Deep Isolated Aquifer Brines Harbor Atypical Halophilic Microbial Communities in Quebec, Canada
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Geochemical and Geological Analyses
2.3. DNA Extraction and Sequencing
2.4. Amplicon Sequence Analyses
2.5. Shotgun Sequence Analyses
2.6. Statistical Analyses Analyses
3. Results
3.1. Geochemical and Physico-Chemical Properties of the Hypersaline Groundwater
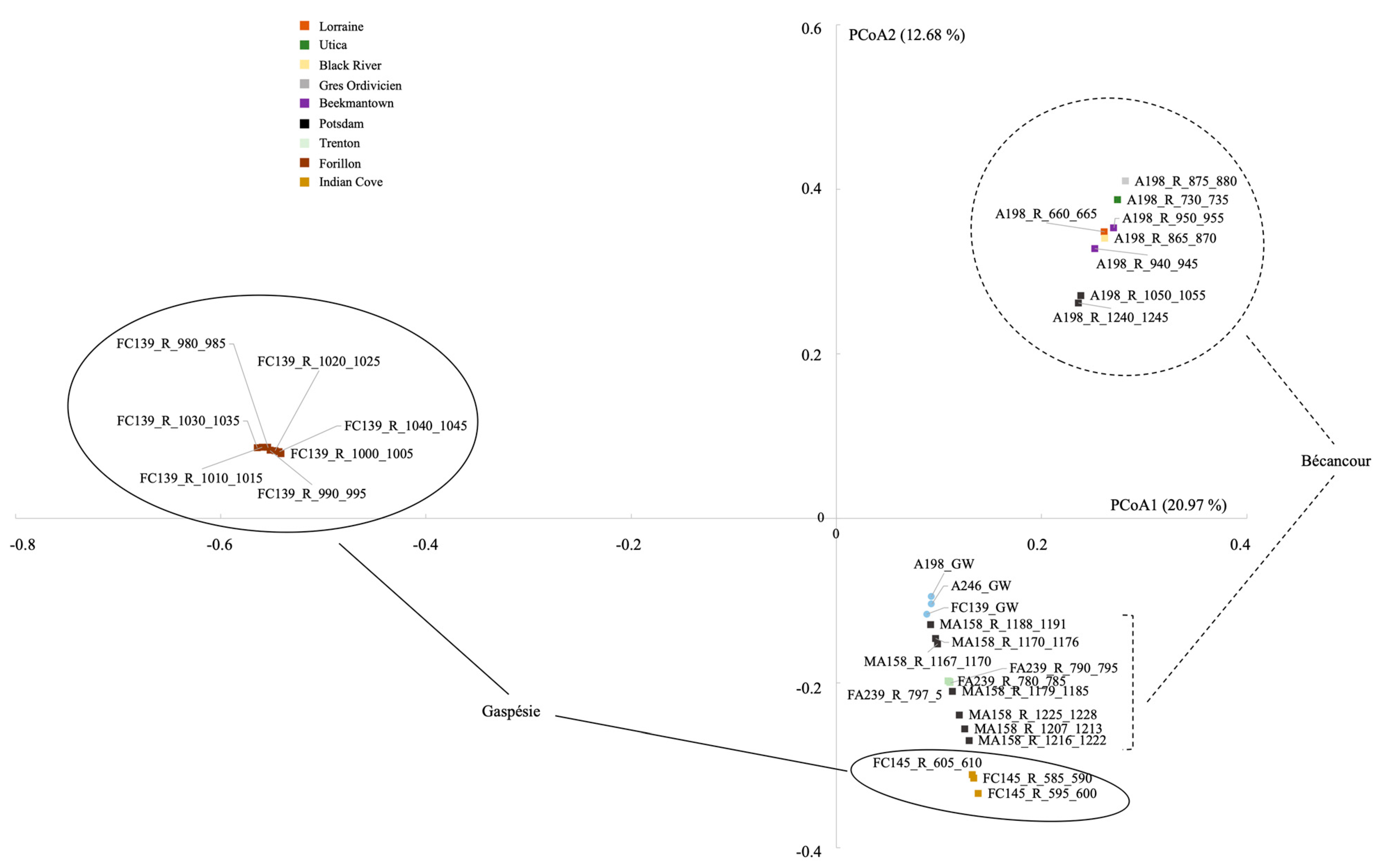
3.2. Microbial 16S rRNA Gene Diversity
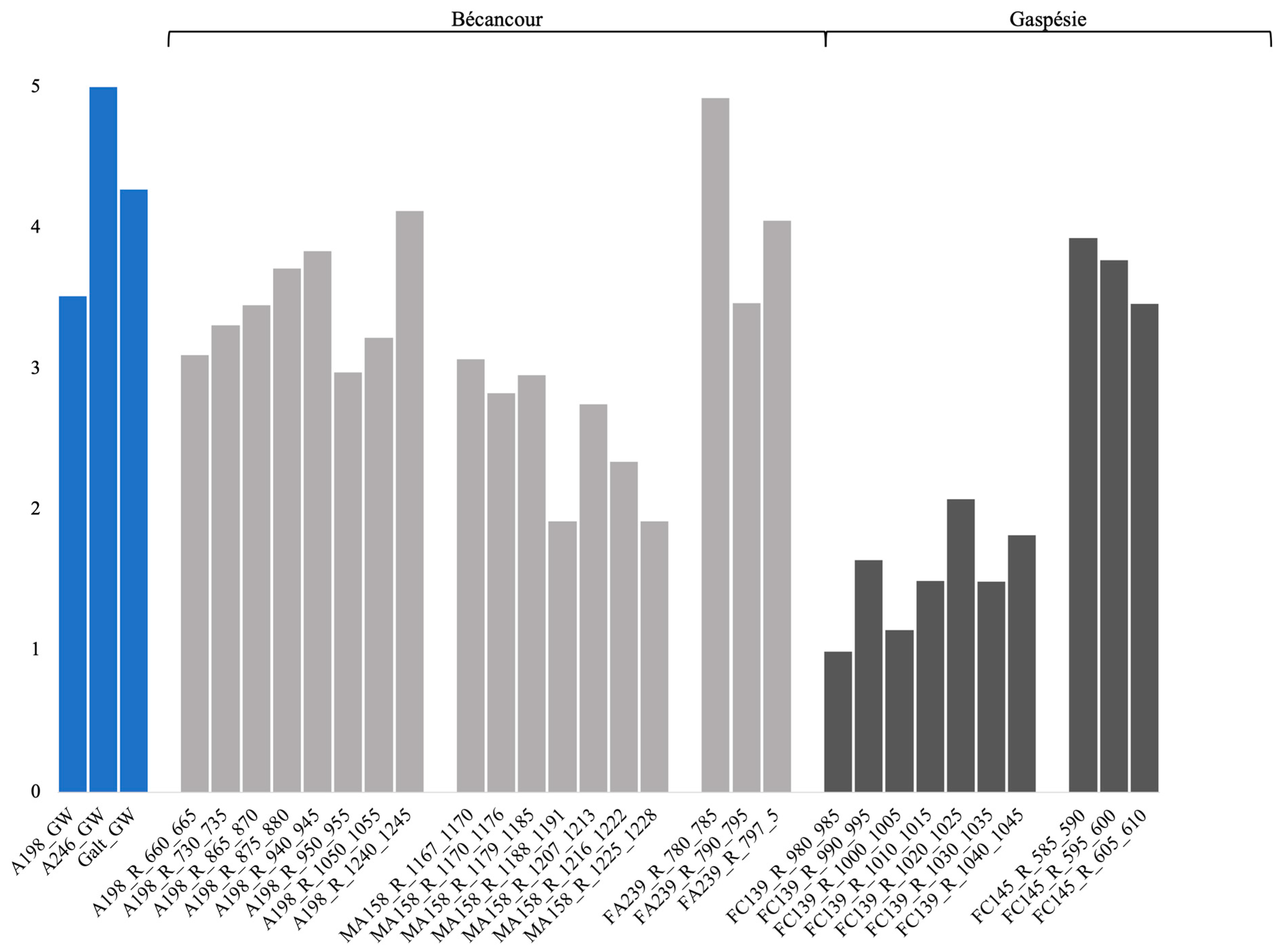
3.3. Bacterial α-Diversity Indices and Comparison
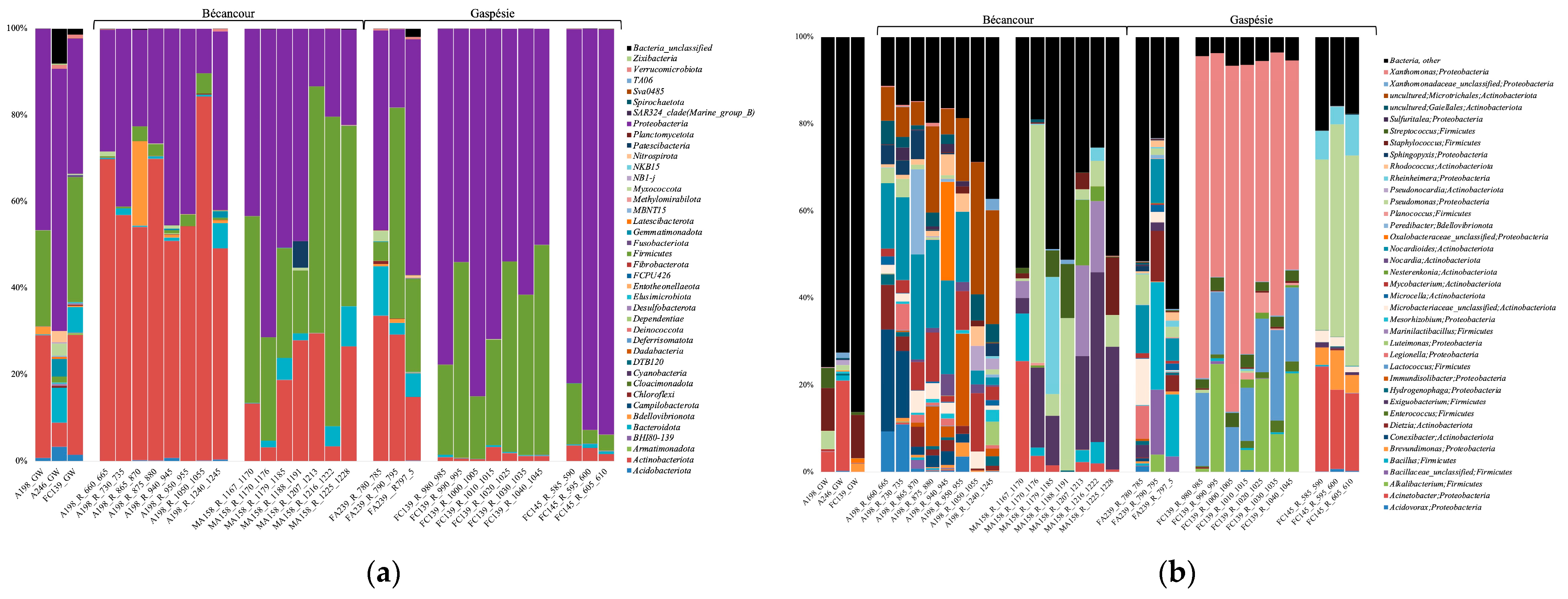
3.4. Bacterial Community Composition
3.6. Rock Bacterial Community Correlation with Environmental Parameters
3.7. Metagenomic Assembly and Predicted Metabolic Pathways in the A246 Groundwater
3.7.1. Information Systems, and Anabolic Pathways
3.7.2. Transporters
3.7.3. Carbon Utilization
3.7.4. Energy Metabolism
3.8. Bacteriophage Identification and Annotation in the A246 Groundwater
4. Discussion
4.1. Bacterial Community Diversity and Structure in the Deep Aquifers
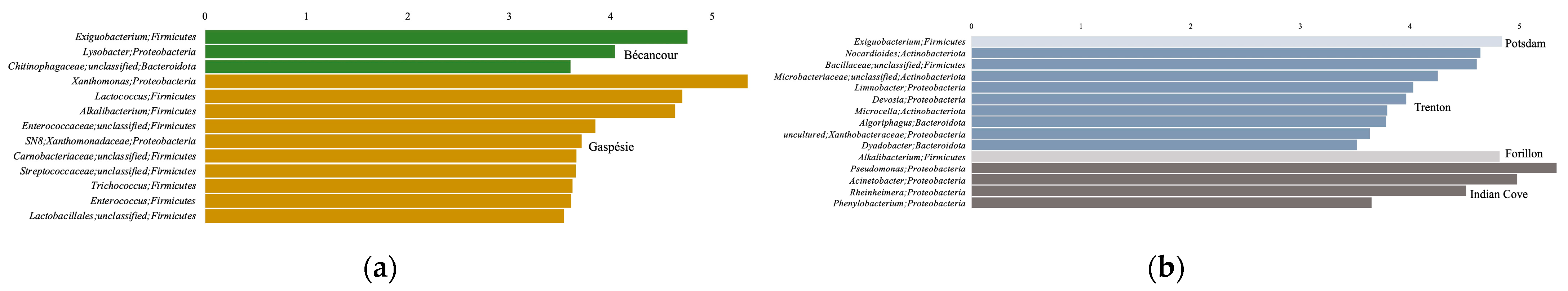
4.1.1. Differences between the Bécancour and Gaspésie Regions
4.1.2. Influence of the Abiotic Environment on the Endolithic Bacterial Community
4.2. Deep Hypersaline Groundwater Microbial Communities
4.2.1. Groundwater Community Taxonomic Diversity
4.2.2. Potential Metabolisms of the Deep Groundwater Bacteria Based on Assembled Genomes
4.2.3. Groundwater Bacteriophages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, H.J.; Zelaya, A.J.; De León, K.B.; Chakraborty, R.; Elias, D.A.; Hazen, T.C.; Arkin, A.P.; Cunningham, A.B.; Fields, M.W. Impact of hydrologic boundaries on microbial planktonic and biofilm communities in shallow terrestrial subsurface environments. FEMS Microbiol. Ecol. 2018, 94, fiy191. [Google Scholar] [CrossRef]
- Bomberg, M.; Ahonen, L. Geomicrobes: Life in Terrestrial Deep Subsurface. Front. Microbiol. 2017, 8, 103. [Google Scholar] [CrossRef]
- Jain, I.D.K.; Providenti, M.; Tanner, C.; Cord, I.; Stroes-Gascoyne, S. Characterization of microbial communities in deep groundwater from granitic rock. Can. J. Microbiol. 1997, 43, 272–283. [Google Scholar] [CrossRef]
- Krumholz, L.R. Microbial communities in the deep subsurface. Hydrogeol. J. 2000, 8, 4–10. [Google Scholar]
- Griebler, C.; Lueders, T. Microbial biodiversity in groundwater ecosystems. Freshw. Biol. 2009, 54, 649–677. [Google Scholar] [CrossRef]
- Stevens, T.O.; McKinley, J.P.; Fredricks, J.K. Bacteria Associated with Deep, Alkaline, Anaerobic Groundwaters in Southeast Washington. Microb. Ecol. 1993, 25, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Kadnikov, V.V.; Mardanov, A.V.; Beletsky, A.V.; Karnachuk, O.V.; Ravin, N.V. Microbial Life in the Deep Subsurface Aquifer Illuminated by Metagenomics. Front. Microbiol. 2020, 11, 572252. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Probst, A.J.; Elling, F.J.; Castelle, C.J.; Zhu, Q.; Elvert, M.; Birarda, G.; Holman, H.-Y.N.; Lane, K.R.; Ladd, B.; et al. Lipid analysis of CO2-rich subsurface aquifers suggests an autotrophy-based deep biosphere with lysolipids enriched in CPR bacteria. ISME J. 2020, 14, 1547–1560. [Google Scholar]
- Pinti, D.L.; Béland-Otis, C.; Tremblay, A.; Castro, M.C.; Hall, C.M.; Marcil, J.-S.; Lavoie, J.-Y.; Lapointe, R. Fossil brines preserved in the St-Lawrence Lowlands, Québec, Canada as revealed by their chemistry and noble gas isotopes. GCA 2011, 75, 4228–4243. [Google Scholar] [CrossRef]
- Globensky, Y. Gaz, Pétrole, Eau Salée—Dans les Puits Forés au Québec Entre 1860 et 1970; Service des Gites Minéraux, Report S-127AF.; Gouvernement du Québec, Ministère des Richesses Naturelles, Direction Générale des Mines: La Sarre, QC, Canada, 1972.
- Junex Inc. Internal Technical Reports. In Drilling History of Wells Galt #4 and #5; Junex Inc.: Quebec City, QC, Canada, 2016. [Google Scholar]
- Lazar, C.S.; Stoll, W.; Lehmann, R.; Herrmann, M.; Schwab, V.F.; Akob, D.M.; Nawaz, A.; Wubet, W.; Buscot, F.; Totsche, K.-U.; et al. Archaeal Diversity and CO2 Fixers in Carbonate-/Siliciclastic-Rock Groundwater Ecosystems. Archaea 2017, 2017, 2136287. [Google Scholar] [CrossRef] [Green Version]
- Direito, S.O.L.; Marees, A.; Rölling, W.F.M. Sensitive life detection strategies for low-biomass environments: Optimizing extraction of nucleic acids adsorbing to terrestrial and Mars analogue minerals. FEMS Microbiol. Ecol. 2012, 81, 111–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gantner, S.; Andersson, A.F.; Alonso-Sáez, L.; Bertilsson, S. Novel primers for 16S rRNA-based archaeal community analyses in environmental samples. J. Microbiol. Methods 2011, 84, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Stahl, D.A.; Amann, R.I. Development and Application of Nucleic Acid Probes. In Nucleic Acid Techniques in Bacterial Systematics; John Wiley & Sons: Hoboken, NJ, USA, 1991; pp. 205–248. [Google Scholar]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D. Reintroducing mothur: 10 Years Later. Appl. Environ. Microbiol. 2020, 86, e02343-19. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene data-base project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Zhou, Z.; Pan, J.; Wang, F.; Gu, J.-D.; Li, M. Bathyarchaeota: Globally distributed metabolic generalists in anoxic environments. FEMS Microbiol. Rev. 2018, 42, 639–655. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Castelle, C.J.; Probst, A.J.; Zhou, Z.; Pan, J.; Liu, Y.; Banfield, J.F.; Gu, J.-D. Insights into the ecology, evolution, and metabolism of the widespread Woesearchaeotal lineages. Microbiome 2018, 6, 102. [Google Scholar] [CrossRef]
- Pereira, M.B.; Wallroth, M.; Jonsson, V.; Kristiansson, E. Comparison of normalization methods for the analysis of metagenomic gene abundance data. BMC Genom. 2018, 19, 274. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.; Fass, J. Sickle: A Sliding-Window, Adaptive, Quality-Based Trimming Tool for FastQ Files. 2011. Available online: https://github.com/najoshi/sickle (accessed on 14 June 2023).
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013. [Google Scholar] [CrossRef]
- Kang, D.D.; Li, F.; Kirton, E.; Thomas, A.; Egan, R.; An, H.; Wang, Z. MetaBAT 2: An adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 2019, 7, e7359. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, M.; Borton, M.A.; McGivern, B.B.; Zayed, A.A.; La Rosa, S.L.; Solden, L.M.; Wrighton, K.C. DRAM for distilling microbial metabolism to automate the curation of microbiome function. Nucleic Acids Res. 2020, 48, 8883–8900. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Mao, X.; Yang, J.; Chen, X.; Mao, F.; Xu, Y. dbCAN: A web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012, 40, W445–W451. [Google Scholar] [CrossRef]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 2019, 36, 2251–2252. [Google Scholar] [CrossRef] [Green Version]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The peptidase database. Nucleic Acids Res. 2010, 38, D227–D233. [Google Scholar] [CrossRef]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Punta, M. Pfam: The protein family database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [Green Version]
- Suzek, B.E.; Huang, H.; McGarvey, P.; Mazumder, R.; Wu, C.H. UniRef: Comprehensive and non-redundant UniProt reference clusters. Bioinformatics 2007, 23, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Tang, X.; Guo, R.; Sun, Y. Accurate identification of bacteriophages from metagenomic data using Transformer. Brief. Bioinform. 2022, 23, bbac258. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Peng, C.; Liao, H.; Tang, X.A.; Sun, Y. PhaBOX: A web server for identifying and characterizing phage contigs in metagenomic data. arXiv 2023, arXiv:2303.15707. [Google Scholar]
- Shang, J.; Jiang, J.; Sun, Y. Bacteriophage classification for assembled contigs using graph convolutional network. Bioinformatics 2021, 37, i25–i33. [Google Scholar] [CrossRef] [PubMed]
- Jiarong. 2022. Virsorter2 (Version 2.2.3). Available online: https://github.com/jiarong/VirSorter2 (accessed on 14 June 2023).
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wackett, L.P.; Dodge, A.G.; Ellis, L.B.M. Microbial Genomics and the Periodic Table. Appl. Environ. Microbiol. 2004, 70, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, D.; Pohl, T.; Walter, J.; Dörner, K.; Kohlstädt, M.; Berger, A.; Spehr, V.; Friedrich, T. Assembly of the Escherichia coli NADH:ubiquinone oxidoreductase (complex I). Biochim. Biophys. Acta BBA 2008, 1777, 735–739. [Google Scholar] [CrossRef] [Green Version]
- Erhardt, H.; Steimle, S.; Muders, V.; Pohl, T.; Walter, J.; Friedrich, T. Disruption of individual nuo-genes leads to the formation of partially assembled NADH:ubiquinone oxidoreductase (complex I) in Escherichia coli. Biochim. Biophys. Acta BBA 2012, 1817, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Scotese, C.R. Atlas of Earth History; Paleogeography; PALEOMAP Project: Alington, TX, USA, 2001; Volume 1. [Google Scholar]
- de Bruijn, I.; Cheng, X.; de Jager, V.; Gómez Expósito, R.; Watrous, J.; Patel, N.; Postma, J.; Dorrestein, P.C.; Kobayashi, D.; Raaijmakers, J.M. Comparative genomics and metabolic profiling of the genus Lysobacter. BMC Genom. 2015, 16, 991. [Google Scholar] [CrossRef] [Green Version]
- Seccareccia, I.; Kost, C.; Nett, M. Quantitative Analysis of Lysobacter Predation. Appl. Environ. Microbiol. 2015, 81, 7098–7105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White III, R.A.; Soles, S.A.; Gavelis, G.; Gosselin, E.; Slater, G.F.; Lim, D.S.S.; Leander, B.; Suttle, C.A. The Complete Genome and Physiological Analysis of the Eurythermal Firmicute Exiguobacterium chiriqhucha Strain RW2 Isolated from a Freshwater Microbialite, Widely Adaptable to Broad Thermal, pH, and Salinity Ranges. Front. Microbiol. 2019, 9, 3189. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, Z.; Li, Y.; Li, X.; Guan, Z.; Zheng, J. Comparative Genomics of Exiguobacterium Reveals What Makes a Cosmopolitan Bacterium. mSystems 2021, 6, e00383-21. [Google Scholar] [CrossRef]
- Fan, X.; Yu, T.; Li, Z.; Zhang, X.-H. Luteimonas abyssi sp. nov., isolated from deep-sea sediment. Int. J. Syst. Evol. Microbiol. 2014, 64, 668–674. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Ojha, A.K.; Kumari, P.; Sundharam, S.S.; Mayilraj, S.; Krishnamurthi, S. Luteimonas padinae sp. nov., an epiphytic bacterium isolated from an intertidal macroalga. Int. J. Syst. Evol. Microbiol. 2016, 66, 5444–5451. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Nakajima, K.; Yanagi, M.; Yamamoto, Y.; Yamasato, K. Marinilactibacillus psychrotolerans gen. nov., sp. nov., a halophilic and alkaliphilic marine lactic acid bacterium isolated from marine organisms in temperate and subtropical areas of Japan. Int. J. Syst. Evol. Microbiol. 2003, 53, 711–720. [Google Scholar] [CrossRef]
- Toffin, L.; Zink, K.; Kato, C.; Pignet, P.; Bidault, A.; Bienvenu, N.; Birrien, J.-L.; Prieur, D. Marinilactibacillus piezotolerans sp. nov., a novel marine lactic acid bacterium isolated from deep sub-seafloor sediment of the Nankai Trough. Int. J. Syst. Evol. Microbiol. 2005, 55, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krick, A.; Kehraus, S.; Eberl, L.; Riedel, K.; Anke, H.; Kaesler, I.; Graeber, I.; Szewzyk, U.; Konig, G.M. A Marine Mesorhizobium sp. Produces Structurally Novel Long-Chain N-Acyl-L-Homoserine Lactones. Appl. Environ. Microbiol. 2007, 73, 3587–3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurahashi, M.; Fukunaga, Y.; Sakiyama, Y.; Harayama, S.; Yokota, A. Iamia majanohamensis gen. nov., sp. nov., an actinobacterium isolated from sea cucumber Holothuria edulis, and proposal of Iamiaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 869–873. [Google Scholar] [CrossRef] [Green Version]
- Rana, R.; Madhavan, V.N.; Saroha, T.; Bansal, K.; Kaur, A.; Sonti, R.V.; Patel, H.K.; Patil, P.B. Xanthomonas indica sp. nov., a Novel Member of Non-Pathogenic Xanthomonas Community from Healthy Rice Seeds. Curr. Microbiol. 2022, 79, 304. [Google Scholar] [CrossRef] [PubMed]
- Barcarolo, M.V.; Gottig, N.; Ottado, J.; Garavaglia, B.S. Participation of two general stress response proteins from Xanthomonas citri subsp. citri in environmental stress adaptation and virulence. FEMS Microbiol. Ecol. 2020, 96, fiaa138. [Google Scholar] [CrossRef]
- Horath, T.; Bachofen, R. Molecular Characterization of an Endolithic Microbial Community in Dolomite Rock in the Central Alps (Switzerland). Microb. Ecol. 2009, 58, 290–306. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Hirota, K.; Nodasaka, Y.; and Yumoto, I. Alkalibacterium iburiense sp. nov., an obligate alkaliphile that reduces an indigo dye. Int. J. Syst. Evol. Microbiol. 2005, 55, 1525–1530. [Google Scholar] [CrossRef]
- Ishikawa, M.; Tanasupawat, S.; Nakajima, K.; Kanamori, H.; Ishizaki, S.; Kodama, K.; Okamoto-Kainuma, A.; Koizumi, Y.; Yamamoto, Y.; Yamasato, K. Alkalibacterium thalassium sp. nov., Alkalibacterium pelagium sp. nov., Alkalibacterium putridalgicola sp. nov. and Alkalibacterium kapii sp. nov., slightly halophilic and alkaliphilic marine lactic acid bacteria isolated from marine organisms and salted foods collected in Japan and Thailand. Int. J. Syst. Evol. Microbiol. 2009, 59, 1215–1226. [Google Scholar]
- Chen, Y.-S.; Yanagida, F.; Shinohara, T. Isolation and identification of lactic acid bacteria from soil using an enrichment procedure. Lett. Appl. Microbiol. 2005, 40, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-R.; Tanner, R.S.; Schumann, P.; Weiss, N.; McKenzie, C.A.; Janssen, P.H.; Seviour, E.M.; Lawson, P.A.; Allen, T.D.; Seviour, R.J. Emended description of the genus Trichococcus, description of Trichococcus collinsii sp. nov., and reclassification of Lactosphaera pasteurii as Trichococcus pasteurii comb. nov. and of Ruminococcus palustris as Trichococcus palustris comb. nov. in the low-GMC Gram-positive bacteria. Int. J. Syst. Evol. Microbiol. 2002, 52, 1113–1126. [Google Scholar]
- Strepis, N.; Naranjo, H.D.; Meier-Kolthoff, J.; Göker, M.; Shapiro, N.; Kyrpides, N.; Klenk, H.-P.; Schaap, P.J.; Stams, A.J.M.; Sousa, D.Z. Genome-guided analysis allows the identification of novel physiological traits in Trichococcus species. BMC Genom. 2020, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Hagadorn, J.W.; Belt, E.S. Stranded in Upstate New York: Cambrian Scyphomedusae from the Potsdam Sandstone. Palaios 2008, 23, 424–441. [Google Scholar] [CrossRef]
- Roed, M.A. Geomorphologic Analysis of Forillon National Park, Quebec, Canada. Atl. Geol. 1979, 15, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, D.; Chi, G.; Fowler, M.G. The Lower Devonian Upper Gaspe Limestones in eastern Gaspe: Carbonate diagenesis and reservoir potential. Bull. Can. Pet. Geol. 2001, 49, 346–365. [Google Scholar] [CrossRef]
- Gutiérrez-Preciado, A.; Vargas-Chávez, C.; Reyes-Prieto, M.; Ordoñez, O.F.; Santos-García, D.; Rosas-Pérez, T.; Valdivia-Anistro, J.; Rebollar, E.A.; Saralegui, A.; Moya, A.; et al. The genomic sequence of Exiguobacterium chiriqhucha str. N139 reveals a species that thrives in cold waters and extreme environmental conditions. PeerJ 2017, 5, e3162. [Google Scholar] [CrossRef] [Green Version]
- Uroz, S.; Kelly, L.C.; Turpault, M.-P.; Lepleux, C.; Frey-Klett, P. The Mineralosphere Concept: Mineralogical Control of the Distribution and Function of Mineral-associated Bacterial Communities. Trends Microbiol. 2015, 23, 751–762. [Google Scholar] [CrossRef]
- Vishnivetskaya, T.A.; Petrova, M.A.; Urbance, J.; Ponder, M.; Moyer, C.L.; Gilichinsky, D.A.; Tiedje, J.A. Bacterial Community in Ancient Siberian Permafrost as Characterized by Culture and Culture-Independent Methods. Astrobiology 2006, 6, 400–414. [Google Scholar] [CrossRef]
- Barton, H.A.; Nicholas, M.; Taylor, N.M.; Kreate, M.P.; Springer, A.C.; Oehrle, S.A.; Bertog, J.L. The Impact of Host Rock Geochemistry on Bacterial Community Structure in Oligotrophic Cave Environments. IJS 2007, 36, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Gontang, E.A.; Fenical, W.; Jensen, P.R. Phylogenetic Diversity of Gram-Positive Bacteria Cultured from Marine Sediments. Appl. Environ. Microbiol. 2007, 73, 3272–3282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, M.; Tamura, T.; Yamamura, H.; Suzuki, K.; Hayakawa, M. Lysinimicrobium mangrovi gen. nov., sp. nov., an actinobacterium isolated from the rhizosphere of a mangrove. Int. J. Syst. Evol. Microbiol. 2012, 62, 1731–1735. [Google Scholar] [CrossRef] [Green Version]
- Phurbu, D.; Wang, H.; Tang, Q.; Lu, H.; Zhu, H.; Jiang, S.; Xing, P.; Wu, Q.L. Tabrizicola alkalilacus sp. nov., isolated from alkaline Lake Dajiaco on the Tibetan Plateau. Int. J. Syst. Evol. Microbiol. 2019, 69, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Spring, S.; Kampfer, P.; Schleifer, K.H. Limnobacter thiooxidans gen. nov., sp. nov., a novel thiosulfate-oxidizing bacterium isolated from freshwater lake sediment. Int. J. Syst. Evol. Microbiol. 2001, 51, 1463–1470. [Google Scholar] [CrossRef] [Green Version]
- Hütz, A.; Schubert, K.; Overmann, J. Thalassospira sp. Isolated from the Oligotrophic Eastern Mediterranean Sea Exhibits Chemotaxis toward Inorganic Phosphate during Starvation. Appl. Environ. Microbiol. 2011, 77, 4412–4421. [Google Scholar] [CrossRef] [Green Version]
- Sahin, N.; Portillo, M.C.; Kato, Y.; Schumann, P. Description of Oxalicibacteriumhorti sp. nov. and Oxalicibacterium faecigallinarum sp. nov., new aerobic, yellow-pigmented, oxalotrophic bacteria. FEMS Microbiol. Lett. 2009, 296, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Gomez, W.S.; Quintana, P.; Gomez-Cornelio, S.; Garcia-Solis, C.; Sierra-Fernandez, A.; Ortega-Morales, O.; De la Rosa-Garcia, S.C. Calcium oxalates in biofilms on limestone walls of Maya buildings in Chichén Itzá, Mexico. Environ. Earth Sci. 2018, 77, 230. [Google Scholar] [CrossRef]
- Sonke, A.; Trembath-Reichert, E. Expanding the taxonomic and environmental extent of an underexplored carbon metabolism—Oxalotrophy. Front. Microbiol. 2023, 14, 1161937. [Google Scholar] [CrossRef]
- Bauman, P. Isolation of Acinetobacter from Soil and Water. J. Bacteriol. 1968, 96, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Lingens, F.; Blecher, R.; Blecher, H.; Blobel, F.; Eberspacher, J.; Froher, C.; Gorisch, H.; Gorisch, H.; Layh, G. Phenylobacterium immobile gen. nov. sp. nov. a Gram-Negative Bacterium That Degrades the Herbicide Chloridazon. IUMS 1985, 35, 26–39. [Google Scholar] [CrossRef]
- Sah, S.; Singh, R. Phylogenetical coherence of Pseudomonas in unexplored soils of Himalayan region. 3 Biotech 2016, 6, 170. [Google Scholar] [CrossRef] [Green Version]
- Markande, A.R.; Vemuluri, V.R.; Shouche, Y.S.; Nerurkar, A.S. Characterization of Solibacillus silvestris strain AM1 that produces amyloid bioemulsifier. J. Basic Microbiol. 2018, 58, 523–531. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Zhang, J.; Lai, Q.; Shao, Z.; Zhu, H. Sphingorhabdus soli sp. nov., isolated from Arctic soil. Int. J. Syst. Evol. Microbiol. 2020, 70, 1610–1616. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, S.; Cheng, Y.; Ding, L.; Li, S.; Peng, N.; He, S. Rheinheimera mangrovi sp. nov., a bacterium isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2020, 70, 6188–6194. [Google Scholar] [CrossRef] [PubMed]
- Loveland-Curtze, J.; Miteva, V.I.; Brenchley, J.E. Herminiimonas glaciei sp. nov., a novel ultramicrobacterium from 3042 m deep Greenland glacial ice. Int. J. Syst. Evol. Microbiol. 2009, 59, 1272–1277. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.; Rainey, F.A.; Nobre, M.F.; Pinhal, I.; Folhasa, F.; da Costaa, M.S. Herminiimonas fonticola gen. nov., sp. nov., a Betaproteobacterium isolated from a source of bottled mineral water. Syst. Appl. Microbiol. 2005, 28, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects—A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Wood, M. A mechanism of aluminium toxicity to soil bacteria and possible ecological implications. Plant Soil 1995, 171, 63–69. [Google Scholar] [CrossRef]
- Marnocha, C.L.; Dixon, J.C. Endolithic bacterial communities in rock coatings from Karkevagge, Swedish Lapland. FEMS Microbiol. Ecol. 2014, 90, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Horiike, T.; Dotsuta, Y.; Nakano, Y.; Ochiai, A.; Utsunomiya, S.; Ohnuki, T.; Yamashita, M. Removal of Soluble Strontium via Incorporation into Biogenic Carbonate Minerals by Halophilic Bacterium Bacillus sp. Strain TK2d in a Highly Saline Solution. Appl. Environ. Microbiol. 2017, 83, e00855-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleary, A.; Lloyd, J.R.; Newsome, L.; Shaw, S.; Boothman, C.; Boshoff, G.; Atherton, N.; Morris, K. Bioremediation of strontium and technetium contaminated groundwater using glycerol phosphate. Chem. Geol. 2019, 509, 213–222. [Google Scholar] [CrossRef]
- Barth-Naftilan, E.; Sohng, J.; Saiers, J.E. Methane in groundwater before, during, and after hydraulic fracturing of the Marcellus Shale. Proc. Natl. Acad. Sci. USA 2018, 115, 6970–6975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, P.B.; Kulongoski, J.T.; Wright, M.T.; Land, M.T.; Landon, M.K.; Cozzarelli, I.M.; Vengosh, A.; Aiken, G.R. Preliminary Results from Exploratory Sampling of Wells for the California Oil, Gas, and Groundwater Program; Open-File Report 2016–1100 Version 1.1 2017; U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 2017. Available online: https://pubs.er.usgs.gov/publication/ofr20161100 (accessed on 14 June 2023).
- Osudar, R.; Matou, A.; Alawi, M.; Wagner, D.; Bussmann, I. Environmental factors affecting methane distribution and bacterial methane oxidation in the German Bight (North Sea). Estuar. Coast. Shelf Sci. 2015, 160, 10–21. [Google Scholar] [CrossRef]
- Pal, S.; Biswas, R.; Misra, A.; Sar, A.; Banerjee, S.; Mukherjee, P.; Dam, B. Poorly known microbial taxa dominate the microbiome of hypersaline Sambhar Lake salterns in India. Extremophiles 2020, 24, 875–885. [Google Scholar] [CrossRef]
- Kubo, M.; Hiroe, J.; Murukam, M.; Fukami, H.; Tachiki, T. Treatment of Hypersaline-Containing Wastewater with Salt-Tolerant Microorganisms. J. Biosci. Bioeng. 2001, 91, 224. [Google Scholar] [CrossRef]
- Vera-Gargallo, B.; Hernandez, M.; Dumont, M.G.; Ventosa, A. Thrive or survive: Prokaryotic life in hypersaline soils. Environ. Microbiome 2023, 18, 17. [Google Scholar] [CrossRef]
- Jacob, J.H.; Hussein, E.I.; Shakhatreh, M.A.K.; Cornelison, C.T. Microbial community analysis of the hypersaline water of the Dead Sea using high-throughput amplicon sequencing. MicrobiologyOpen 2017, 6, e500. [Google Scholar] [CrossRef] [Green Version]
- Farias, M.E.; Revale, S.; Mancini, E.; Ordonez, O.; Turjanski, A.; Cortez, N.; Vazquez, M.P. Genome Sequence of Sphingomonas sp. S17, Isolated from an Alkaline, Hyperarsenic, and Hypersaline Volcano-Associated Lake at High Altitude in the Argentinean Puna. J. Bacteriol. 2011, 193, 3686–3687. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Han, R.; Long, Q.; Gao, X.; Xing, J.; Shen, G.; Li, Y.; Wang, R. An evaluation of the core bacterial communities associated with hypersaline environments in the Qaidam Basin, China. Arch. Microbiol. 2020, 202, 2093–2103. [Google Scholar] [CrossRef]
- Didari, M.; Bagheri, M.; Amoozegar, M.A.; Bouzari, S.; Babavalian, H.; Tebyanian, H.; Hassanshahian, M.; Ventosa, A. Diversity of halophilic and halotolerant bacteria in the largest seasonal hypersaline lake (Aran-Bidgol-Iran). J. Environ. Health Sci. Eng. 2020, 18, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.Z.; Choi, G.-M.; Kim, S.Y.; Choi, K.D.; Im, W.-T. Sphingomonas agri sp. nov., a bacterium isolated from soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Olaya-Abril, A.; Hidalgo-Carrillo, J.; Luque-Almagro, V.M.; Fuentes-Almagro, C.; Urbano, F.J.; Moreno-Vivián, C.; David, J.; Richardson, D.J.; Roldán, M.D. Effect of pH on the denitrification proteome of the soil bacterium Paracoccus denitrificans PD1222. Sci. Rep. 2021, 11, 17276. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Y.; Wang, X.; Wang, G. Hyphomicrobium album sp. nov., isolated from mountain soil and emended description of genus Hyphomicrobium. Arch. Microbiol. 2021, 203, 5931–5936. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Anand, U.; Jha, N.K.; Korzeniewska, E.; Bontempi, E.; Prockow, J.; Dey, A. The soil bacterium, Corynebacterium glutamicum, from biosynthesis of value-added products to bioremediation: A master of many trades. Environ. Res. 2022, 2013, 113622. [Google Scholar] [CrossRef]
- Luef, B.; Frischkorn, K.R.; Wrighton, K.C.; Holman, H.-Y.N.; Birarda, G.; Thomas, B.C.; Singh, A.; Williams, K.H.; Siegerist, C.E.; Tringe, S.G.; et al. Diverse uncultivated ultra-small bacterial cells in groundwater. Nat. Commun. 2015, 6, 6372. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, A.-G.U.; Thomsen, T.R.; Lomstein, B.A.; Jørgensen, N.O.G. Bacterial influence on amino acid enantiomerization in a coastal marine sediment. Limnol. Oceanogr. 2001, 46, 1358–1369. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, Y.; Zhou, R.; Feng, J.; Zhang, K.; Li, X.; Ma, X.; Dietrich, A.M. Occurrence of Free Amino Acids in the Source Waters of Zhejiang Province, China, and Their Removal and Transformation in Drinking Water Systems. Water 2020, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Xie, E.; Wei, X.; Ding, A.; Zheng, L.; Wu, X.; Anderson, B. Short-Term Effects of Salt Stress on the Amino Acids of Phragmites australis Root Exudates in Constructed Wetlands. Water 2020, 12, 569. [Google Scholar] [CrossRef] [Green Version]
- Valk, L.C.; Luttik, M.A.H.; de Ram, C.; Pabst, M.; van den Broek, M.; van Loosdrecht, M.C.M.; Pronk, J.T. A Novel D-Galacturonate Fermentation Pathway in Lactobacillus suebicus Links Initial Reactions of the Galacturonate-Isomerase Route with the Phosphoketolase Pathway. Front. Microbiol. 2020, 10, 3027. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Laroche, C.; Michaud, P. Galactans and Its Applications. In Polysaccharides; Ramawat, K., Mérillon, J.M., Eds.; Springer: Cham, Switzerland, 2014; pp. 1–37. [Google Scholar]
- Schroder, R.; Atkinson, R.G.; Redgwell, R.G. Re-interpreting the role of endo-b-mannanases as mannan endotransglycosylase/hydrolases in the plant cell wall. Ann. Bot. 2009, 104, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef] [Green Version]
- Eberta, B.; Rautengarten, C.; Heazlewood, J.L. GDP-L-fucose transport in plants: The missing piece. Channels 2017, 11, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Curry, T.M.; Pena, M.J.; Urbanowicz, B.R. An update on xylan structure, biosynthesis, and potential commercial applications. Cell Surf. 2023, 9, 100101. [Google Scholar] [CrossRef]
- Kim, S.-J.; Chandrasekar, B.; Rea, A.C.; Keegstra, K. The synthesis of xyloglucan, an abundant plant cell wall polysaccharide, requires CSLC function. Proc. Natl. Acad. Sci. USA 2020, 117, 20316–20324. [Google Scholar] [CrossRef]
- Fernando, L.D.; Widanage, M.C.D.; Penfield, J.; Lipton, A.S.; Washton, N.; Latgé, J.-P.; Wang, P.; Liqun Zhang, L.; Wang, T. Structural Polymorphism of Chitin and Chitosan in Fungal Cell Walls from Solid-State NMR and Principal Component Analysis. Front. Mol. Biosci. 2021, 8, 727053. [Google Scholar] [CrossRef]
- Gibson, T.M. Precise age of Bangioporpha pubescens dates the origin of eukaryotic photosynthesis. Geology 2018, 46, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.L.; Puttick, M.N.; Clark, J.W.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Yang, Z.; Schneider, H.; Donoghue, P.C.J. The timescale of early land plant evolution. Proc. Natl. Acad. Sci. USA 2018, 115, E2274–E2283. [Google Scholar] [CrossRef] [Green Version]
- Bottomley, D.J. A Review of Theories on the Origins of Saline Waters and Brines in the Canadian Precambrian Shield (INFO—0631). Canada; International Nuclear Information System (INIS): Vienna, Austria, 1996; Volume 27, 32p. [Google Scholar]
- Carignan, J.; Garirpy, C.; Hillaire-Marcel, C. Hydrothermal fluids during Mesozoic reactivation of the St. Lawrence rift system, Canada: C, O, Sr and Pb isotopic characterization. Chem. Geol. 1997, 137, 1–21. [Google Scholar] [CrossRef]
- Jojima, T.; Igari, T.; Noburyu, R.; Watanabe, A.; Suda, M.; Inui, M. Coexistence of the Entner–Doudoroff and Embden–Meyerhof–Parnas pathways enhances glucose consumption of ethanol-producing Corynebacterium glutamicum. Biotechnol. Biofuels 2021, 14, 45. [Google Scholar] [CrossRef]
- Pastor, J.M.; Borges, N.; Pagán, J.P.; Castano-Cerezo, S.; Csonka, L.N.; Goodner, B.W.; Reynolds, K.A.; Gonçalves, L.G.; Argandoña, M.; Nieto, J.J.; et al. Fructose metabolism in Chromohalobacter salexigens: Interplay between the Embden–Meyerhof–Parnas and Entner–Doudoroff pathways. Microb. Cell Factories 2019, 18, 134. [Google Scholar] [CrossRef] [Green Version]
- Brunner, F.; Dean, L.; Mireault, R. Estimate of Reservoir Pressure, Formation Permeability and Wellbore “Skin” for DST TESTS for 5 Wells of Bécancour Area; St. Lawrence Platform: Quebec City, QC, USA, 2010. [Google Scholar]
- Huber, H.; Gallenberger, M.; Jahn, U.; Eylert, E.; Berg, I.A.; Kockelkorn, D.; Eisenreich, W.; Fuchs, G. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis. Proc. Natl. Acad. Sci. USA 2008, 105, 7851–7856. [Google Scholar] [CrossRef]
- Kim, S.; Lindner, S.N.; Aslan, S.; Yishai, O.; Wenk, S.; Schann, K.; Bar-Even, A. Growth of E. coli on formate and methanol via the reductive glycine pathway. Nat. Chem. Biol. 2020, 16, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Guedes, I.A.; Hornung, B.; Boeren, S.; Lawson, C.E.; Sousa, D.Z.; Bar-Even, A.; Claassens, N.J.; Stams, A.J.M. The reductive glycine pathway allows autotrophic growth of Desulfovibrio desulfuricans. Nat. Commun. 2020, 11, 5090. [Google Scholar] [CrossRef]
- Mohammadipanah, F.; Hamedi, J.; Dehhaghi, M. Halophilic Bacteria: Potentials and Applications in Biotechnology. In Halophiles, Sustainable Development and Biodiversity 6; Maheshwari, D.K., Saraf, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 277–321. [Google Scholar]
- Roberts, M.F. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005, 1, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imhoff, J.F. Osmoregulation and compatible solutes in eubacteria. FEMS Microbiol. Rev. 1986, 39, 57–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, D.; Liao, Z.; Zhaoa, B. Draft Genome Sequence of Alkalicoccus halolimnae BZ-SZXJ29T, a Moderately Halophilic Bacterium Isolated from a Salt Lake. Microbiol. Resour. Announc. 2020, 9, e00500-20. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.-W. Tailed bacteriophages: The order Caudovirales. Adv. Virus Res. 1999, 51, 135–201. [Google Scholar]
- Antunes, A.; Alam, I.; Simoes, M.F.; Daniels, C.; Ferreira, A.J.S.; Siam, R.; El-Dorry, H.; Baji, V.B. First Insights into the Viral Communities of the Deep-sea Anoxic Brines of the Red Sea. Genom. Proteom. Bioinform. 2015, 13, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Motlagh, A.M.; Bhattacharjee, A.S.; Coutinho, F.H.; Dutilh, B.E.; Casjens, S.R.; Goel, R.K. Insights of Phage-Host Interaction in Hypersaline Ecosystem through Metagenomics Analyses. Front. Microbiol. 2017, 8, 352. [Google Scholar] [CrossRef] [Green Version]
- Hylling, O.; Carstens, A.B.; Kot, W.; Hansen, M.; Neve, H.; Franz, C.M.A.P.; Johansen, A.; Lea Ellegaard-Jensen, L.; Hansen, L.H. Two novel bacteriophage genera from a groundwater reservoir highlight subsurface environments as underexplored biotopes in bacteriophage ecology. Sci. Rep. 2020, 10, 11879. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Roux, S.; Zhang, H.; Prieto, A.; Soneja, D.; Chandonia, J.-M.; Spencer, S.; Wu, X.; Altenburg, S.; Fields, M.W.; et al. Ecogenomics of Groundwater Phages Suggests Niche Differentiation Linked to Specific Environmental Tolerance. mSystems 2021, 6, e00537-21. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, B.; Bhattacharjee, A.S.; Coutinho, F.H.; Goel, R.K. Viruses and Their Interactions with Bacteria and Archaea of Hypersaline Great Salt Lake. Front. Microbiol. 2021, 12, 701414. [Google Scholar] [CrossRef] [PubMed]
| Sample Name | Depth (mbs) | Geological Layer |
|---|---|---|
| Bécancour A198 rock | 660–665 | Lorraine |
| 730–735 | Utica | |
| 865–870 | Black River | |
| 875–880 | Grès Ordovicien | |
| 940–945 | Beekmantown | |
| 950–955 | Beekmantown | |
| 1050–1055 | Potsdam | |
| 1240–1245 | Potsdam | |
| Bécancour A198 groundwater | 937–955 | Beekmantown |
| Bécancour A246 groundwater | 875 | Black River |
| Bécancour MA158 rock | 1167–1170 | Potsdam |
| 1170–1176 | Potsdam | |
| 1179–1185 | Potsdam | |
| 1188–1191 | Potsdam | |
| 1207–1213 | Potsdam | |
| 1216–1222 | Potsdam | |
| 1225–1228 | Potsdam | |
| Bécancour FA239 rock | 780–785 | Trenton |
| 790–795 | Trenton | |
| 797.5 | Trenton | |
| Gaspésie FC139 rock | 980–985 | Forillon |
| 990–995 | Forillon | |
| 1000–1005 | Forillon | |
| 1010–1015 | Forillon | |
| 1020–1025 | Forillon | |
| 1030–1035 | Forillon | |
| 1040–1045 | Forillon | |
| Gaspésie FC139 groundwater | 1002 | Forillon |
| Gaspésie FC145 rock | 585–590 | Indian Cove |
| 595–600 | Indian Cove | |
| 605–610 | Indian Cove |
| A198 | A246 | |
|---|---|---|
| pH | 6.01 | 8.45 |
| Temperature (°C) | 17.5 | 25.6 |
| Dissolved oxygen (%) | 13.4 | 15.5 |
| Dissolved organic carbon (mg/L) | 0.09 | 16.2 |
| CO2 (ppm) | 616.97 | 858.36 |
| CH4 (ppm) | 189,148.77 | 1,198,300.5 |
| δ13C CH4 (permil) | −39.74 | −35.097 |
| Shannon | Chao1 | Evenness | |
|---|---|---|---|
| All samples | 0.05389 | 0.02076 | 0.0002886 |
| Rocks | 0.03583 | 0.04089 | 3 × 10−4 |
| All Samples | |||||
|---|---|---|---|---|---|
| Df | SumOfSqs | R2 | F | Pr(>F) | |
| Habitat | 1 | 0.6373 | 0.04651 | 1.6170 | 0.038 |
| Site | 1 | 2.0283 | 0.14804 | 5.1463 | 0.001 |
| Residual | 28 | 11.0355 | 0.80545 | ||
| Total | 30 | 13.7010 | 1.00000 | ||
| Rock samples | |||||
| Df | SumOfSqs | R2 | F | Pr(>F) | |
| Habitat | 1 | 0.8697 | 0.09383 | 2.3047 | 0.022 |
| Geology | 2 | 1.6067 | 0.17335 | 2.1289 | 0.002 |
| Residual | 18 | 6.7922 | 0.73282 | ||
| Total | 21 | 9.2686 | 1.00000 | ||
| Major Cations and Biological Transition Metals | |||||
|---|---|---|---|---|---|
| Df | SumOfSqs | R2 | F | Pr(>F) | |
| Mg | 1 | 0.4238 | 0.9852 | 0.464 | 0.464 |
| S | 1 | 0.5869 | 1.3644 | 0.091 | 0.091 |
| K | 1 | 0.7321 | 1.7018 | 0.024 | 0.024 |
| Ca | 1 | 0.4033 | 0.9375 | 0.561 | 0.561 |
| V | 1 | 0.4341 | 1.0091 | 0.395 | 0.395 |
| Mn | 1 | 0.3992 | 0.9279 | 0.518 | 0.518 |
| Fe | 1 | 0.5528 | 1.2851 | 0.144 | 0.144 |
| Ni | 1 | 0.4296 | 0.9987 | 0.445 | 0.445 |
| Cu | 1 | 0.5852 | 1.3604 | 0.095 | 0.095 |
| Zn | 1 | 0.3560 | 0.8275 | 0.743 | 0.743 |
| Residual | 14 | 6.0226 | |||
| Specialized uses | |||||
| Df | SumOfSqs | R2 | F | Pr(>F) | |
| Al | 1 | 0.8212 | 2.0807 | 0.005 | 0.005 |
| Si | 1 | 0.4860 | 1.2315 | 0.165 | 0.165 |
| Ti | 1 | 0.5320 | 1.3481 | 0.096 | 0.096 |
| Cr | 1 | 0.5855 | 1.4837 | 0.062 | 0.062 |
| As | 1 | 0.4826 | 1.2228 | 0.154 | 0.154 |
| Rb | 1 | 0.3459 | 0.8766 | 0.679 | 0.679 |
| Sr | 1 | 0.8256 | 2.0919 | 0.002 | 0.002 |
| Y | 1 | 0.4166 | 1.0557 | 0.364 | 0.364 |
| Pb | 1 | 0.5104 | 1.2933 | 0.120 | 0.120 |
| Residual | 15 | 5.9197 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagnon, J.-C.; Beauregard-Tousignant, S.; Marcil, J.-S.; Lazar, C.S. Deep Isolated Aquifer Brines Harbor Atypical Halophilic Microbial Communities in Quebec, Canada. Genes 2023, 14, 1529. https://doi.org/10.3390/genes14081529
Gagnon J-C, Beauregard-Tousignant S, Marcil J-S, Lazar CS. Deep Isolated Aquifer Brines Harbor Atypical Halophilic Microbial Communities in Quebec, Canada. Genes. 2023; 14(8):1529. https://doi.org/10.3390/genes14081529
Chicago/Turabian StyleGagnon, Jean-Christophe, Samuel Beauregard-Tousignant, Jean-Sébastien Marcil, and Cassandre Sara Lazar. 2023. "Deep Isolated Aquifer Brines Harbor Atypical Halophilic Microbial Communities in Quebec, Canada" Genes 14, no. 8: 1529. https://doi.org/10.3390/genes14081529






