Enhancing Healthcare Outcomes and Modulating Apoptosis- and Antioxidant-Related Genes through the Nano-Phytosomal Delivery of Phenolics Extracted from Allium ampeloprasum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Phenolic Rich Fraction (PRF) Preparation
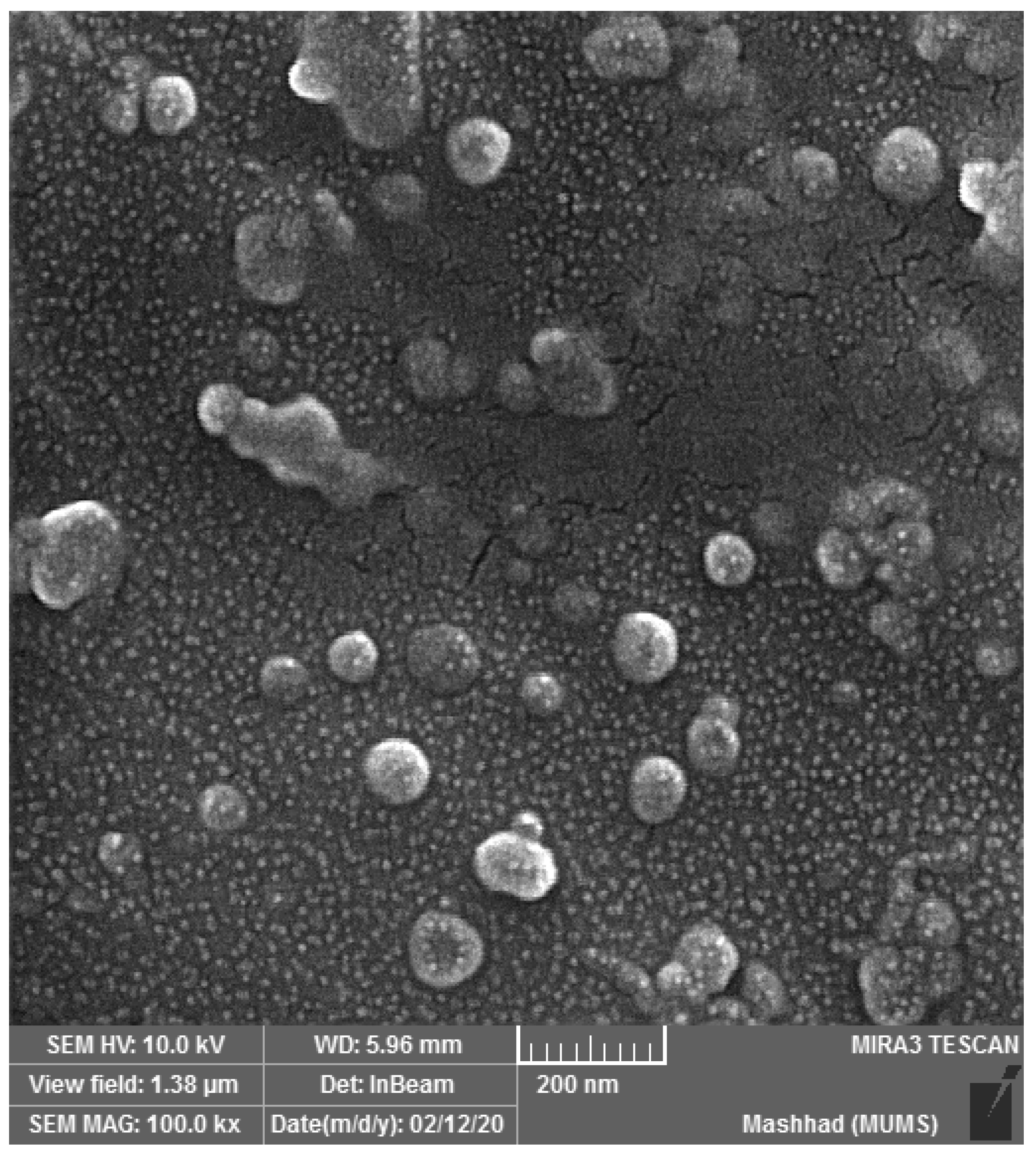
2.4. Nano-Phytosome Preparation and Characterization
2.5. Phenolic Profiling of Nano-phytosome
2.6. Animal Trials
2.7. Sample Collection
2.8. Gene Expression Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fractionation Analysis
3.2. Phytosome Synthesis and Characterization
3.3. Body Weight and Food Intake Analyses in Animals
3.4. Liver Enzyme and Lipid Peroxidation Assessment
3.5. Histopathology, Size, and Weight of Tumor-Bearing Mice
3.6. Antioxidant Gene Expression Pattern Analysis
3.7. Anticancer Gene Analysis in Mice Tumors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Rezatabar, S.; Karimian, A.; Rameshknia, V.; Parsian, H.; Majidinia, M.; Kopi, T.A.; Bishayee, A.; Sadeghinia, A.; Yousefi, M.; Monirialamdari, M.; et al. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J. Cell. Physiol. 2019, 234, 14951–14965. [Google Scholar] [PubMed]
- Lu, Q.-B.; Du, Q.; Wang, H.-P.; Tang, Z.-H.; Wang, Y.-B.; Sun, H.-J.J.R.B. Salusin-β mediates tubular cell apoptosis in acute kidney injury: Involvement of the PKC/ROS signaling pathway. Redox Biol. 2020, 30, 101411. [Google Scholar] [PubMed]
- Khatoon, E.; Banik, K.; Harsha, C.; Sailo, B.L.; Thakur, K.K.; Khwairakpam, A.D.; Vikkurthi, R.; Devi, T.B.; Gupta, S.C.; Kunnumakkara, A.B. Phytochemicals in cancer cell chemosensitization: Current knowledge and future perspectives. Semin. Cancer Biol. 2022, 80, 306–339. [Google Scholar] [CrossRef]
- Mazurakova, A.; Koklesova, L.; Samec, M.; Kudela, E.; Kajo, K.; Skuciova, V.; Csizmár, S.H.; Mestanova, V.; Pec, M.; Adamkov, M. Anti-breast cancer effects of phytochemicals: Primary, secondary, and tertiary care. EPMA J. 2022, 13, 315–334. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: Overcoming pharmacokinetic limitations for nutraceutical applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 1863–1873. [Google Scholar] [CrossRef]
- Jia, W.; Zhou, L.; Li, L.; Zhou, P.; Shen, Z. Nano-based drug delivery of polyphenolic compounds for cancer treatment: Progress, opportunities, and challenges. Pharmaceuticals 2023, 16, 101. [Google Scholar] [CrossRef]
- Lu, M.; Qiu, Q.; Luo, X.; Liu, X.; Sun, J.; Wang, C.; Lin, X.; Deng, Y.; Song, Y. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2019, 14, 265–274. [Google Scholar] [CrossRef]
- Kaur, P.; Mandal, U.K. Phytosomes: Preparations, Characterization, and Future Uses. In Medicinal Plants; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 319–335. [Google Scholar]
- Anandharamakrishnan, C.; Dutta, S.; Moses, J. Introductory overview on liposomes. In Liposomal Encapsulation in Food Science and Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–14. [Google Scholar]
- Telange, D.R.; Jain, S.P.; Pethe, A.M.; Dave, V.S. Phytosomes and phytosomal nanoparticles: A promising drug delivery system for flavonoids and nutraceuticals. In Nutraceutical Delivery Systems; Apple Academic Press: Palm Bay, FL, USA, 2023; pp. 153–171. [Google Scholar]
- Deka, B.; Manna, P.; Borah, J.C.; Talukdar, N.C. A review on phytochemical, pharmacological attributes and therapeutic uses of Allium hookeri. Phytomedicine Plus 2022, 2, 100262. [Google Scholar] [CrossRef]
- Alam, A.; Al Arif Jahan, A.; Bari, M.S.; Khandokar, L.; Mahmud, M.H.; Junaid, M.; Chowdhury, M.S.; Khan, M.F.; Seidel, V.; Haque, M.A. Allium vegetables: Traditional uses, phytoconstituents, and beneficial effects in inflammation and cancer. Crit. Rev. Food Sci. Nutr. 2022, 16, 1–35. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.; Ghasemzadeh, A. Chemical composition, antioxidant and anticancer potential of Labisia pumila variety alata under CO2 enrichment. Wagening. J. Life Sci. 2016, 78, 85–91. [Google Scholar] [CrossRef]
- Oskoueian, E.; Karimi, E.; Noura, R.; Ebrahimi, M.; Shafaei, N.; Karimi, E. Nanoliposomes Encapsulation of Enriched-Phenolic Fraction from Pistachio Hulls and It’s Antioxidant, Anti-Inflammatory and Anti-Melanogenic Activities. J. Microencapsul. 2019, 37, 1–34. [Google Scholar] [PubMed]
- Hassirian, N.; Karimi, E.; Oskoueian, E. Nanoliposome-encapsulated Phenolic Rich Fraction From Alcea Rosea as a Dietary Phytobiotic in Mice Challenged by Escherichia Coli. Ann. Microbiol. 2022, 72, 6. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Souto, E.B.; Silva, A.M. Nanophytosomes: A novel approach for the delivery of herbal drugs. In Systems of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 239–257. [Google Scholar]
- Shamansoori, M.T.; Karimi, E.; Oskoueian, E.J.B.; Biochemistry, A. Rheum ribes extract-loaded nanoliposome as a novel phytogenic antibiotic alternative in mice challenged by Escherichia coli (O157: H7). Biotechnol. Appl. Biochem. 2022, 69, 2540–2549. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar]
- Moazzen, A.; Öztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, e10467. [Google Scholar] [PubMed]
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil–water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [PubMed]
- Pal, P.; Dave, V.; Paliwal, S.; Sharma, M.; Potdar, M.B.; Tyagi, A. Phytosomes—nanoarchitectures’ promising clinical applications and therapeutics. In Nanopharmaceutical Advanced Delivery Systems; Wiley and Sons: Hoboken, NJ, USA, 2021; pp. 187–216. [Google Scholar]
- Zhang, S.-Q.; Fu, Q.; Zhang, Y.-J.; Pan, J.-X.; Zhang, L.; Zhang, Z.-R.; Liu, Z.-M. Surface loading of nanoparticles on engineered or natural erythrocytes for prolonged circulation time: Strategies and applications. Acta Pharmacol. Sin. 2021, 42, 1040–1054. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Rahim, M.A.; Jan, N.; Shah, H.; Rawas-Qalaji, M.; Khan, S.; Sohail, M.; Thu, H.E.; Ramli, N.A.; Sarfraz, R.M. Cell membrane cloaked nanomedicines for bio-imaging and immunotherapy of cancer: Improved pharmacokinetics, cell internalization and anticancer efficacy. J. Control. Release 2021, 335, 130–157. [Google Scholar] [PubMed]
- Mohammadi, K.; Sani, M.A.; Azizi-Lalabadi, M.; McClements, D.J. Recent progress in the application of plant-based colloidal drug delivery systems in the pharmaceutical sciences. Adv. Colloid Interface Sci. 2022, 307, 102734. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, N.; Chaudhary, B.; Umakanthan, S.; Chattu, V.K.; Kazmi, I.; Al-Abbasi, F.A.; Alzarea, S.I.; Afzal, O.; Altamimi, A.S.A. Lipid Nanocapsule: A Novel Approach to Drug Delivery System Formulation Development. Curr. Pharm. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Cai, J.; Wen, H.; Zhou, H.; Zhang, D.; Lan, D.; Liu, S.; Li, C.; Dai, X.; Song, T.; Wang, X. Naringenin: A flavanone with anti-inflammatory and anti-infective properties. Biomed. Pharmacother. 2023, 164, 114990. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Maeda, K.; Murotani, K.; Shimizu, A.; Ueshima, J.; Nagano, A.; Sonoi, N.; Inoue, T.; Mori, N. Association of body mass index and weight change with death in patients with advanced cancer. Nutrition 2023, 112152. [Google Scholar] [CrossRef]
- Gad, M.; Hassouna, H.Z.; Mahmoud, K.; Abd-Rabou, A.A.; Abdel-Azeem, A.S.; Hegazy, A.M.; Abdel-Lattife, M.S.; Ahmed, F.A.; Oz, F.; Proestos, C. Pinus roxburghii and Nauplius graveolens Extracts Elevate Apoptotic Gene Markers in C26 Colon Carcinoma Cells Induced in a BALB/c Mouse Model. Separations 2022, 9, 277. [Google Scholar]
- Bhattacharjee, S.; Deb, P.K.; Devi, R. Phytoantioxidants and Their Role in Cellular Oxidative Stress. In Phytoantioxidants and Nanotherapeutics; Wiley and Sons: Hoboken, NJ, USA, 2022; pp. 77–98. [Google Scholar]
- Abdelkader, N.F.; Elyamany, M.; Gad, A.M.; Assaf, N.; Fawzy, H.M.; Elesawy, W.H. Ellagic acid attenuates liver toxicity induced by valproic acid in rats. J. Pharmacol. Sci. 2020, 143, 23–29. [Google Scholar] [CrossRef]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.J.N. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meca, A.-D.; Turcu-Stiolica, A.; Stanciulescu, E.C.; Andrei, A.M.; Nitu, F.M.; Banita, I.M.; Matei, M.; Pisoschi, C.-G. Variations of serum oxidative stress biomarkers under first-line antituberculosis treatment: A pilot study. J. Pers. Med. 2021, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.-R.; Kuo, C.-Y.; Tsai, M.-Y.; Lin, W.-L.; Lin, T.-C.; Liao, H.-J.; Chen, C.-H.; Wang, Y.-C.J.M. Anti-cancer effects of zotarolimus combined with 5-fluorouracil treatment in HCT-116 colorectal cancer-bearing BALB/c nude mice. Molecules 2021, 26, 4683. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [CrossRef] [Green Version]
- Aborehab, N.M.; Elnagar, M.R.; Waly, N.E.; Toxicology, M. Gallic acid potentiates the apoptotic effect of paclitaxel and carboplatin via overexpression of Bax and P53 on the MCF-7 human breast cancer cell line. J. Biochem. Mol. Toxicol. 2021, 35, e22638. [Google Scholar] [CrossRef]
- Hafezi, K.; Hemmati, A.A.; Abbaszadeh, H.; Valizadeh, A.; Makvandi, M. Anticancer activity and molecular mechanisms of α-conidendrin, a polyphenolic compound present in Taxus yunnanensis, on human breast cancer cell lines. Phytother. Res. 2020, 34, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-G.; Hwang, K.-A.; Choi, K.-C. Rosmarinic acid, a component of rosemary tea, induced the cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer cell lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.-H.; Bouyahya, A.J.F. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef] [PubMed]

| Genes | Forward (5′ to 3′) | Reverse (5′to 3′) | |
|---|---|---|---|
| Liver | SOD | GAGACCTGGGCAATGTGACT | GTTTACTGCGCAATCCCAAT |
| GPX | GTCCACCGTGTATGCCTTCTCC | TCTCCTGATGTCCGAACTGATTGC | |
| GAPDH | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG | |
| Tunor | bax | TTTGCTTCAGGGTTTCATCCA | CTCCATGTTACTGTCCAGTTCGT |
| bcl2 | CATGTGTGTGGAGAGCGTCAAC | CAGATAGGCACCCAGGGTGAT | |
| caspase-3 | CTGGACTGTGGCATTGAGAC | ACAAAGCGACTGGATGAACC | |
| GAPDH | CCGGATCGACCACTACCTGGGCAAC | GTTCCCCACGTACTGGCCCAGGACCA | |
| Phenolic Compounds (µg/g) | ||
|---|---|---|
| Gallic acid | Pyrogallol | Ferulic acid |
| 129.5 ± 1.7 | 203.8 ± 3.1 | 431.5 ± 4.1 |
| Ellagic acid | Syringic acid | Naringin |
| 811 ± 6.7 | 507.2 ± 3.5 | 975.6 ± 5.2 |
| Average | T1 | T2 | T3 | SEM |
|---|---|---|---|---|
| Average daily weight gain (mg) | 163.5 c | 185.2 b | 228.6 a | 8.21 |
| Average daily feed intake (g) | 2.95 c | 3.21 b | 3.35 a | 0.04 |
| Parameters | T1 | T2 | T3 | SEM |
|---|---|---|---|---|
| ALT (IU/L) | 183 a | 177 b | 155 c | 9.4 |
| AST (IU/L) | 76 a | 63 b | 54 c | 4.2 |
| ALP (IU/L) | 489 a | 407 b | 382 b | 19.5 |
| MDA * (%) | 100.0 a | 83.46 b | 74.19 c | 3.2 |
| Tumor | T1 | T2 | T3 | SEM |
|---|---|---|---|---|
| Weight (g) | 3.1 a | 2.61 b | 2.37 c | 0.14 |
| Size (mm) | 26.03 a | 24.95 b | 22.53 c | 0.36 |
| Gene Expression (Fold Changes) | S.E.M | |||
|---|---|---|---|---|
| Genes | T1 | T2 | T3 | |
| SOD | 1.0 c | 1.6 b | 2.4 a | 0.08 |
| GPx | 1.0 c | 1.5 b | 1.8 a | 0.09 |
| Gene Expression (Fold Changes) | S.E.M | |||
|---|---|---|---|---|
| Genes | T1 | T2 | T3 | |
| Upregulated genes | ||||
| Bax | 1.0 c | 2.69 b | 3.48 a | 0.32 |
| Caspase-3 | 1.0 c | 1.76 b | 2.24 a | 0.18 |
| Downregulated gene | ||||
| Bcl2 | 1.0 c | 2.27 b | 2.94 a | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoeibi, A.; Karimi, E.; Zareian, M.; Oskoueian, E. Enhancing Healthcare Outcomes and Modulating Apoptosis- and Antioxidant-Related Genes through the Nano-Phytosomal Delivery of Phenolics Extracted from Allium ampeloprasum. Genes 2023, 14, 1547. https://doi.org/10.3390/genes14081547
Shoeibi A, Karimi E, Zareian M, Oskoueian E. Enhancing Healthcare Outcomes and Modulating Apoptosis- and Antioxidant-Related Genes through the Nano-Phytosomal Delivery of Phenolics Extracted from Allium ampeloprasum. Genes. 2023; 14(8):1547. https://doi.org/10.3390/genes14081547
Chicago/Turabian StyleShoeibi, Ali, Ehsan Karimi, Mohsen Zareian, and Ehsan Oskoueian. 2023. "Enhancing Healthcare Outcomes and Modulating Apoptosis- and Antioxidant-Related Genes through the Nano-Phytosomal Delivery of Phenolics Extracted from Allium ampeloprasum" Genes 14, no. 8: 1547. https://doi.org/10.3390/genes14081547





