The 3′ Non-Coding Sequence Negatively Regulates PD-L1 Expression, and Its Regulators Are Systematically Identified in Pan-Cancer
Abstract
:1. Introduction
2. Material and Method
2.1. Cell Culture and Treatment
2.2. Plasmids
2.3. Establishment of Stable Cell Lines
2.4. RNA Isolation and Quantitative Real-Time PCR
2.5. Western Blot Analysis
2.6. Cellular Immunofluorescence
2.7. Flow Cytometry
2.8. Data Acquisition
2.9. Prognostic Analysis
2.10. Immune Microenvironment and Immune Cell Infiltration Analysis
2.11. Immunotherapy Response Prediction
2.12. Statistical Analysis
2.13. MicroRNA Network Analysis
3. Results
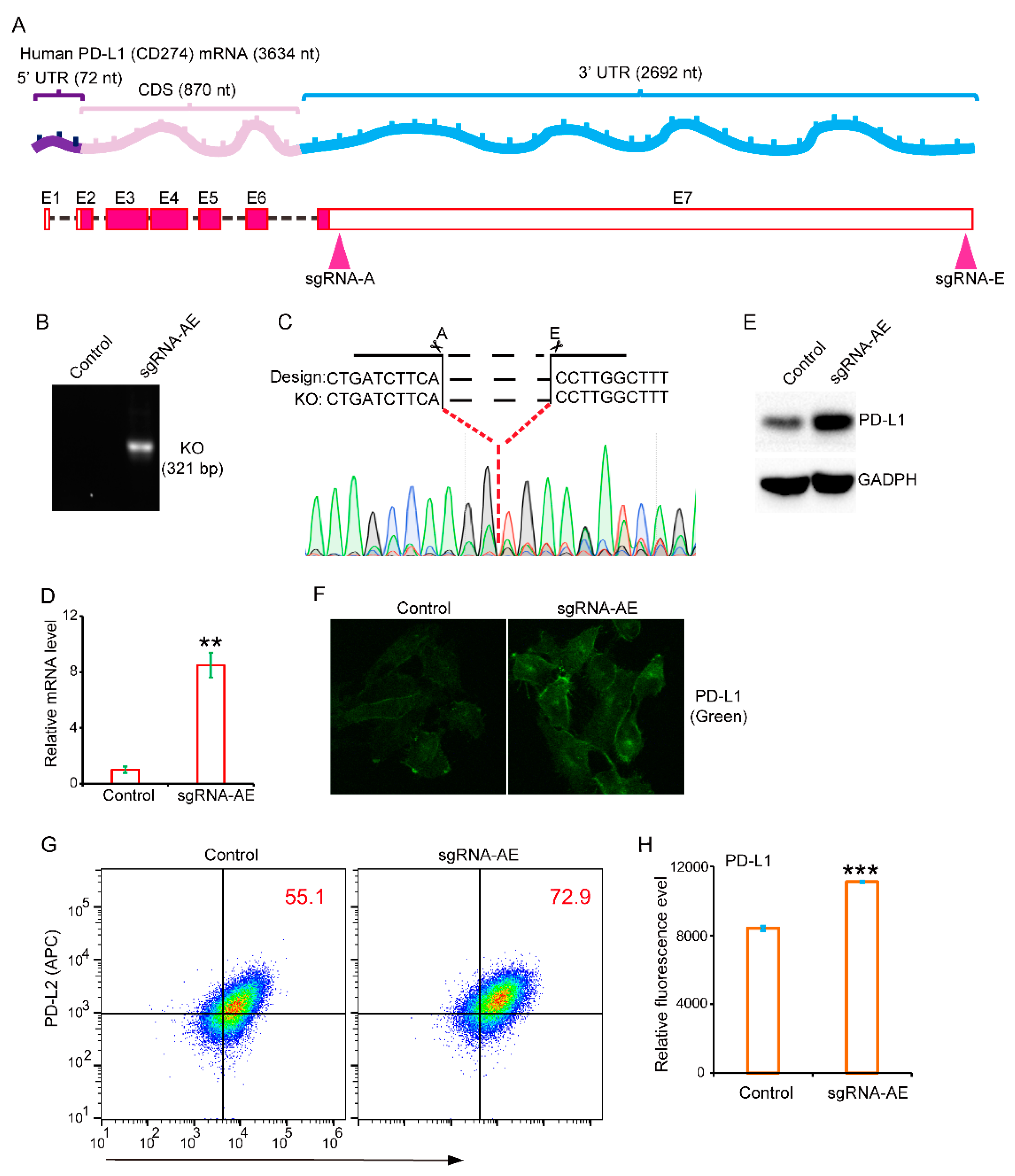
3.1. The 3′-Untranslated Region Strongly Restricts PD-L1 Expression
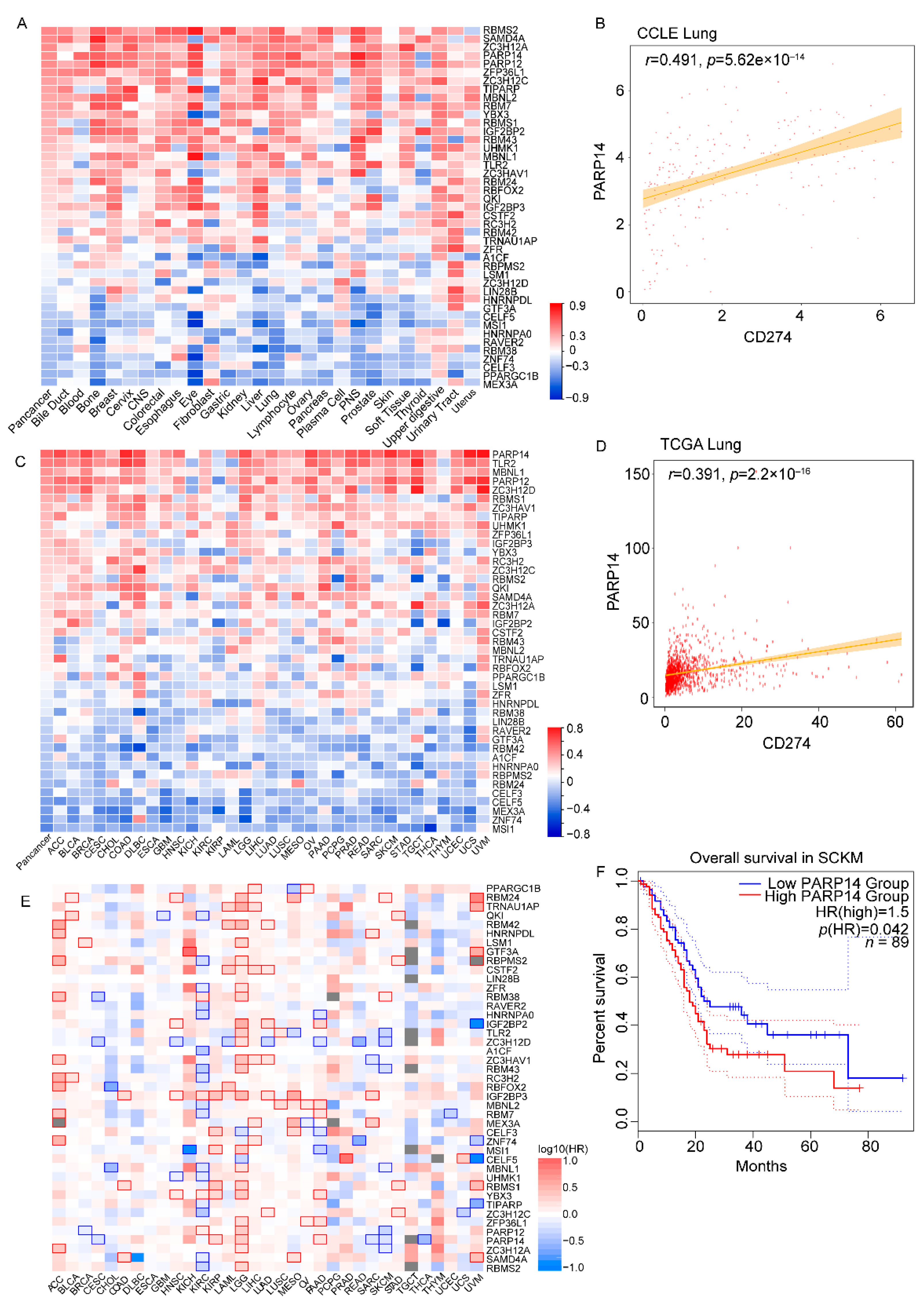
3.2. Identification of a Panel of 43 RNA-Binding Proteins That Correlated with PD-L1 in a Majority of Cancer Cell Lines and Tumor Tissues
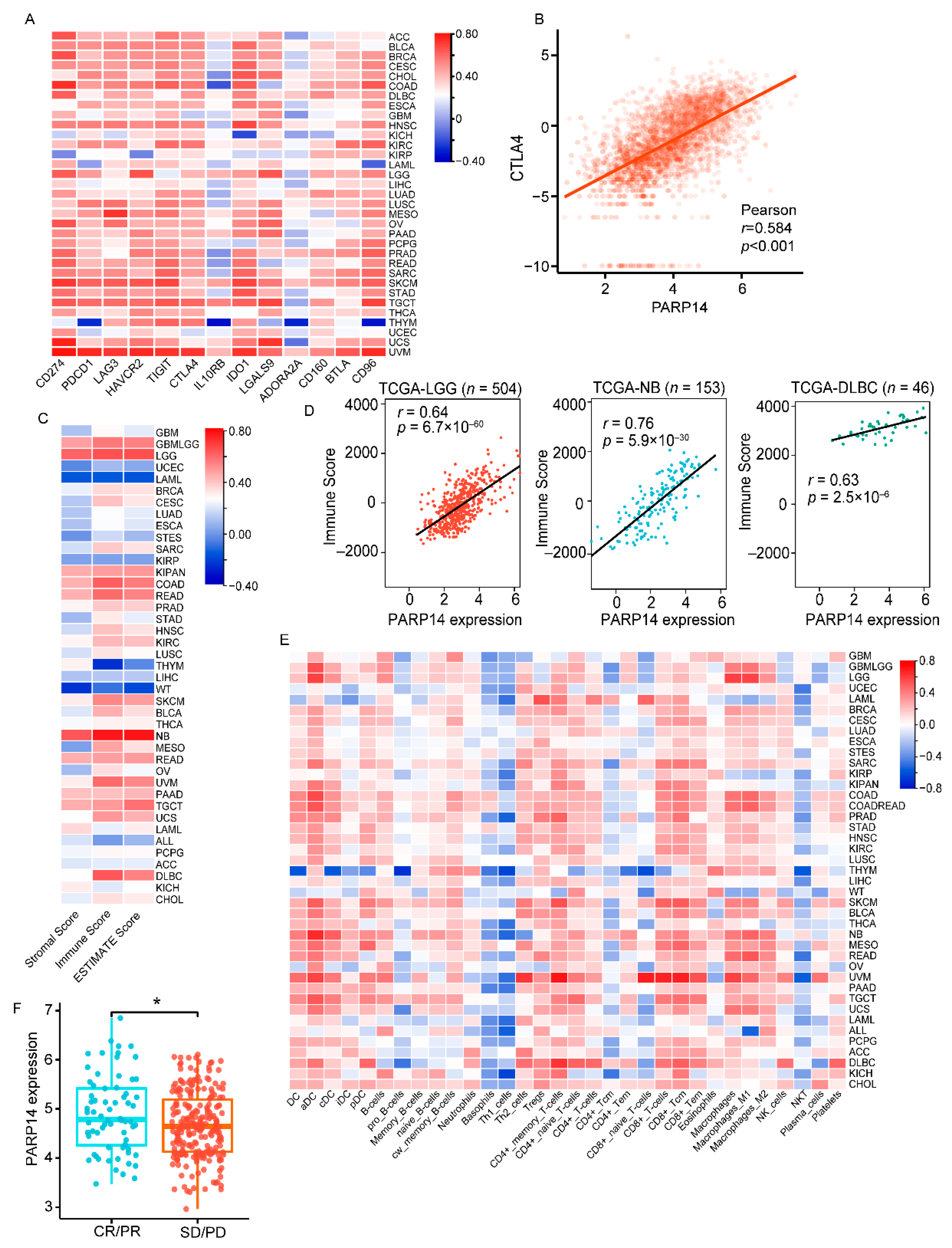
3.3. PARP14 Is Widely Associated with Immune Checkpoints, Tumor Microenvironment, and Immune-Infiltrating Cells in Various Cancer Types
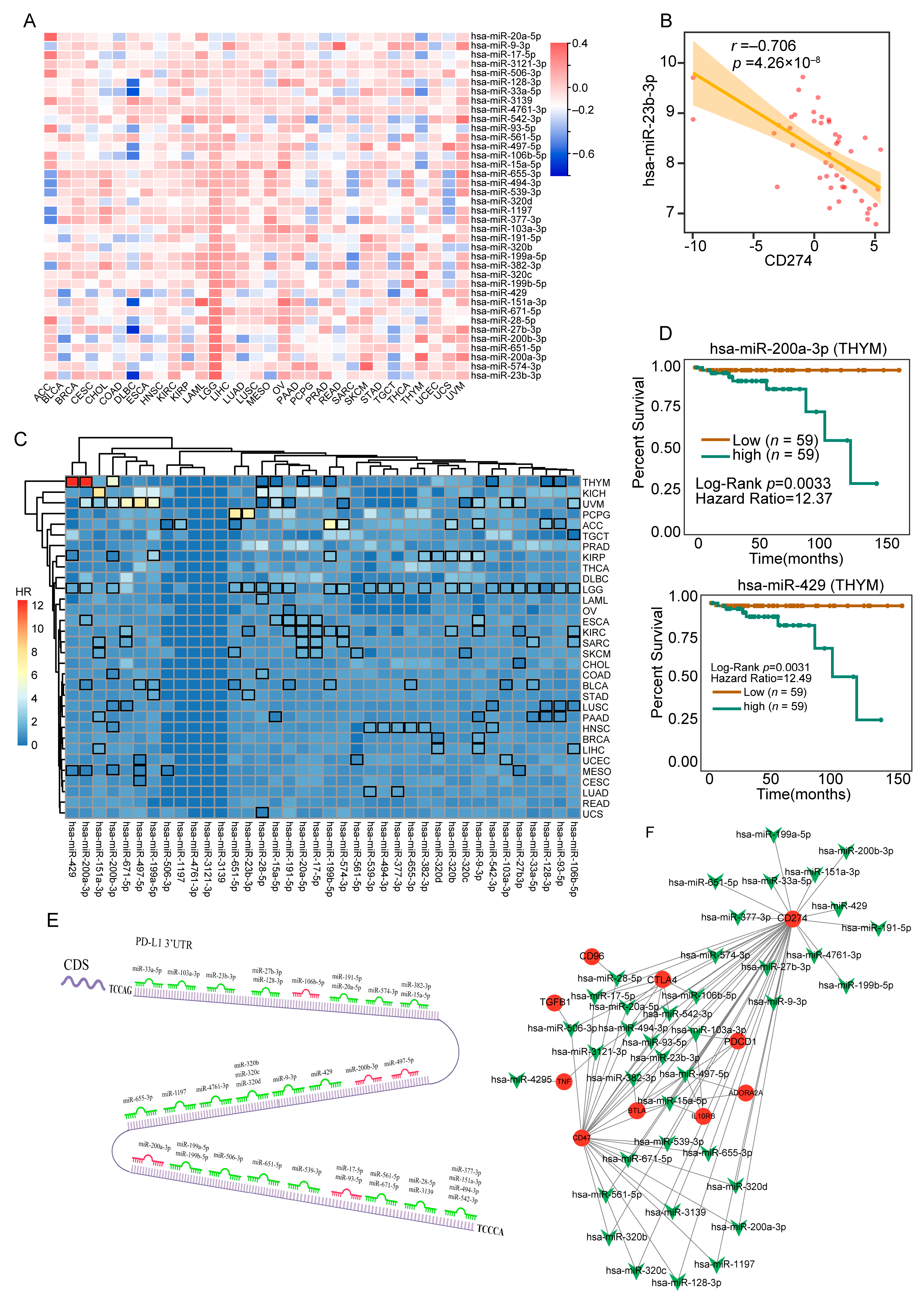
3.4. Comprehensively Identification of 38 MicroRNAs Associated with PD-L1 in a Majority of Tumor Tissues
3.5. Comprehensive Analysis of m6A Regulators in the Control of PD-L1 Expression
3.6. Combination of Different PD-L1-3′-UTR Regulators Allowed Us to Establish The Predictive Immune Signature (RBMS1, QKI, ZC3HAV1, RBM38) in STAD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, H.; Togashi, Y. Aging, cancer, and antitumor immunity. Int. J. Clin. Oncol. 2022, 27, 316–322. [Google Scholar] [CrossRef]
- Ancevski Hunter, K.; Socinski, M.A.; Villaruz, L.C. PD-L1 Testing in Guiding Patient Selection for PD-1/PD-L1 Inhibitor Therapy in Lung Cancer. Mol. Diagn. Ther. 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, J.; Zhang, X.; Yuan, Z.; Cheng, J.; Chen, B. Efficacy and safety of PD-1/PD-L1 immune checkpoint inhibitors in treating non-Hodgkin lymphoma: A systematic review and meta-analysis of clinical trials. Medicine 2022, 101, e32333. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, J.H.; Chan, L.C.; Li, C.W.; Hsu, J.L.; Hung, M.C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wu, C.; Chen, M.; Jiang, Y.; Wu, Y.; Mao, R.; Fan, Y. The generation of PD-L1 and PD-L2 in cancer cells: From nuclear chromatin reorganization to extracellular presentation. Acta Pharm. Sin. B 2022, 12, 1041–1053. [Google Scholar] [CrossRef]
- Kataoka, K.; Shiraishi, Y.; Takeda, Y.; Sakata, S.; Matsumoto, M.; Nagano, S.; Maeda, T.; Nagata, Y.; Kitanaka, A.; Mizuno, S.; et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature 2016, 534, 402–406. [Google Scholar] [CrossRef]
- Qin, H.; Ni, H.; Liu, Y.; Yuan, Y.; Xi, T.; Li, X.; Zheng, L. RNA-binding proteins in tumor progression. J. Hematol. Oncol. 2020, 13, 90. [Google Scholar] [CrossRef]
- Pereira, B.; Billaud, M.; Almeida, R. RNA-Binding Proteins in Cancer: Old Players and New Actors. Trends Cancer 2017, 3, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Hong, S. RNA Binding Protein as an Emerging Therapeutic Target for Cancer Prevention and Treatment. J. Cancer Prev. 2017, 22, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Erson-Bensan, A.E. RNA-biology ruling cancer progression? Focus on 3′UTRs and splicing. Cancer Metastasis Rev. 2020, 39, 887–901. [Google Scholar] [CrossRef]
- Mayr, C. Evolution and Biological Roles of Alternative 3′UTRs. Trends Cell Biol. 2016, 26, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, G.; Zhang, W.; Bai, L.; Wang, L.; Li, T.; Yan, L.; Xu, Y.; Chen, D.; Gao, W.; et al. Loss of RBMS1 promotes anti-tumor immunity through enabling PD-L1 checkpoint blockade in triple-negative breast cancer. Cell Death Differ. 2022, 29, 2247–2261. [Google Scholar] [CrossRef] [PubMed]
- Skaftnesmo, K.O.; Prestegarden, L.; Micklem, D.R.; Lorens, J.B. MicroRNAs in tumorigenesis. Curr. Pharm. Biotechnol. 2007, 8, 320–325. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Ye, J.; Pan, X.; Wang, J.; Gan, S.; Chu, C.; Chu, J.; Zhang, X.; Liu, M.; He, H.; et al. MicroRNA-497-5p down-regulation increases PD-L1 expression in clear cell renal cell carcinoma. J. Drug Target. 2019, 27, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Wang, G.X.; Lin, B.A.; Huang, J.S. MicroRNA-93-5p expression in tumor tissue and its tumor suppressor function via targeting programmed death ligand-1 in colorectal cancer. Cell Biol. Int. 2020, 44, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lin, J.; Yu, T.; Lou, J.; Qian, C.; Xu, A.; Liu, B.; Tao, H.; Jin, L. The regulation of N6-methyladenosine modification in PD-L1-induced anti-tumor immunity. Immunol. Cell Biol. 2023, 101, 204–215. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, S.; Wang, S.; Wu, J.; Zheng, B.; Wang, K.; Shen, S.; Jeong, S.; Li, Z.; Zhu, Y.; et al. M6A Demethylase ALKBH5 Regulates PD-L1 Expression and Tumor Immunoenvironment in Intrahepatic Cholangiocarcinoma. Cancer Res. 2021, 81, 4778–4793. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Pi, H.; Zheng, W.; Guo, X.; Shi, C.; Wang, Z.; Zhang, J.; Qu, X.; Liu, L.; Shen, H.; et al. The 3′ Non-Coding Sequence Negatively Regulates PD-L1 Expression, and Its Regulators Are Systematically Identified in Pan-Cancer. Genes 2023, 14, 1620. https://doi.org/10.3390/genes14081620
Chen Z, Pi H, Zheng W, Guo X, Shi C, Wang Z, Zhang J, Qu X, Liu L, Shen H, et al. The 3′ Non-Coding Sequence Negatively Regulates PD-L1 Expression, and Its Regulators Are Systematically Identified in Pan-Cancer. Genes. 2023; 14(8):1620. https://doi.org/10.3390/genes14081620
Chicago/Turabian StyleChen, Zike, Hui Pi, Wen Zheng, Xiaohong Guo, Conglin Shi, Zhiyang Wang, Jie Zhang, Xuanhao Qu, Lehan Liu, Haoliang Shen, and et al. 2023. "The 3′ Non-Coding Sequence Negatively Regulates PD-L1 Expression, and Its Regulators Are Systematically Identified in Pan-Cancer" Genes 14, no. 8: 1620. https://doi.org/10.3390/genes14081620
APA StyleChen, Z., Pi, H., Zheng, W., Guo, X., Shi, C., Wang, Z., Zhang, J., Qu, X., Liu, L., Shen, H., Lu, Y., Chen, M., Zhang, W., Sun, R., & Fan, Y. (2023). The 3′ Non-Coding Sequence Negatively Regulates PD-L1 Expression, and Its Regulators Are Systematically Identified in Pan-Cancer. Genes, 14(8), 1620. https://doi.org/10.3390/genes14081620




