gurha, P.; Marian, A.J. DNA damage response/TP53 pathway is activated and contributes to the pathogenesis of dilated cardiomyopathy associated with LMNA (Lamin A/C) mutations. Circ. Res. 2019, 124, 856–873. [Google Scholar] [CrossRef] [PubMed]
- Tatman, P.D.; Woulfe, K.C.; Karimpour-Fard, A.; Jeffrey, D.A.; Jaggers, J.; Cleveland, J.C.; Nunley, K.; Taylor, M.R.; Miyamoto, S.D.; Stauffer, B.L. Pediatric dilated cardiomyopathy hearts display a unique gene expression profile. JCI Insight 2017, 2, e94249. [Google Scholar] [CrossRef] [Green Version]
- Vigil-Garcia, M.; Demkes, C.J.; Eding, J.E.C.; Versteeg, D.; de Ruiter, H.; Perini, I.; Kooijman, L.; Gladka, M.M.; Asselbergs, F.W.; Vink, A.; et al. Gene expression profiling of hypertrophic cardiomyocytes identifies new players in pathological remodelling. Cardiovasc. Res. 2021, 117, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Z.; Song, T.; Jia, Y.; Qi, L.; Ren, L.; Chen, S. Proteomics study on the effect of silybin on cardiomyopathy in obese mice. Sci. Rep. 2021, 11, 7136. [Google Scholar] [CrossRef]
- Merlo, M.; Cannata, A.; Gobbo, M.; Stolfo, D.; Elliott, P.M.; Sinagra, G. Evolving concepts in dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Li, L. Identification of Biomarkers Associated with Heart Failure Caused by Idiopathic Dilated Cardiomyopathy Using WGCNA and Machine Learning Algorithms. Int. J. Genom. 2023, 2023, 2250772. [Google Scholar] [CrossRef]
- Liu, Z.; Song, Y.-N.; Chen, K.-Y.; Gao, W.-L.; Chen, H.-J.; Liang, G.-Y. Bioinformatics prediction of potential mechanisms and biomarkers underlying dilated cardiomyopathy. World J. Cardiol. 2022, 14, 282–296. [Google Scholar] [CrossRef]
- Si, W. Analysis of Associated Genes and Biological Pathways Between Inflammatory Dilated Cardiomyopathy and Ischemic Cardiomyopathy by Bioinformatics. Eur. J. Cardiovasc. Med. 2023, 11, 31–38. [Google Scholar] [CrossRef]
- Luo, X.; Luo, P.; Zhang, Y. Identification of differentially expressed long non-coding RNAs associated with dilated cardiomyopathy using integrated bioinformatics approaches. Drug Discov. Ther. 2020, 14, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fan, M.; Cao, X.; Geng, H.; Su, Y.; Wu, C.; Pan, H.; Pan, M. Integrated Bioinformatics Algorithms and Experimental Validation to Explore Robust Biomarkers and Landscape of Immune Cell Infiltration in Dilated Cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 809470. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Jin, X.; Liu, Y.; Fan, T.; Zhu, Z.; Jin, J.; Zheng, G.; Chen, Z.; Lu, M.; Wang, Z. The Bioinformatical Identification of Potential Biomarkers in Heart Failure Diagnosis and Treatment. Genet. Res. 2022, 2022, 8727566. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, T.; Singh, P.; Jairajpuri, D.S.; Al-Keridis, L.A.; Alshammari, N.; Adnan, M.; Dohare, R.; Hassan, M.I. Differential Gene Expression and Weighted Correlation Network Dynamics in High-Throughput Datasets of Prostate Cancer. Front. Oncol. 2022, 12, 881246. [Google Scholar] [CrossRef]
- Zhao, B.; Erwin, A.; Xue, B. How many differentially expressed genes: A perspective from the comparison of genotypic and phenotypic distances. Genomics 2018, 110, 67–73. [Google Scholar] [CrossRef]
- Dalman, M.R.; Deeter, A.; Nimishakavi, G.; Duan, Z.-H. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinform. 2012, 13, S11. [Google Scholar] [CrossRef] [Green Version]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular pharmacology of VEGF-A isoforms: Binding and signalling at VEGFR2. International journal of molecular sciences 2018, 19, 1264. [Google Scholar] [CrossRef] [Green Version]
- Feucht, M.; Christ, B.; Wilting, J. VEGF induces cardiovascular malformation and embryonic lethality. Am. J. Pathol. 1997, 151, 1407–1416. [Google Scholar]
- Arif, M.; Alam, P.; Ahmed, R.P.H.; Pandey, R.; Faridi, H.M.; Sadayappan, S. Upregulated Angiogenesis Is Incompetent to Rescue Dilated Cardiomyopathy Phenotype in Mice. Cells 2021, 10, 771. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Inoue, K.; Hara, Y.; Morioka, N.; Ohshima, K.; Suzuki, J.; Ogimoto, A.; Shigematsu, Y.; Higaki, J. Serum markers of angiogenesis and myocardial ultrasonic tissue characterization in patients with dilated cardiomyopathy. Eur. J. Heart Fail. 2005, 7, 689–695. [Google Scholar] [CrossRef]
- Roura, S.; Planas, F.; Prat-Vidal, C.; Leta, R.; Soler-Botija, C.; Carreras, F.; Llach, A.; Hove-Madsen, L.; Pons Lladó, G.; Farré, J.; et al. Idiopathic dilated cardiomyopathy exhibits defective vascularization and vessel formation. Eur. J. Heart Fail. 2007, 9, 995–1002. [Google Scholar] [CrossRef]
- Tham, E.; Wang, J.; Piehl, F.; Weber, G. Upregulation of VEGF-A without angiogenesis in a mouse model of dilated cardiomyopathy caused by mitochondrial dysfunction. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2002, 50, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saygili, E.; Noor-Ebad, F.; Schröder, J.W.; Mischke, K.; Saygili, E.; Rackauskas, G.; Marx, N.; Kelm, M.; Rana, O.R. Autoantibodies in dilated cardiomyopathy induce vascular endothelial growth factor expression in cardiomyocytes. Biochem. Biophys. Res. Commun. 2015, 465, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Hofbauer, R.; Schäfer, R.; Blumer, R.; Paulus, P.; Miksovsky, A.; Traxler, H.; Kocher, A.; Aharinejad, S. Selective downregulation of VEGF-A(165), VEGF-R(1), and decreased capillary density in patients with dilative but not ischemic cardiomyopathy. Circ. Res. 2000, 87, 644–647. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, J.; Domal-Kwiatkowska, D.; Mazurek, U.; Zembala, M.; Michalski, B.; Zembala, M. Post-transcriptional modifications of VEGF-A mRNA in non-ischemic dilated cardiomyopathy. Cell. Mol. Biol. Lett. 2007, 12, 331–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecollari, V.; Nieuwenhuis, B.; Verhaagen, J. A perspective on the role of class III semaphorin signaling in central nervous system trauma. Front. Cell. Neurosci. 2014, 8, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; He, G.; Hou, M.; Chen, L.; Chen, S.; Xu, A.; Fu, Y. Cell cycle regulation by alternative polyadenylation of CCND1. Scientific reports 2018, 8, 6824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Qin, L.; Han, L.; Zhao, Y.; Jing, H.; Song, W.; Shi, H. Role of MicroRNA-93 I in pathogenesis of left ventricular remodeling via targeting cyclin-D1. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruetten, H.; Dimmeler, S.; Gehring, D.; Ihling, C.; Zeiher, A.M. Concentric left ventricular remodeling in endothelial nitric oxide synthase knockout mice by chronic pressure overload. Cardiovasc. Res. 2005, 66, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, R.; Lin, F.; Zhang, S.; Zhang, G.; Hu, S.; Zheng, Z. MicroRNA: Novel regulators involved in the remodeling and reverse remodeling of the heart. Cardiology 2009, 113, 81–88. [Google Scholar] [CrossRef]
- Harmelink, C.; Peng, Y.; DeBenedittis, P.; Chen, H.; Shou, W.; Jiao, K. Myocardial Mycn is essential for mouse ventricular wall morphogenesis. Dev. Biol. 2013, 373, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cognet, M.; Nougayrede, A.; Malan, V.; Callier, P.; Cretolle, C.; Faivre, L.; Genevieve, D.; Goldenberg, A.; Heron, D.; Mercier, S.; et al. Dissection of the MYCN locus in Feingold syndrome and isolated oesophageal atresia. Eur. J. Hum. Genet. 2011, 19, 602–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, D.; Chen, W.; Tan, C.Y.; Wong, J.X.; Chan, P.S.; Tan, L.W.; Foo, R.; Jiang, J. Upregulation of Yy1 Suppresses Dilated Cardiomyopathy caused by Ttn insufficiency. Sci. Rep. 2019, 9, 16330. [Google Scholar] [CrossRef] [Green Version]
- Deo, R.C. Alternative Splicing, Internal Promoter, Nonsense-Mediated Decay, or All Three: Explaining the Distribution of Truncation Variants in Titin. Circ. Cardiovasc. Genet. 2016, 9, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Haggerty, C.M.; Damrauer, S.M.; Levin, M.G.; Birtwell, D.; Carey, D.J.; Golden, A.M.; Hartzel, D.N.; Hu, Y.; Judy, R.; Kelly, M.A.; et al. Genomics-first evaluation of heart disease associated with Titin-truncating variants. Circulation 2019, 140, 42–54. [Google Scholar] [CrossRef]
- Herman, D.S.; Lam, L.; Taylor, M.R.G.; Wang, L.; Teekakirikul, P.; Christodoulou, D.; Conner, L.; DePalma, S.R.; McDonough, B.; Sparks, E.; et al. Truncations of titin causing dilated cardiomyopathy. New Engl. J. Med. 2012, 366, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Linke, W.A. Titin gene and protein functions in passive and active muscle. Annu. Rev. Physiol. 2018, 80, 389–411. [Google Scholar] [CrossRef]
- Foncea, R.; Andersson, M.; Ketterman, A.; Blakesley, V.; Sapag-Hagar, M.; Sugden, P.H.; LeRoith, D.; Lavandero, S. Insulin-like growth factor-I rapidly activates multiple signal transduction pathways in cultured rat cardiac myocytes. J. Biol. Chem. 1997, 272, 19115–19124. [Google Scholar] [CrossRef] [Green Version]
- Jing, Z.; Hou, X.; Wang, Y.; Yang, G.; Wang, B.; Tian, X.; Zhao, S.; Wang, Y. Association between insulin-like growth factor-1 and cardiovascular disease risk: Evidence from a meta-analysis. Int. J. Cardiol. 2015, 198, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Carlzon, D.; Svensson, J.; Petzold, M.; Karlsson, M.K.; Ljunggren, Ö.; Tivesten, A.; Mellström, D.; Ohlsson, C. Both low and high serum IGF-1 levels associate with increased risk of cardiovascular events in elderly men. J. Clin. Endocrinol. Metab. 2014, 99, E2308–E2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Giorgi, A.; Marra, A.M.; Iacoviello, M.; Triggiani, V.; Rengo, G.; Cacciatore, F.; Maiello, C.; Limongelli, G.; Masarone, D.; Perticone, F.; et al. Insulin-like growth factor-1 (IGF-1) as predictor of cardiovascular mortality in heart failure patients: Data from the T.O.S.CA. registry. Intern. Emerg. Med. 2022, 17, 1651–1660. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, H. Long non-coding RNA HAND2-AS1 downregulation predicts poor survival of patients with end-stage dilated cardiomyopathy. J. Int. Med. Res. 2019, 47, 3690–3698. [Google Scholar] [CrossRef]
- Mao, C.; Hou, X.; Wang, B.; Chi, J.; Jiang, Y.; Zhang, C.; Li, Z. Intramuscular injection of human umbilical cord-derived mesenchymal stem cells improves cardiac function in dilated cardiomyopathy rats. Stem Cell Res. Ther. 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastasilakis, A.D.; Koulaxis, D.; Upadhyay, J.; Pagkalidou, E.; Kefala, N.; Perakakis, N.; Polyzos, S.A.; Economou, F.; Mantzoros, C.S. Free IGF-1, intact IGFBP-4, and PicoPAPP-A are altered in acute myocardial infarction compared to stable coronary artery disease and healthy controls. Horm. Metab. Res. 2019, 51, 112–119. [Google Scholar] [CrossRef]
- Guo, S.; Gong, M.; Tse, G.; Li, G.; Chen, K.-Y.; Liu, T. The Value of IGF-1 and IGFBP-1 in Patients With Heart Failure With Reduced, Mid-range, and Preserved Ejection Fraction. Front. Cardiovasc. Med. 2021, 8, 772105. [Google Scholar] [CrossRef]
- Larsson, S.C.; Michaëlsson, K.; Burgess, S. IGF-1 and cardiometabolic diseases: A Mendelian randomisation study. Diabetologia 2020, 63, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Chang, Y.H.; Lo, H.J.; Isfandiari, M.A.; Martini, S.; Hou, W.H.; Li, C.Y. Incidence of idiopathic cardiomyopathy in patients with type 2 diabetes in Taiwan: Age, sex, and urbanization status-stratified analysis. Cardiovasc. Diabetol. 2020, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Schüler, R.; Markova, M.; Osterhoff, M.A.; Arafat, A.; Pivovarova, O.; Machann, J.; Hierholzer, J.; Hornemann, S.; Rohn, S.; Pfeiffer, A.F.H. Similar dietary regulation of IGF-1- and IGF-binding proteins by animal and plant protein in subjects with type 2 diabetes. Eur. J. Nutr. 2021, 60, 3499–3504. [Google Scholar] [CrossRef]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Archacki, S.R.; Zhang, T.; Wang, Q.K. Induction of high STAT1 expression in transgenic mice with LQTS and heart failure. Biochem. Biophys. Res. Commun. 2007, 358, 454. [Google Scholar] [CrossRef] [Green Version]
- Olson, T.M.; Michels, V.V.; Ballew, J.D.; Reyna, S.P.; Karst, M.L.; Herron, K.J.; Horton, S.C.; Rodeheffer, R.J.; Anderson, J.L. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA 2005, 293, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zicha, S.; Maltsev, V.A.; Nattel, S.; Sabbah, H.N.; Undrovinas, A.I. Post-transcriptional alterations in the expression of cardiac Na+ channel subunits in chronic heart failure. J. Mol. Cell. Cardiol. 2004, 37, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yong, S.L.; Drinko, J.K.; Popović, Z.B.; Shryock, J.C.; Belardinelli, L.; Wang, Q.K. LQTS Mutation N1325S in Cardiac Sodium Channel Gene SCN5A Causes Cardiomyocyte Apoptosis, Cardiac Fibrosis and Contractile Dysfunction in Mice. Int. J. Cardiol. 2011, 147, 239–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, R.-Z.; Rao, L.; Chen, Z.; Strash, N.; Bursac, N. Loss of sarcomeric proteins via upregulation of JAK/STAT signaling underlies interferon-γ-induced contractile deficit in engineered human myocardium. Acta Biomater. 2021, 126, 144–153. [Google Scholar] [CrossRef]
- Boscardin, S.B.; Torrecilhas, A.C.T.; Manarin, R.; Revelli, S.; Rey, E.G.; Tonelli, R.R.; Silber, A.M. Chagas’ disease: An update on immune mechanisms and therapeutic strategies. J. Cell. Mol. Med. 2010, 14, 1373–1384. [Google Scholar] [CrossRef] [Green Version]
- Stahl, P.; Ruppert, V.; Schwarz, R.T.; Meyer, T. Trypanosoma cruzi Evades the Protective Role of Interferon-Gamma-Signaling in Parasite-Infected Cells. PLoS ONE 2014, 9, e110512. [Google Scholar] [CrossRef] [Green Version]



| Primer | Sequence (5′→3′) | |
|---|---|---|
| VEGFA | F | GGCAGAAGGAGGAGGGCAGAAT |
| R | CATCGCATCAGGGGCACACA | |
| CCND1 | F | AGGCGGAGGAGAACAAACAGA |
| R | TGAGGCGGTAGTAGGACAGGA | |
| MYH11 | F | GGACAAACTGCAAGCAAAGGTGA |
| R | TTGTGTGAACCTCCCTGCTCT | |
| MYH10 | F | ATCTCGGCGTAAACTCCAGCG |
| R | TCTGTGTCATCGTCGGAGAGC | |
| STAT1 | F | GTGGCAGGATGTCTCAGTGGT |
| R | AACATCATTGGCAGCGTGCTC | |
| IGF1 | F | ACCATGTCCTCCTCGCATCTCT |
| R | ACTGCTGGAGCCATACCCTGT | |
| APP | F | TGGTGGGCGGTGTTGTCATAG |
| R | GCCGTTCTGCTGCATCTTGGA | |
| GAPDH (Housekeeping) | F | ATTATTCTCTGATTTGGTCGTAT |
| R | CTCCTGGAAGATGGTGAT | |
| ID | Description | Adjusted p-Value | Gene Symbol |
|---|---|---|---|
| hsa05165 | Human papillomavirus infection | 0.000771289 | ATP6V0E2/CCND1/CCND2/CDKN1B/COL1A1/COL1A2/COL6A1/COMP/EIF2AK2/FZD1/FZD7/HEY1/HLA-B/HLA-E/ITGB5/JAG1/LAMA4/LAMB1/LAMB2/MX1/NOTCH2/NOTCH3/PRKACB/STAT1/TCF7L2/THBS2/THBS4/TNXB/VEGFA/VWF |
| hsa05416 | Viral myocarditis | 0.000833702 | CCND1/DMD/HLA-B/HLA-DMA/HLA-DMB/HLA-DPA1/HLA-DPB1/HLA-DRA/HLA-DRB1/HLA-E/SGCG |
| hsa04510 | Focal adhesion | 0.000833702 | CCND1/CCND2/COL1A1/COL1A2/COL6A1/COMP/IGF1/ITGB5/LAMA4/LAMB1/LAMB2/MYLK3/PDGFC/PDGFD/PPP1R12B/THBS2/THBS4/TNXB/VEGFA/VEGFB/VWF |
| hsa04512 | ECM receptor interaction | 0.000833702 | AGRN/COL1A1/COL1A2/COL6A1/COMP/ITGB5/LAMA4/LAMB1/LAMB2/THBS2/THBS4/TNXB/VWF |
| hsa04020 | Calcium signaling pathway | 0.000833702 | ASPH/CALM3/CAMK2B/CASQ2/EDNRA/F2R/FGF1/GRM1/HRC/ITPR1/MYLK3/NTRK2/PDE1A/PDE1C/PDGFC/PDGFD/PLCB4/PLCE1/PRKACB/TPCN1/VDAC3/VEGFA/VEGFB |
| ID | Description | Gene Symbol | Adjusted p-Value | Database |
|---|---|---|---|---|
| C0349782 | Ischemic cardiomyopathy | IGF1/VEGFA | 0.000957133 | DisGeNET |
| C0235527 | Right-sided heart failure | IGF1/VEGFA | 0.001407601 | DisGeNET |
| C4552322 | Obesity cardiomyopathy | IGF1 | 0.001552258 | DisGeNET |
| C0264716 | Chronic heart failure | IGF1/VEGFA | 0.002243913 | DisGeNET |
| C1168330 | Non-ischemic dilated cardiomyopathy | VEGFA | 0.003639825 | DisGeNET |
| C0007787 | Transient ischemic attack | IGF1/VEGFA | 0.003972576 | DisGeNET |
| C4509223 | Heart failure with reduced ejection fraction (HFrEF) | IGF1 | 0.004298851 | DisGeNET |
| C0018802 | Congestive heart failure | IGF1/STAT1/VEGFA | 0.004923199 | DisGeNET |
| C0007193 | Cardiomyopathy, dilated | IGF1/VEGFA | 0.006671016 | DisGeNET |
| C0007194 | Hypertrophic cardiomyopathy | IGF1/VEGFA | 0.007424915 | DisGeNET |
| C0746731 | Acute myocardial ischemia | VEGFA | 0.009968188 | DisGeNET |
| C4509226 | Heart failure with preserved ejection fraction (HFpEF) | IGF1 | 0.020442736 | DisGeNET |
| C0023212 | Left-sided heart failure | VEGFA | 0.026246253 | DisGeNET |
| C0018801 | Heart failure | IGF1/VEGFA | 0.029611829 | DisGeNET |
| C4551472 | Hypertrophic obstructive cardiomyopathy | IGF1 | 0.044684056 | DisGeNET |
| DOID:6000 | Congestive heart failure | IGF1/STAT1/VEGFA | 0.000875442 | DO |
| DOID:11984 | Hypertrophic cardiomyopathy | IGF1 | 0.038275847 | DO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehghani, K.; Stanek, A.; Bagherabadi, A.; Atashi, F.; Beygi, M.; Hooshmand, A.; Hamedi, P.; Farhang, M.; Bagheri, S.; Zolghadri, S. CCND1 Overexpression in Idiopathic Dilated Cardiomyopathy: A Promising Biomarker? Genes 2023, 14, 1243. https://doi.org/10.3390/genes14061243
Dehghani K, Stanek A, Bagherabadi A, Atashi F, Beygi M, Hooshmand A, Hamedi P, Farhang M, Bagheri S, Zolghadri S. CCND1 Overexpression in Idiopathic Dilated Cardiomyopathy: A Promising Biomarker? Genes. 2023; 14(6):1243. https://doi.org/10.3390/genes14061243
Chicago/Turabian StyleDehghani, Khatereh, Agata Stanek, Arash Bagherabadi, Fatemeh Atashi, Mohammad Beygi, Amirreza Hooshmand, Pezhman Hamedi, Mohsen Farhang, Soghra Bagheri, and Samaneh Zolghadri. 2023. "CCND1 Overexpression in Idiopathic Dilated Cardiomyopathy: A Promising Biomarker?" Genes 14, no. 6: 1243. https://doi.org/10.3390/genes14061243
APA StyleDehghani, K., Stanek, A., Bagherabadi, A., Atashi, F., Beygi, M., Hooshmand, A., Hamedi, P., Farhang, M., Bagheri, S., & Zolghadri, S. (2023). CCND1 Overexpression in Idiopathic Dilated Cardiomyopathy: A Promising Biomarker? Genes, 14(6), 1243. https://doi.org/10.3390/genes14061243








