Clinical and Genetic Features of NR2E3-Associated Retinopathy: A Report of Eight Families with a Longitudinal Study and Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probands and Family Members
2.2. Bioinformatics Analysis of NR2E3 Variants
2.3. Phenotypic Analysis
2.4. Literature Review of NR2E3 Variants
2.5. Statistical Analysis
3. Results
3.1. Molecular Analysis of NR2E3 in Our Cohort
3.2. Clinical Data and Follow-Up of the Patients with NR2E3 Associated ADRP in Our Cohort
3.3. Follow-Up of Patients with NR2E3 Associated ARRP in Our Cohort
3.4. Genotype-Phenotype Correlation Analysis of NR2E3-Associated Retinopathy
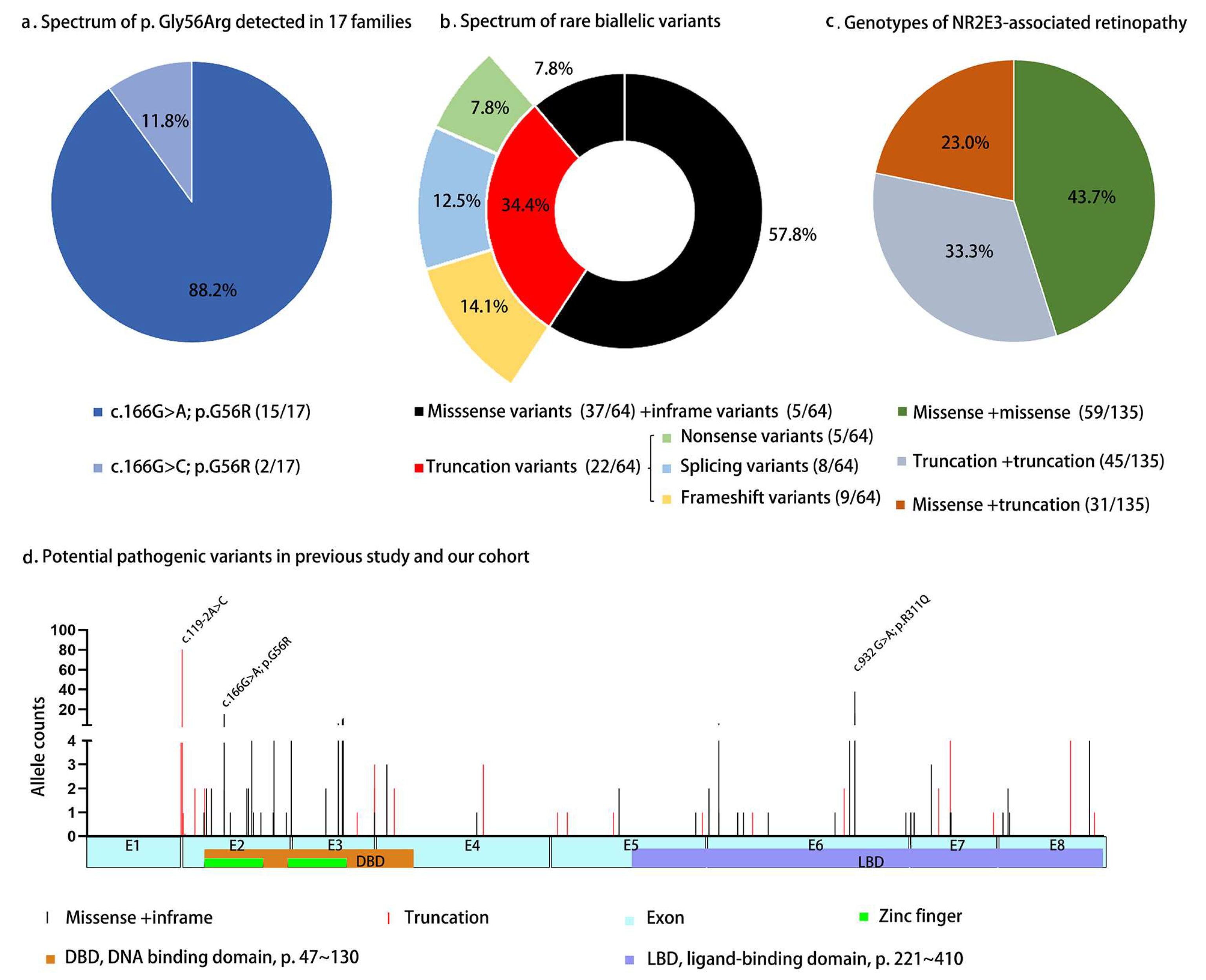
3.5. Literatures Review of NR2E3
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerber, S.; Rozet, J.M.; Takezawa, S.I.; Dos, S.L.; Lopes, L.; Gribouval, O.; Penet, C.; Perrault, I.; Ducroq, D.; Souied, E.; et al. The photoreceptor cell-specific nuclear receptor gene (PNR) accounts for retinitis pigmentosa in the Crypto-Jews from Portugal (Marranos), survivors from the Spanish Inquisition. Hum. Genet. 2000, 107, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.B.; Jacobson, S.G.; Cideciyan, A.V.; Swiderski, R.; Streb, L.M.; Searby, C.; Beck, G.; Hockey, R.; Hanna, D.B.; Gorman, S.; et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat. Genet. 2000, 24, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Takezawa, S.I.; Hara, K.; Yu, R.T.; Umesono, Y.; Agata, K.; Taniwaki, M.; Yasuda, K.; Umesono, K. Identification of a photoreceptor cell-specific nuclear receptor. Proc. Natl. Acad. Sci. USA 1999, 9, 4814–4819. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, F.; Leroy, B.P.; Beysen, D.; Hellemans, J.; De Bosscher, K.; Haegeman, G.; Robberecht, K.; Wuyts, W.; Coucke, P.J.; De Baere, E. Recurrent Mutation in the First Zinc Finger of the Orphan Nuclear Receptor NR2E3 Causes Autosomal Dominant Retinitis Pigmentosa. Am. J. Hum. Genet. 2007, 81, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gire, A.I.; Sullivan, L.S.; Bowne, S.J.; Birch, D.G.; Hughbanks-Wheaton, D.; Heckenlively, J.R.; Daiger, S.P. The Gly56Arg mutation in NR2E3 accounts for 1-2% of autosomal dominant retinitis pigmentosa. Mol. Vis. 2007, 13, 1970–1975. [Google Scholar]
- Blanco-Kelly, F.; García Hoyos, M.; Lopez Martinez, M.A.; Lopez-Molina, M.I.; Riveiro-Alvarez, R.; Fernandez-San Jose, P.; Avila-Fernandez, A.; Corton, M.; Millan, J.M.; García Sandoval, B.; et al. Dominant Retinitis Pigmentosa, p.Gly56Arg Mutation in NR2E3: Phenotype in a Large Cohort of 24 Cases. PLoS ONE 2016, 11, e149473. [Google Scholar] [CrossRef] [Green Version]
- Escher, P.; Tran, H.V.; Vaclavik, V.; Borruat, F.X.; Schorderet, D.F.; Munier, F.L. Double concentric autofluorescence ring in NR2E3-p.G56R-linked autosomal dominant retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4754–4764. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, S.G.; Roman, A.J.; Roman, M.I.; Gass, J.D.; Parker, J.A. Relatively enhanced S cone function in the Goldmann-Favre syndrome. Am. J. Ophthalmol. 1991, 111, 446–453. [Google Scholar] [CrossRef]
- Schorderet, D.F.; Escher, P. NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP). Hum. Mutat. 2009, 30, 1475–1485. [Google Scholar] [CrossRef]
- Marmor, M.F.; Jacobson, S.G.; Foerster, M.H.; Kellner, U.; Weleber, R.G. Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am. J. Ophthalmol. 1990, 110, 124–134. [Google Scholar] [CrossRef]
- Sharon, D.; Sandberg, M.A.; Caruso, R.C.; Berson, E.L.; Dryja, T.P. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch. Ophthalmol. (1960) 2003, 121, 1316. [Google Scholar] [CrossRef] [Green Version]
- Tsang, S.H.; Sharma, T. Enhanced S-Cone Syndrome (Goldmann-Favre Syndrome). Adv. Exp. Med. Biol. 2018, 1085, 153–156. [Google Scholar] [CrossRef]
- de Carvalho, E.R.; Robson, A.G.; Arno, G.; Boon, C.J.F.; Webster, A.A.; Michaelides, M. Enhanced S-Cone Syndrome. Ophthalmol. Retin. 2021, 5, 195–214. [Google Scholar] [CrossRef]
- Vincent, A.; Robson, A.G.; Holder, G.E. PATHOGNOMONIC (DIAGNOSTIC) ERGs A Review and Update. Retin.-J. Retin. Vitr. Dis. 2013, 33, 5–12. [Google Scholar] [CrossRef]
- Kawasaki, A.; Crippa, S.V.; Kardon, R.; Leon, L.; Hamel, C. Characterization of pupil responses to blue and red light stimuli in autosomal dominant retinitis pigmentosa due to NR2E3 mutation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5562–5569. [Google Scholar] [CrossRef] [Green Version]
- Da, P.M.; Marrra, M.; Pennesi, M.E. A double hyperautofluorescent ring in a 33-year-old-female patient. Retin. Cases Brief Rep. 2022, 17, S15–S18. [Google Scholar] [CrossRef]
- Smirnov, V.M.D.C. Triple hyperautofluorescent retinal ring. Pathognomonic appearance of c.166G>A NR2E3-related inherited retinal degeneration. J. Fr. D’Ophtalmol. 2019, 42, 332–333. [Google Scholar] [CrossRef]
- Murro, V.; Mucciolo, D.P.; Sodi, A.; Passerini, I.; Giorgio, D.; Virgili, G.; Rizzo, S. Novel clinical findings in autosomal recessive NR2E3-related retinal dystrophy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 9–22. [Google Scholar] [CrossRef]
- Wang, P.; Li, S.; Sun, W.; Xiao, X.; Jia, X.; Liu, M.; Xu, L.; Long, Y.; Zhang, Q. An Ophthalmic Targeted Exome Sequencing Panel as a Powerful Tool to Identify Causative Mutations in Patients Suspected of Hereditary Eye Diseases. Transl. Vis. Sci. Technol. 2019, 8, 21. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [Green Version]
- Grover, S.; Fishman, G.A.; Anderson, R.J.; Tozatti, M.S.; Heckenlively, J.R.; Weleber, R.G.; Edwards, A.O.; Brown, J.J. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology 1999, 106, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Escher, P.; Gouras, P.; Roduit, R.L.; Tiab, L.; Bolay, S.; Delarive, T.; Chen, S.; Tsai, C.; Hayashi, M.; Zernant, J.; et al. Mutations in NR2E3 can cause dominant or recessive retinal degenerations in the same family. Hum. Mutat. 2009, 30, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Naessens, S.; Ruysschaert, L.; Lefever, S.; Coppieters, F.; De Baere, E. Antisense Oligonucleotide-Based Downregulation of the G56R Pathogenic Variant Causing NR2E3-Associated Autosomal Dominant Retinitis Pigmentosa. Genes 2019, 10, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roduit, R.; Escher, P.; Schorderet, D.F. Mutations in the DNA-binding domain of NR2E3 affect in vivo dimerization and interaction with CRX. PLoS ONE 2009, 4, e7379. [Google Scholar] [CrossRef]
- Nakagawa, S.; Oishi, A.; Ogino, K.; Morooka, S.; Oishi, M.; Sugahara, M.; Yoshimura, N. Asymmetric Cone Distribution and Its Clinical Appearance in Retinitis Pigmentosa. Retin.-J. Retin. Vitr. Dis. 2016, 36, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef]
- Sohn, E.H.; Chen, F.K.; Rubin, G.S.; Moore, A.T.; Webster, A.R.; Maclaren, R.E. Macular Function Assessed by Microperimetry in Patients with Enhanced S-Cone Syndrome. Ophthalmology 2010, 117, 1199–1206. [Google Scholar] [CrossRef]
- Hull, S.; Arno, G.; Sergouniotis, P.I.; Tiffin, P.; Borman, A.D.; Chandra, A.; Robson, A.G.; Holder, G.E.; Webster, A.R.; Moore, A.T. Clinical and Molecular Characterization of Enhanced S-Cone Syndrome in Children. JAMA Ophthalmol. 2014, 132, 1341. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Gekka, T.; Tsuneoka, H. Spontaneous Resolution of Large Macular Retinoschisis in Enhanced S-Cone Syndrome. Ophthalmic Surg. Lasers Imag. Retin. 2016, 47, 187–190. [Google Scholar] [CrossRef]
- Iannaccone, A.; Fung, K.H.; Eyestone, M.E.; Stone, E.M. Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am. J. Ophthalmol. 2009, 147, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Genead, M.A.; Fishman, G.A.; Mcanany, J.J. Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc. Ophthalmol. 2010, 121, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Hajali, M.; Fishman, G.A. Dorzolamide use in the management of macular cysts in a patient with enhanced s-cone syndrome. Retin. Cases Brief Rep. 2009, 3, 121–124. [Google Scholar] [CrossRef]
- Sato, T.; Kuniyoshi, K.; Nakao, A.; Shimomura, Y.; Tomemori, R. Long-term observation of two cases of enhanced S-cone syndrome. Nippon Ganka Gakkai Zasshi 2009, 113, 980–990. [Google Scholar]
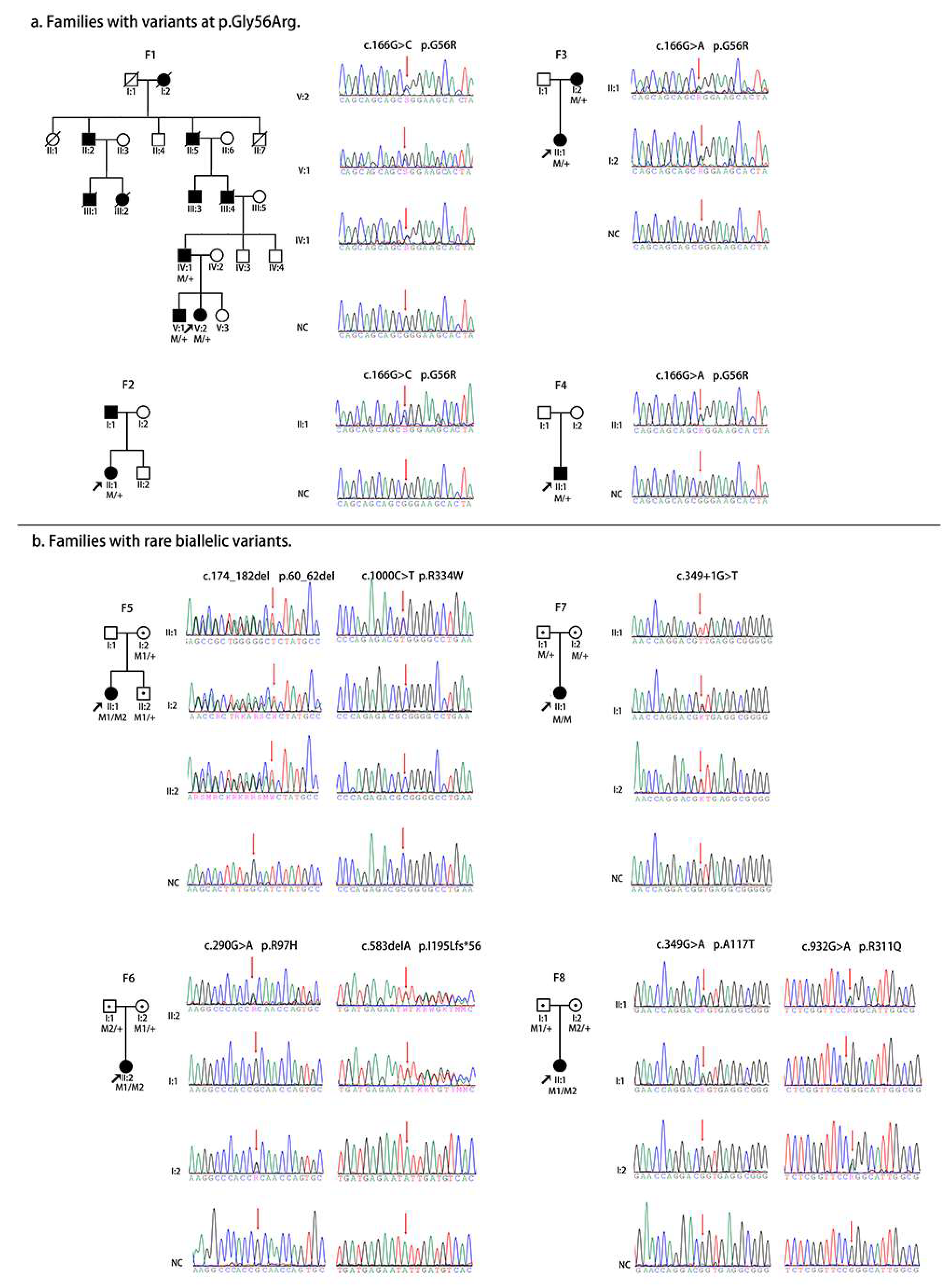
| No | Family | Position | Change | Effect | ① | ACMG Evidence | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | HGMD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | at Chr15 | NM_014249 | NM_014249 | Total | EA | ||||||||||
| Dominant | |||||||||||||||
| 1 | F1, F2 | 72103870 | c.166G>C | p.G56R | LPV | PS1, PP1, PP3, PP4 | D* | D | D | 0.57 | 16.00 | 0 | 0 | DM | |
| 2 | F3, F4 | 72103870 | c.166G>A | p.G56R | PV | PS1, PS3, PM2, PP1, PP3, PP4 | D* | D | D | 0.57 | 15.66 | 1/152182 | 0 | DM | |
| Recessive | |||||||||||||||
| 3 | F5 | 72103878 | c.174_182del | p.60_62del | LPV | PM2, PM4, PP1, PP4 | / | / | / | / | / | 0 | 0 | novel | |
| 4 | F6 | 72104150 | c.290G>A | p.R97H | LPV | PS1, PM2, PP3, PP4 | D* | D | D | 0.65 | 15.58 | 0 | 0 | DM | |
| 6 | F7 | 72104210 | c.349+1G>T | SD | LPV | PM2, PM4, PP1, PP4 | / | D | D | / | 25.3 | SSC | 1/151650 | 0 | novel |
| 5 | F8 | 72104209 | c.349G>A | p.A117T | LPV | PM2, PP1, PP3, PP4 | D* | D | D | 0.59 | 16.00 | 0 | 0 | novel | |
| 7 | F6 | 72104687 | c.583delA | p.I195Lfs*56 | LPV | PM2, PM4, PP1, PP4 | / | / | / | / | 0 | 0 | novel | ||
| 8 | F8 | 72105913 | c.932G>A | p.R311Q | LPV | PS1, PP4, PP5 | P | D | D | 0.11 | 16.82 | 94/230880 | 2/17158 | DM | |
| 9 | F5 | 72106358 | c.1000C>T | p.R334W | LPV | PM2, PM5, PP3, PP4 | D* | D | D | 0.5 | 17.08 | 35/275146 | 0 | novel | |
| Family | Change | Effect | Gender | Age/Years | BCVA of FV | BCVA of LV | First | NB | Macular Change | Retinal Change | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Onset | FV | LV | OD | OS | OD | OS | Symptom | FV | LV | |||||
| Dominant | |||||||||||||||
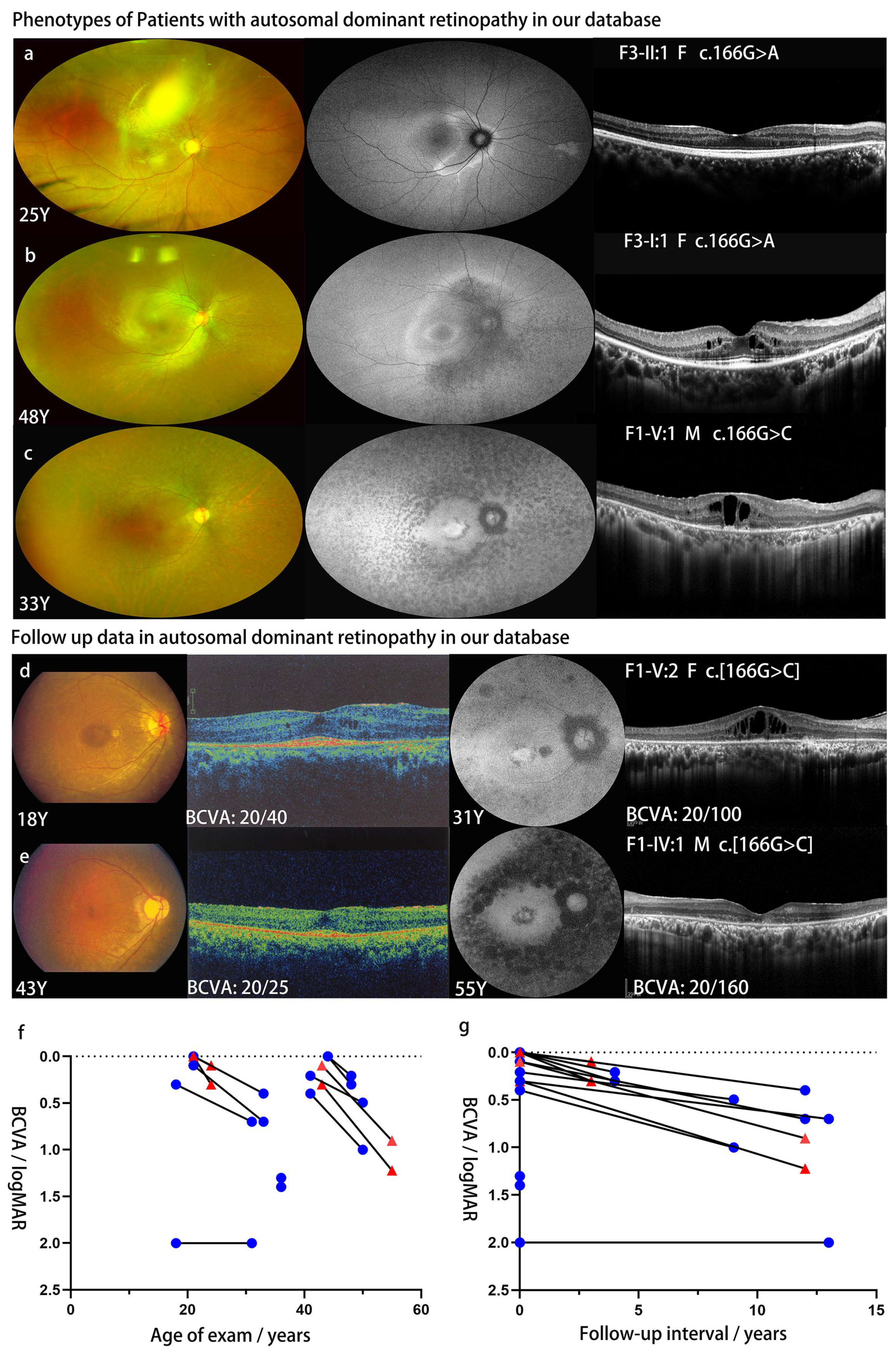
| F1-V:1 | c.166G>C | p.G56R | F | ECH | 18 | 31 | 20/40 | 20/2000 | 20/100 | 20/2000 | NB | Y | MS | MS, MA | WD, MP-RPD |
| F1-IV:1 | c.166G>C | p.G56R | M | NA | 43 | 55 | 20/25 | 20/40 | 20/160 | 20/333 | NA | Y | MS | MS, MA | MP-RPD |
| F1-V:1 | c.166G>C | p.G56R | M | NA | 21 | 33 | 20/25 | 20/20 | 20/100 | 20/50 | NB | Y | NL | MS, MA | WD, MP-RPD |
| F2-II:1 | c.166G>C | p.G56R | F | ECH | 41 | 50 | 20/32 | 20/50 | 20/63 | 20/200 | PV | Y | NA | MA | MP-RPD |
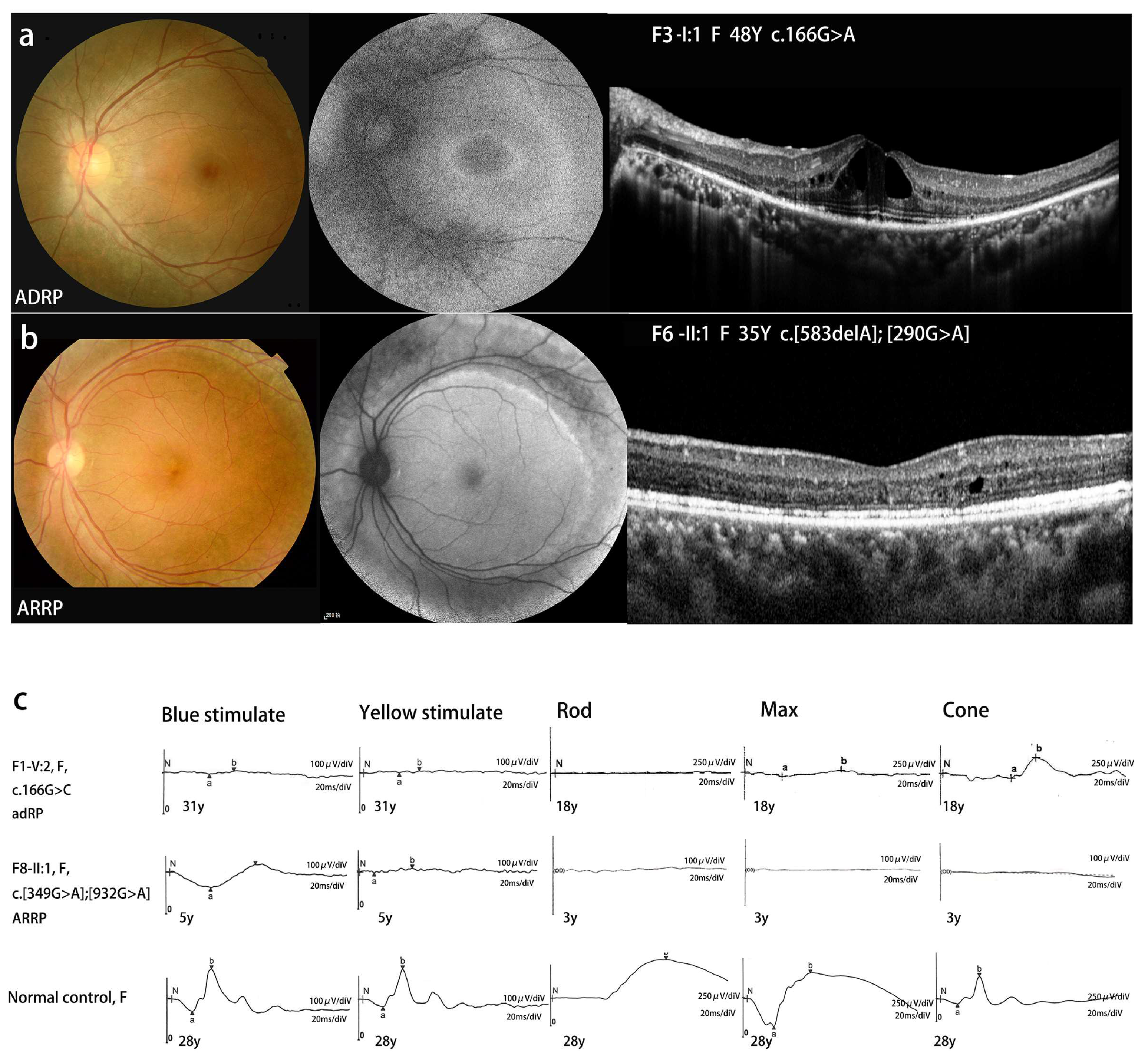
| F3-II:1 | c.166G>A | p.G56R | F | 21 | 21 | 24 | 20/20 | 20/20 | 20/40 | 20/25 | high IOP | N | NL | NL | VA-RPD |
| F3-I:2 | c.166G>A | p.G56R | F | NA | 44 | 48 | 20/20 | 20/20 | 20/32 | 20/40 | NB | Y | NL | MS | MP-RPD |
| F4-II:1 | c.166G>A | p.G56R | M | 28 | 36 | NA | 20/400 | 20/500 | NA | NA | PV | Y | NA | NA | MP-RPD |
| Recessive | |||||||||||||||
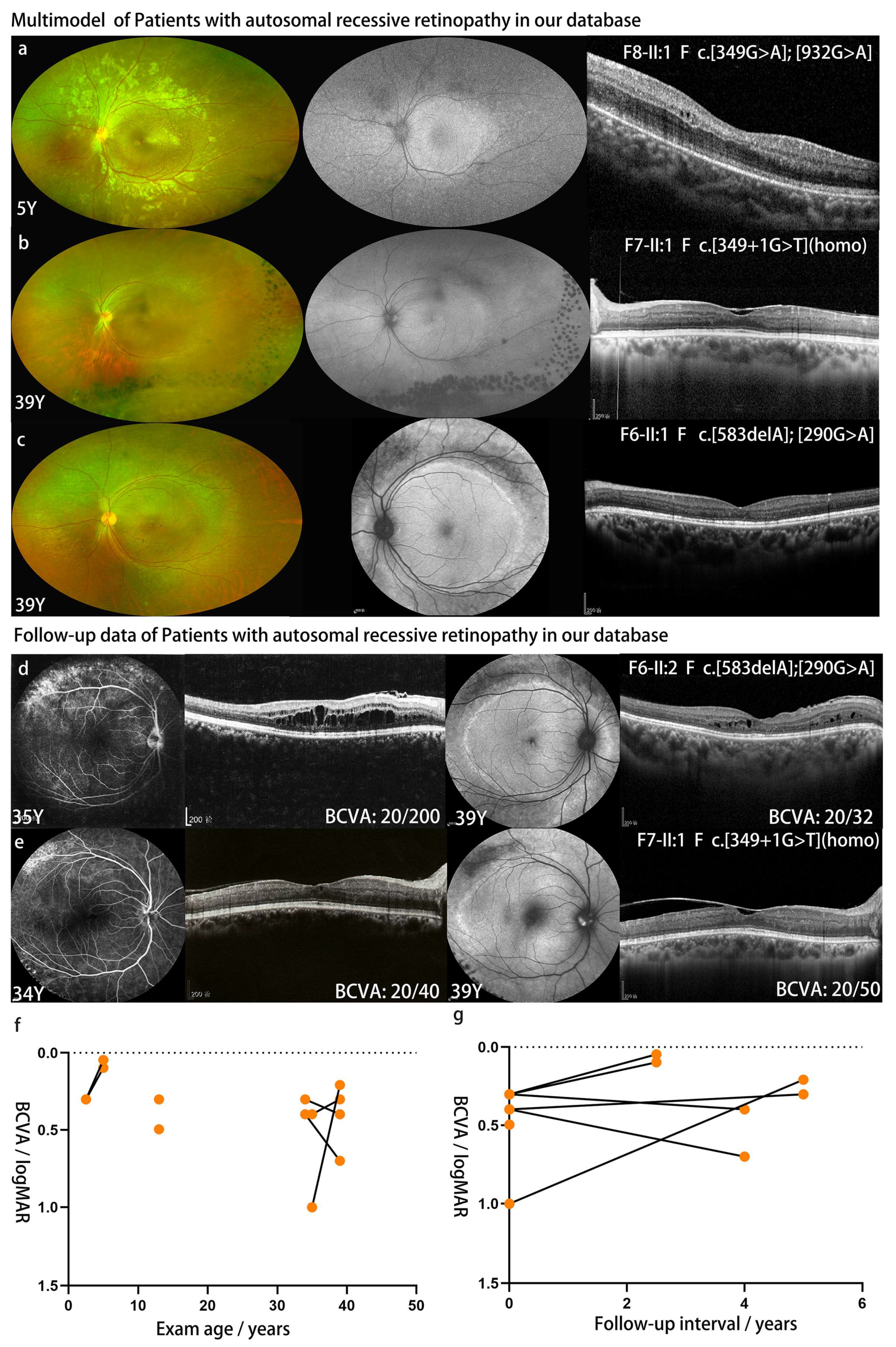
| F5-II:1 | c.174_182del | p.60_62del | F | ECH | 13 | NA | 20/63 | 20/40 | NA | NA | PV | Y | MS | NA | VA-RPD |
| c.1000C>T | p.R334W | ||||||||||||||
| F6-II:1 | c.290G>A | p.R97H] | F | 32 | 35 | 39 | 20/200 | 20/50 | 20/32 | 20/40 | PV | N | MS | MS | VA-RPD |
| c.583delA | p.I195Lfs*56 | ||||||||||||||
| F7-II:1 | c.349+1G>T (hom) | SD | F | ECH | 34 | 39 | 20/40 | 20/50 | 20/50 | 20/100 | NB | Y | MS | MS | PRS |
| F8-II:1 | c.349G>A | p.A117T | |||||||||||||
| c.932G>A | p.R311Q | F | ECH | 2.5 | 5 | 20/40 | 20/40 | 20/22 | 20/25 | PV | Y | NL | MS | VA-RPD | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Yi, Z.; Xiao, X.; Li, S.; Jia, X.; Lian, P.; Sun, W.; Wang, P.; Lu, L.; Zhang, Q. Clinical and Genetic Features of NR2E3-Associated Retinopathy: A Report of Eight Families with a Longitudinal Study and Literature Review. Genes 2023, 14, 1525. https://doi.org/10.3390/genes14081525
Xiao S, Yi Z, Xiao X, Li S, Jia X, Lian P, Sun W, Wang P, Lu L, Zhang Q. Clinical and Genetic Features of NR2E3-Associated Retinopathy: A Report of Eight Families with a Longitudinal Study and Literature Review. Genes. 2023; 14(8):1525. https://doi.org/10.3390/genes14081525
Chicago/Turabian StyleXiao, Sainan, Zhen Yi, Xueshan Xiao, Shiqiang Li, Xiaoyun Jia, Ping Lian, Wenmin Sun, Panfeng Wang, Lin Lu, and Qingjiong Zhang. 2023. "Clinical and Genetic Features of NR2E3-Associated Retinopathy: A Report of Eight Families with a Longitudinal Study and Literature Review" Genes 14, no. 8: 1525. https://doi.org/10.3390/genes14081525





