Assessing the Differential Abundance of Maternal Circulating MicroRNAs or Interferon-Stimulated Genes with Early Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Pregnancy Detection
2.3. RNA Isolation
2.4. RT-qPCR for INF Stimulated Genes
2.5. RT-qPCR for Specific miRNA
2.6. Statistical Analysis
3. Results
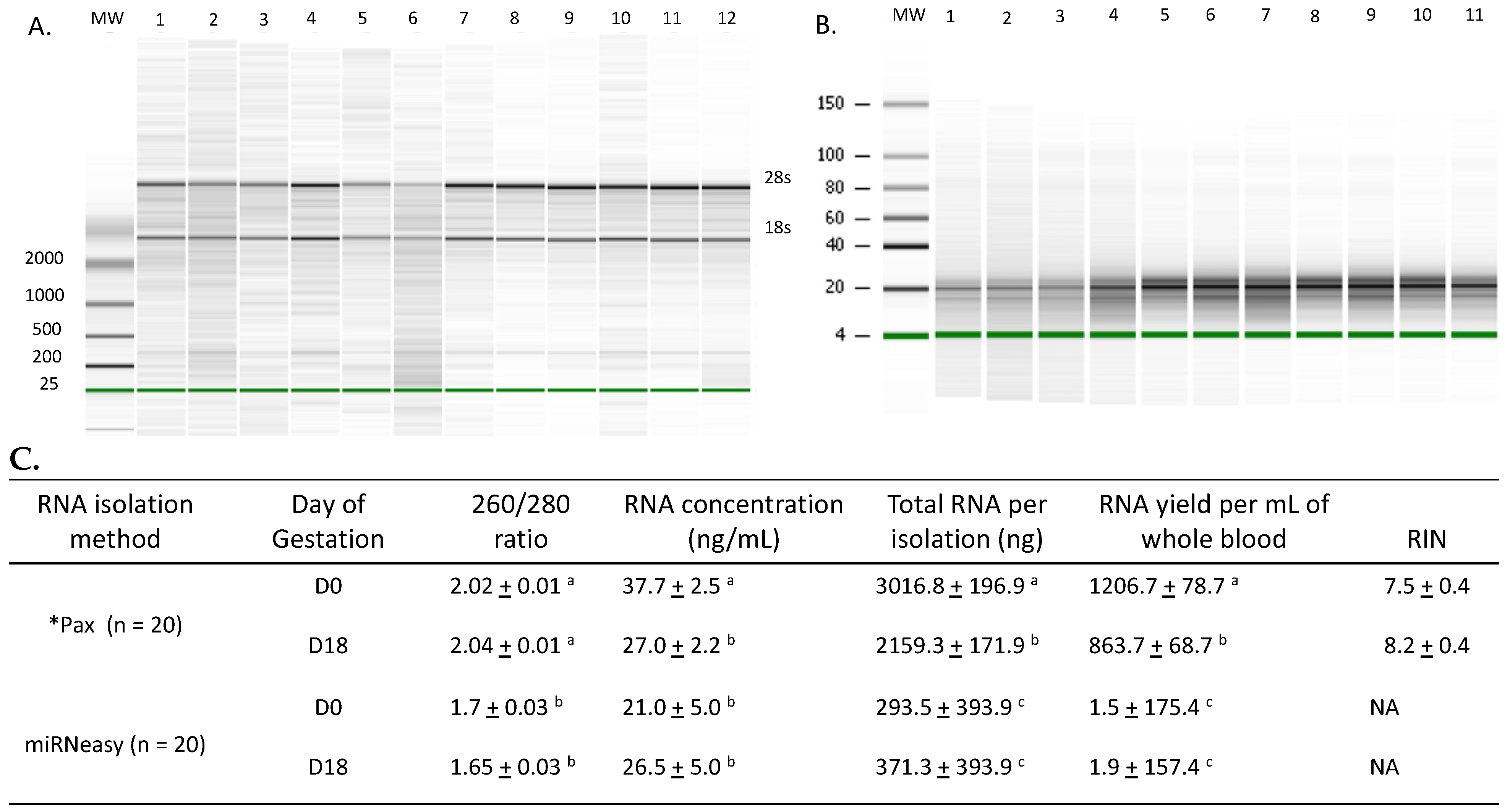
3.1. RNA Isolation
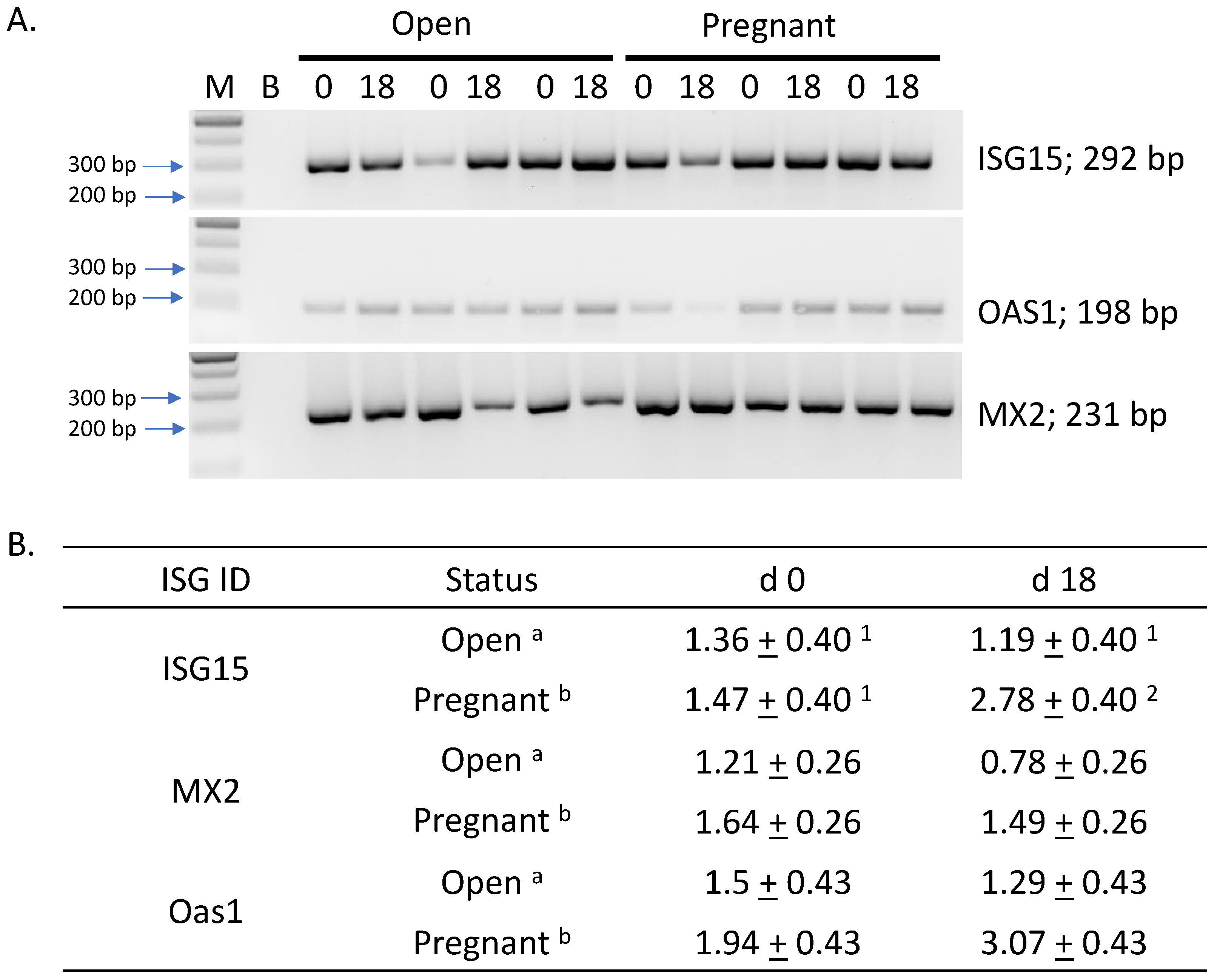
3.2. RT-PCR Analysis of INF Stimulated Genes
3.3. RT-qPCR Analysis of miRNA Abundance
3.4. Receiver Operator Characteristic Curves
4. Discussion
4.1. miRNA as Markers for Pregnancy Detection and at Risk Pregnancies
4.2. Assessment of Isolated RNA Quality
4.3. Relative Abundance and Predictive Value of Interferon Stimulated Genes
4.4. Relative Abundance and Predictive Value of Circulating miRNA
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, D.K.; van Leeuwen, J.; Beaumont, S.; Berg, M.; Pfeffer, P.L. Embryo loss in cattle between Days 7 and 16 of pregnancy. Therioenology 2010, 73, 250–260. [Google Scholar] [CrossRef]
- Diskin, M.G.; Waters, S.M.; Parr, M.H.; Kenny, D.A. Pregnancy losses in cattle: Potential for improvement. Reprod. Fertil. Dev. 2016, 28, 83–93. [Google Scholar] [CrossRef]
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. BOARD INVITED REVIEW: Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef]
- Alexopoulos, N.I.; Maddox-Hyttel, P.; Tveden-Nyborg, P.; D’Cruz, N.T.; Tecirlioglu, T.R.; Cooney, M.A.; Schauser, K.; Holland, M.K.; French, A.J. Developmental disparity between in vitro-produced and somatic cell nuclear transfer bovine days 14 and 21 embryos: Implications for embryonic loss. Reproduction 2008, 136, 433–445. [Google Scholar] [PubMed]
- Alexopoulos, N.I.; French, A.J. The prevalence of embryonic remnants following the recovery of post-hatching bovine embryos produced in vitro or by somatic cell nuclear transfer. Anim. Reprod. Sci. 2009, 114, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.; Donadeu, F.X. Circulating miRNA signatures of early pregnancy in cattle. BMC Genom. 2016, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- De Bem, T.H.C.; da Silveira, J.C.; Sampaio, R.V.; Sangalli, J.R.; Oliveira, M.L.F.; Ferreira, R.M.; Silva, L.A.; Perecin, F.; King, W.A.; Meirelles, F.V.; et al. Low levels of exosomal-miRNAs in maternal blood are associated with early pregnancy loss in cloned cattle. Sci. Rep. 2017, 7, 14319. [Google Scholar] [CrossRef] [PubMed]
- Pohler, K.G.; Green, J.A.; Moley, L.A.; Gunewardena, S.; Hung, W.T.; Payton, R.R.; Hong, X.; Christenson, L.K.; Geary, T.W.; Smith, M.F. Circulating microRNA as candidates for early embryonic viability in cattle. Mol. Reprod. Dev. 2017, 84, 731–743. [Google Scholar] [CrossRef]
- Markkandan, K.; Ahn, K.; Lee, D.J.; Kim, T.I.; Dang, C.; Hong, S.E.; Yoon, H.B.; Lim, H.J.; Hong, C.P. Profiling and identification of pregnancy-associated circulating microRNAs in dairy cattle. Genes Genom. 2018, 40, 1111–1117. [Google Scholar] [CrossRef]
- Dalmaso de Melo, G.; Mello, B.P.; Ferreira, C.A.; Souto Godoy Filho, C.A.; Rocha, C.C.; Silva, A.G.; Reese, S.T.; Madureira, E.H.; Pohler, K.G.; Pugliesi, G. Applied use of interferon-tau stimulated genes expression in polymorphonuclear cells to detect pregnancy compared to other early predictors in beef cattle. Theriogenology 2020, 152, 94–105. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Czerniawska-Piatkowska, E.; Wrzecinska, M. The Importance of Interferon-Tau in the Diagnosis of Pregnancy. Biomed. Res. Int. 2021, 2021, 9915814. [Google Scholar] [CrossRef]
- Thatcher, W.W.; Bartol, F.F.; Knickerbocker, J.J.; Curl, J.S.; Wolfenson, D.; Bazer, F.W.; Roberts, R.M. Maternal recognition of pregnancy in cattle. J. Dairy Sci. 1984, 67, 2797–2811. [Google Scholar] [CrossRef]
- Helmer, S.D.; Hansen, P.J.; Thatcher, W.W.; Johnson, J.W.; Bazer, F.W. Intrauterine infusion of highly enriched bovine trophoblast protein-1 complex exerts an antiluteolytic effect to extend corpus luteum lifespan in cyclic cattle. J. Reprod. Fertil. 1989, 87, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Hansen, T.R.; Malathy, P.V.; Anthony, R.V.; Polites, H.G.; Marotti, K.R.; Roberts, R.M. Molecular cloning and characterization of complementary deoxyribonucleic acids corresponding to bovine trophoblast protein-1: A comparison with ovine trophoblast protein-1 and bovine interferon-α II. Mol. Endocrinol. 1989, 3, 127–139. [Google Scholar] [PubMed]
- Thatcher, W.W.; Hansen, P.J.; Gross, T.S.; Helmer, S.D.; Plante, C.; Bazer, F.W. Antiluteolytic effects of bovine trophoblast protein-1. J. Reprod. Fertil. Suppl. 1989, 37, 91–99. [Google Scholar] [PubMed]
- Gifford, C.A.; Racicot, K.; Clark, D.S.; Austin, K.J.; Hansen, T.R.; Lucy, M.C.; Davies, C.J.; Ott, T.L. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J. Dairy Sci. 2007, 90, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Green, J.C.; Okamura, C.S.; Poock, S.E.; Lucy, M.C. Measurement of interferon-tau (IFN-tau) stimulated gene expression in blood leukocytes for pregnancy diagnosis within 18–20d after insemination in dairy cattle. Anim. Reprod. Sci. 2010, 121, 24–33. [Google Scholar] [CrossRef]
- Yoshino, H.; Toji, N.; Sasaki, K.; Koshi, K.; Yamagishi, N.; Takahashi, T.; Ishiguro-Oonuma, T.; Matsuda, H.; Yamanouchi, T.; Hashiyada, Y.; et al. A predictive threshold value for the diagnosis of early pregnancy in cows using interferon-stimulated genes in granulocytes. Theriogenolgy 2018, 107, 188–193. [Google Scholar] [CrossRef]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Plasterk, R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids—The mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef]
- Xu, L.; Yang, B.F.; Ai, J. MicroRNA transport: A new way in cell communication. J. Cell. Phys. 2013, 228, 1713–1719. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Ding, X.; Xiong, C. Presence and characterization of cell-free seminal RNA in healthy individuals: Implications for noninvasive disease diagnosis and gene expression studies of the male reproductive system. Clin. Chem. 2009, 55, 1967–1976. [Google Scholar] [PubMed]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 2010, 50, 298–301. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Jiang, H.; Wen, Y.; Hu, L.; Miao, T.; Zhang, M.; Dong, J. Serum MicroRNAs as Diagnostic Biomarkers for Macrosomia. Reprod. Sci. 2015, 22, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Miura, S.; Yamasaki, K.; Higashijima, A.; Kinoshita, A.; Yoshiura, K.; Masuzaki, H. Identification of pregnancy-associated microRNAs in maternal plasma. Clin. Chem. 2010, 56, 1767–1771. [Google Scholar] [CrossRef]
- Ioannidis, J.; Donadeu, F.X. Changes in circulating microRNA levels can be identified as early as day 8 of pregnancy in cattle. PLoS ONE 2017, 12, e0174892. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhn, S.; Salilew-Wondim, D.; Hoelker, M.; Held-Hoelker, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Tesfaye, D. Exploring maternal serum microRNAs during early pregnancy in cattle. Theriogenology 2018, 121, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Guo, S.; Jiang, K.; Zhang, T.; Wu, H.; Qiu, C.; Deng, G. MiRNA profiling of plasma-derived exosomes from dairy cows during gestation. Theriogenology 2019, 130, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Hammerle-Fickinger, A.; Riedmaier, I.; Pfaffl, M.W. mRNA and microRNA quality control for RT-qPCR analysis. Methods 2010, 50, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Blondal, T.; Jensby Nielsen, S.; Baker, A.; Andreasen, D.; Mouritzen, P.; Wrang Teilum, M.; Dahlsveen, I.K. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M.B.; van Zandwijk, N.; Reid, G. Cell-free microRNAs: Potential biomarkers in need of standardized reporting. Front. Genet. 2013, 4, 56. [Google Scholar] [CrossRef]
- Calcatera, S.M.; Reicks, D.; Pratt, S.L. Novel and differentially abundant microRNAs in sperm cells, seminal plasma, and serum of boars due to porcine reproduction and respiratory syndrome virus infection. Anim. Reprod. Sci. 2018, 199, 60–71. [Google Scholar] [CrossRef]
- Sasser, R.G.; Crock, J.; Ruder-Montgomery, C.A. Characteristics of pregnancy-specific protein B in cattle. J. Reprod. Fertil. Suppl. 1989, 37, 109–113. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Mandrekar, J.N.; Mandrekar, S.J. Statistical Methods in Diagnostic Medicine Using SAS Software. In Proceedings of the Thirtieth SAS Users Group International Conference, Philidelphi, PA, USA, 10–13 April 2005. [Google Scholar]
- Bae, I.S.; Chung, K.Y.; Yi, J.; Kim, T.I.; Choi, H.S.; Cho, Y.M.; Choi, I.; Kim, S.H. Identification of reference genes for relative quantification of circulating microRNAs in bovine serum. PLoS ONE 2015, 10, e0122554. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Han, L.; Zhou, R.; Yan, J.; Zhao, G.; Liu, L. Expression of miRNA-146b-5p in patients with thyroid cancer in combination with Hashimoto’s disease and its clinical significance. Oncol. Lett. 2019, 17, 4871–4876. [Google Scholar]
- Zhao, G.; Yang, C.; Yang, J.; Liu, P.; Jiang, K.; Shaukat, A.; Wu, H.; Deng, G. Placental exosome-mediated Bta-miR-499-Lin28B/let-7 axis regulates inflammatory bias during early pregnancy. Cell Death Dis. 2018, 9, 704. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 626. [Google Scholar] [CrossRef]
- Han, H.; Austin, K.J.; Rempel, L.A.; Hansen, T.R. Low blood ISG15 mRNA and progesterone levels are predictive of non-pregnant dairy cows. J. Endocrinol. 2006, 191, 505–512. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Henkes, L.E.; Ashley, R.L.; Purcell, S.H.; Smirnova, N.P.; Veeramachaneni, D.N.; Anthony, R.V.; Hansen, T.R. Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-tau release from the uterine vein. Endocrinology 2008, 149, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, P.A.; Filho, C.; Rocha, C.C.; Neto, A.L.; de Andrade Bruni, G.; Oshiro, T.S.I.; Baruselli, P.S.; Lima, F.S.; Pugliesi, G. Feasibility and accuracy of using different methods to detect pregnancy by conceptus-stimulated genes in dairy cattle. JDS Commun. 2021, 2, 153–158. [Google Scholar] [CrossRef]
- Stowe, H.M.; Calcatera, S.M.; Dimmick, M.A.; Andrae, J.G.; Duckett, S.K.; Pratt, S.L. The bull sperm microRNAome and the effect of fescue toxicosis on sperm microRNA expression. PLoS ONE 2014, 9, e113163. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Wilfinger, W.W.; Eghbalnia, H.R.; Kennedy, A.; Rymaszewski, M.; Mackey, K. Inter-Individual Differences in RNA Levels in Human Peripheral Blood. PLoS ONE 2016, 11, e0148260. [Google Scholar] [CrossRef] [PubMed]
- Bastien, P.; Procop, G.W.; Reischl, U. Quantitative real-time PCR is not more sensitive than “conventional” PCR. J. Clin. Microbiol. 2008, 46, 1897–1900. [Google Scholar] [CrossRef]


| Gene | Accession Number | Forward Primer Sequence | Reverse Primer Sequence | R2 | Slope | Primer Efficiency | Product Size (bp) |
|---|---|---|---|---|---|---|---|
| Cyclophilin | NM_178320 | 5′-CACCGTGTTCTTCGACATCG | 5′-ACAGCTCAAAAGAGACGCGG | 0.93 | −3.37 | 1.98 | 60 |
| MX2 | NM_173941 | 5′-CTTCAGAGACGCCTCAGTCG | 5′-TGAAGCAGCCAGGAATAGTG | 0.99 | −3.6 | 1.9 | 232 |
| Oas1 | NM_001040606 | 5′-ACCCTCTCCAGGAATCCAGT | 5′-GATTCTGGTCCCAGGTCTGA | 0.99 | −2.9 | 2.21 | 198 |
| Isg15 | NM_174366 | 5′-CAGCCAACCAGTGTCTGCAGAGA | 5′-CCAGGATGGAGATGCAGTTCTGC | 0.99 | −3.17 | 2.07 | 292 |
| microRNA | miRNA Identified in Bovine Serum | Chromosome Location | Mature Sequence (5′ to 3′) | miRbase Accession Number | Taqman Assay ID # |
|---|---|---|---|---|---|
| miR-25 * | [6,8] | chr25: 37072717-37072800 [+] | CAUUGCACUUGUCUCGGUCUGA | MI0005067 | 000403 |
| miR-15b * | [8] | chr1: 108321741-108321838 [-] | UAGCAGCACAUCAUGGUUUACA | MI0005012 | 000390 |
| miR16 * | [8,29] | chr12: 19660386-19660474 [-] | UAGCAGCACGUAAAUAUUGGUG | MI0009753 | 007149-mat |
| Let-7a | [29] | chr1: 19984844-19984927 [-] | UGAGGUAGUAGGUUGUAUGGUU | MI0005454 | 000379 |
| miR-26a * | [6,29] | chr22: 11513974-11514063 [+] | UUCAAGUAAUCCAGGAUAGGCU | MI0009784 | 000405 |
| miR-127 * | [8,39] | chr21: 67708504-67708598 [+] | UCGGAUCCGUCUGAGCUUGGCU | MI0005008 | 000452 |
| miR-128 | [29] | chr2: 62228129-62228210 [-] | UCACAGUGAACCGGUCUCUUU | MI0004755 | 002216 |
| miR-93 | [39] | chr25: 37072517-37072593 [+] | CAAAGUGCUGUUCGUGCAGGUA | MI0005050 | 007615_mat |
| PaxGene | Qiagen | ||||
|---|---|---|---|---|---|
| miR ID | Status | d 0 | d 18 | d 0 | d 18 |
| Bta-Let-7a * | Open | 5.36 ± 1.71 | 7.43 ± 1.71 | 0.58 ± 1.71 | 0.718 ± 1.63 |
| Pregnant | 4.49 ± 1.71 | 7.73 ± 1.71 | 1.61 ± 2.04 | 0.89 ± 2.04 | |
| Bta-miR-16a | Open | 3.09 ± 0.89 | 1.98 ± 0.89 | 2.47 ± 0.81 | 0.32 ± 0.85 |
| Pregnant | 3.16 ± 0.89 | 2.47 ± 0.89 | 4.47 ± 0.99 | 2.06 ± 0.99 | |
| Bta-miR-15b * | Open | 13.08 ± 2.82 1 | 10.38 ± 2.82 2 | 0.39 ± 2.57 1 | 0.53 ± 2.57 1 |
| Pregnant | 16.13 ± 2.82 1 | 10.18 ± 2.82 2 | 0.80 ± 3.14 1 | 0.36 ± 3.14 1 | |
| Bta-miR-25 * | Open | 4.12 ± 6.29 a | 22.33 ± 6.29 b | 5.77 ± 5.74 a | 2.78 ± 5.74 a |
| Pregnant | 8.95 ± 6.29 a | 18.21 ± 6.29 b | 4.53 ± 7.03 a | 3.25 ± 7.51 a | |
| Bta-miR-26 * | Open | 6.73 ± 2.02 | 6.34 ± 2.02 | 0.93 ± 1.85 | 1.32 ± 1.85 |
| Pregnant | 8.03 ± 2.02 | 9.45 ± 2.02 | 1.69 ± 2.26 | 1.18 ± 2.26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeCarlo, A.N.; Parrish, J.; Quarles, J.D.; Long, N.M.; Pratt, S.L. Assessing the Differential Abundance of Maternal Circulating MicroRNAs or Interferon-Stimulated Genes with Early Pregnancy. Genes 2023, 14, 1532. https://doi.org/10.3390/genes14081532
DeCarlo AN, Parrish J, Quarles JD, Long NM, Pratt SL. Assessing the Differential Abundance of Maternal Circulating MicroRNAs or Interferon-Stimulated Genes with Early Pregnancy. Genes. 2023; 14(8):1532. https://doi.org/10.3390/genes14081532
Chicago/Turabian StyleDeCarlo, Andrea N., Joseph Parrish, Jasmine D. Quarles, Nathan M. Long, and Scott L. Pratt. 2023. "Assessing the Differential Abundance of Maternal Circulating MicroRNAs or Interferon-Stimulated Genes with Early Pregnancy" Genes 14, no. 8: 1532. https://doi.org/10.3390/genes14081532
APA StyleDeCarlo, A. N., Parrish, J., Quarles, J. D., Long, N. M., & Pratt, S. L. (2023). Assessing the Differential Abundance of Maternal Circulating MicroRNAs or Interferon-Stimulated Genes with Early Pregnancy. Genes, 14(8), 1532. https://doi.org/10.3390/genes14081532




