Genetic Structuring of One of the Main Vectors of Sylvatic Yellow Fever: Haemagogus (Conopostegus) leucocelaenus (Diptera: Culicidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of the Study Area
2.2. Collection of Hg. leucocelaenus Specimens
2.3. Sample Preparation and Genotyping by Sequencing (GBS)
2.4. Population Diversity and Stratification Analysis
2.5. Landscape Descriptors
3. Results
3.1. Genetic Diversity
3.2. Neutrality Test
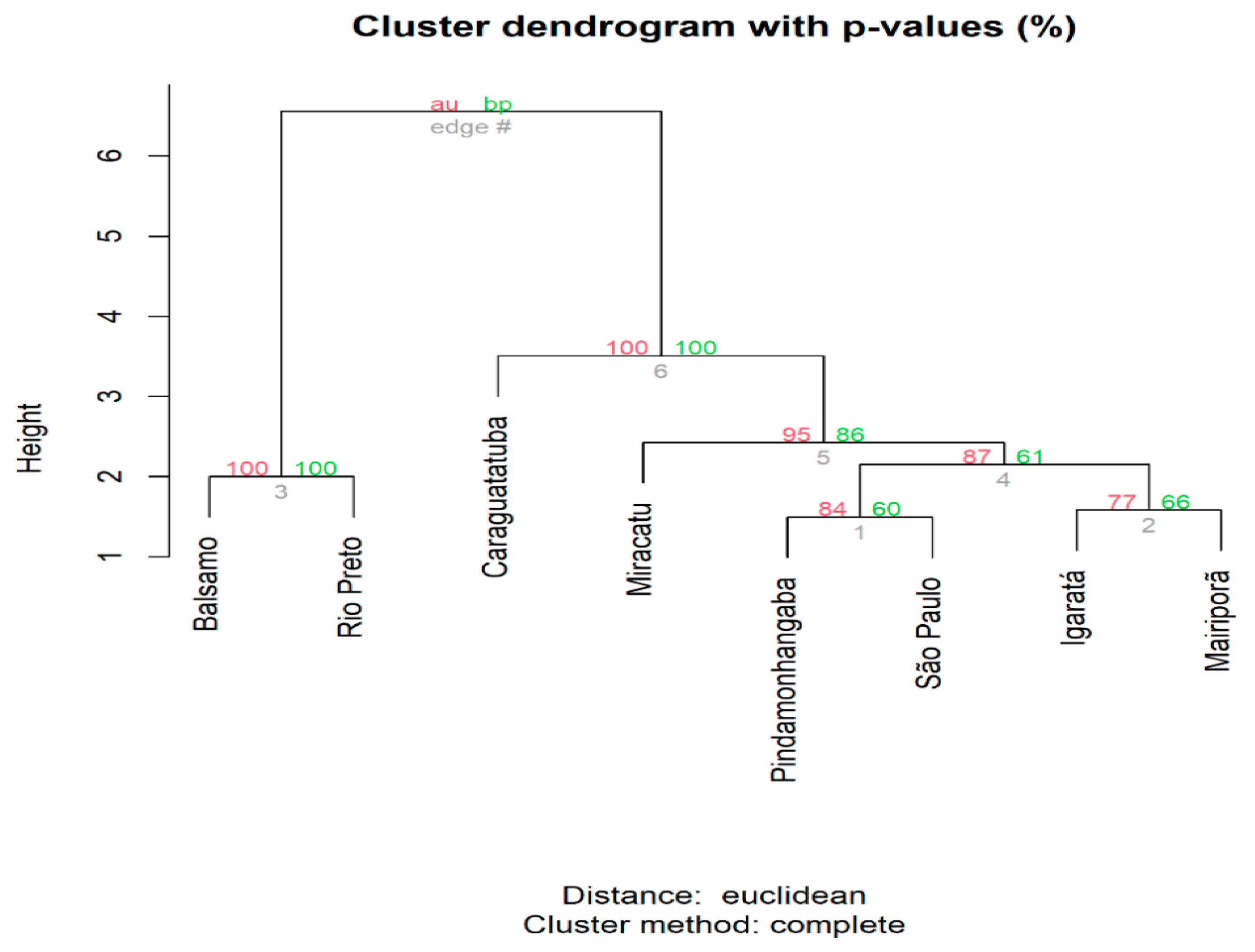
3.3. Genetic Structure
3.4. Landscape Metrics and Generalized Linear Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, S.C.; Reibold, R.; Cavaleri, M.A.; Alonso-Rodríguez, A.M.; Berberich, M.E.; Wood, T.E. Soil biogeochemical responses of a tropical forest to warming and hurricane disturbance. In Advances in Ecological Research; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Betts, R.A.; Malhi, Y.; Roberts, J.T. The future of the Amazon: New perspectives from climate, ecosystem and social sciences. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- MEA. Millennium Ecosystem Assessment: Ecosystems and Human Well-being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Charles, H.; Dukes, J.S. Impacts of Invasive Species on Ecosystem Services. In Biological Invasions; Springer: Berlin/Heidelberg, Germany, 2007; pp. 217–237. [Google Scholar]
- Giam, X. Global biodiversity loss from tropical deforestation. Proc. Natl. Acad. Sci. USA 2017, 114, 5775–5777. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- WHO. Zoonoses Key Facts. 2021. Available online: www.who.int/news-room/fact-sheets/detail/zoonoses (accessed on 10 May 2023).
- Dantas-Torres, F. Vector-Borne Zoonoses. In Zoonoses-Infections Affecting Humans and Animals: Focus on Public Health Aspects; Sing, A., Ed.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [PubMed]
- Gottdenker, N.L.; Streicker, D.G.; Faust, C.L.; Carroll, C.R. Anthropogenic Land Use Change and Infectious Diseases: A Review of the Evidence. EcoHealth 2014, 11, 619–632. [Google Scholar] [CrossRef]
- Mishra, J.; Mishra, P.; Arora, N.K. Linkages between environmental issues and zoonotic diseases: With reference to COVID-19 pandemic. Environ. Sustain. 2021, 4, 455–467. [Google Scholar] [CrossRef]
- Loh, E.H.; Zambrana-Torrelio, C.; Olival, K.J.; Bogich, T.L.; Johnson, C.K.; Mazet, J.A.K.; Karesh, W.; Daszak, P. Targeting Transmission Pathways for Emerging Zoonotic Disease Surveillance and Control. Vector-Borne Zoonotic Dis. 2015, 15, 432–437. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow fever. J. Cli. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- Chippaux, J.-P.; Chippaux, A. Yellow fever in Africa and the Americas: A historical and epidemiological perspective. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 20. [Google Scholar] [CrossRef]
- Sacchetto, L.; Drumond, B.P.; Han, B.A.; Nogueira, M.L.; Vasilakis, N. Re-emergence of yellow fever in the neotropics—Quo vadis? Emerg. Top. Life Sci. 2020, 4, 411–422. [Google Scholar]
- Arnell, J.H. Mosquito studies (Diptera, Culicidae). A revision of the genus Haemagogus. Contrib. Am. Entomol. Inst. 1973, 10, 1–174. [Google Scholar]
- Abreu, F.V.S.d.; Ribeiro, I.P.; Ferreira-de-Brito, A.; dos Santos, A.A.C.; de Miranda, R.M.; Bonelly, I.d.S.; Neves, M.S.A.S.; Bersot, M.I.; dos Santos, T.P.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef]
- Forattini, O.P. Culicidologia Médica: Identificação, Biologia e Epidemiologia; EdUSP: São Paulo, Brazil, 2002; Volume 2. [Google Scholar]
- da Cardoso, J.C.; de Almeida, M.A.B.; dos Santos, E.; da Fonseca, D.F.; Sallum, M.A.M.; Noll, C.A.; Monteiro, H.A.d.O.; Cruz, A.C.R.; Carvalho, V.L.; Pinto, E.V.; et al. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus Mosquitoes, Southern Brazil, 2008. Emerg. Infect. Dis. 2010, 16, 1918–1924. [Google Scholar] [CrossRef]
- Cunha, M.d.P.; Duarte-Neto, A.N.; Pour, S.Z.; Ortiz-Baez, A.S.; Černý, J.; Pereira, B.B.d.S.; Braconi, C.T.; Ho, Y.-L.; Perondi, B.; Sztajnbok, J.; et al. Origin of the São Paulo Yellow Fever epidemic of 2017–2018 revealed through molecular epidemiological analysis of fatal cases. Sci. Rep. 2019, 9, 20418. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.C.; Costa, Z.G.; Travassos Da Rosa, E.S.; Luna, E.; Rodrigues, S.G.; Barros, V.L.R.S.; Dias, J.P.; Monteiro, H.A.O.; Oliva, O.F.P.; Vasconcelos, H.B.; et al. Epidemic of Jungle Yellow Fever in Brazil, 2000: Implications of Climatic Alterations in Disease Spread. J. Med. Virol. 2001, 65, 598–604. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.d.C. Yellow fever in Brazil: Thoughts and hypotheses on the emergence in previously free areas. Rev. Saude Publica 2010, 44, 1144–1149. [Google Scholar] [CrossRef]
- Ministério da Saúde. Secretaria de Vigilância em Saúde: Nota Informativa N°169 de 2019-CGARB/DEIDT/SVS/MS; Ministério da Saúde: Brasilia, Brazil, 2019.
- Ministério da Saúde. Monitoramento dos Casos de Arboviroses Urbanas Transmitidas Pelo Aedes, Boletim Epidemiológico Arboviroses; Ministério da Saúde: Brasilia, Brazil, 2020.
- Ministério da Saúde. Boletim Epidemiológico-Situação Epidemiológica da Febre Amarela–Monitoramento 2020/2021, Boletim Epidemiológico; Ministério da Saúde: Brasilia, Brazil, 2021.
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Wilk-da-Silva, R.; Mucci, L.F.; Ceretti-Junior, W.; Duarte, A.M.R.d.C.; Marrelli, M.T.; Medeiros-Sousa, A.R. Influence of landscape composition and configuration on the richness and abundance of potential sylvatic yellow fever vectors in a remnant of Atlantic Forest in the city of São Paulo, Brazil. Acta Trop. 2020, 204, 105385. [Google Scholar] [CrossRef]
- Bolt, L.M.; Russell, D.G.; Schreier, A.L. Anthropogenic edges impact howler monkey (Alouatta palliata) feeding behaviour in a Costa Rican rainforest. Primates 2021, 62, 647–657. [Google Scholar] [CrossRef]
- Mbora, D.N.M.; McPeek, M.A. Host density and human activities mediate increased parasite prevalence and richness in primates threatened by habitat loss and fragmentation. J. Anim. Ecol. 2009, 78, 210–218. [Google Scholar] [CrossRef]
- Plowright, R.K.; Parrish, C.R.; Mccallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef]
- Prist, P.; Tambosi, L.; Mucci, L.; Pinter, A.; de Souza, R.; Muylaert, R.; Rhodes, J.; Comin, C.; Costa, L.; D’Agostini, T.; et al. Roads and forest edges facilitate yellow fever virus dispersion. J. Appl. Ecol. 2021, 59, 4–17. [Google Scholar] [CrossRef]
- Wilk-da-Silva, R.; Medeiros-Sousa, A.R.; Laporta, G.Z.; Mucci, L.F.; Prist, P.R.; Marrelli, M.T. The influence of landscape structure on the dispersal pattern of yellow fever virus in the state of São Paulo. Acta Trop. 2022, 228, 106333. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.D.; Fahrig, L.; With, K.A. Landscape connectivity: A return to the basics. In Connectivity Conservation; Cambridge University Press: Cambridge, UK, 2006; pp. 29–43. [Google Scholar]
- Klinga, P.; Mikoláš, M.; Smolko, P.; Tejkal, M.; Höglund, J.; Paule, L. Considering landscape connectivity and gene flow in the Anthropocene using complementary landscape genetics and habitat modelling approaches. Landsc. Ecol. 2019, 34, 521–536. [Google Scholar] [CrossRef]
- Cheng, J.; Kao, H.; Dong, S. Population genetic structure and gene flow of rare and endangered Tetraena mongolica Maxim. revealed by reduced representation sequencing. BMC Plant. Biol. 2020, 20, 391. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, F.; Coltman, D.W. Will human influences on evolutionary dynamics in the wild pervade the Anthropocene? BMC Biol. 2018, 16, 7. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Braschler, B.; Rusterholz, H.-P.; Baur, B. Genetic effects of anthropogenic habitat fragmentation on remnant animal and plant populations: A meta-analysis. Ecosphere 2018, 9, e02488. [Google Scholar] [CrossRef]
- IBGE. Instituto Brasileiro de Geografia e Estatística: Cidades e Estados 2021. Available online: https://www.ibge.gov.br/cidades-e-estados/sp/ (accessed on 9 May 2023).
- MapBiomas. MapBiomas, Projeto Maobiomas: Coleção 5.0 da Série Anual de Mapas de Cobertura e Uso de Solo do Brasil, Período em 1985 e 2019. 2020. Available online: https://plataforma.brasil.mapbiomas.org/ (accessed on 9 May 2023).
- SOSMA. Qual é a área de Cobertura da Mata Atlântica? Fundação SOS Mata Atl. 2020. Available online: https://www.sosma.org.br/artigos/qual-e-area-de-cobertura-da-mata-atlantica/ (accessed on 9 May 2023).
- Consoli, R.A.G.B.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária No Brasil, Cadernos de Saúde Pública; Editora FIOCRUZ: Rio de Janeiro, Brazil, 1994. [Google Scholar]
- Alvarez, M.V.N.; Alonso, D.P.; Kadri, S.M.; Rufalco-Moutinho, P.; Bernardes, I.A.F.; de Mello, A.C.F.; Souto, A.C.; Carrasco-Escobar, G.; Moreno, M.; Gamboa, D.; et al. Nyssorhynchus darlingi genome-wide studies related to microgeographic dispersion and blood-seeking behavior. Parasit. Vec. 2022, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.P.; Alvarez, M.V.N.; Amorim, J.A.; de Sá, I.L.R.; de Carvalho, D.P.; Ribeiro, K.A.N.; Ribolla, P.E.M.; Sallum, M.A.M. Mansonia spp. population genetics based on mitochondrion whole-genome sequencing alongside the Madeira River near Porto Velho, Rondonia, Brazil. Infect. Genet. Evol. 2022, 103, 105341. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics: Cambridge, UK, 2010. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef]
- Alvarez, M.V. LCVCFtools v1.0.2-Alpha (v1.0.2-Alpha). 2021. Available online: https://doi.org/10.5281/zenodo.5259931 (accessed on 9 May 2023).
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Tajima, F. Evolutionary relationship of DNA sequences in finite populations. Genetics 1983, 105, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Fu, Y.-X. Statistical Tests of Neutrality of Mutations Against Population Growth, Hitchhiking and Background Selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F -Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar]
- Gillespie, J.H. Population Genetics: A Concise Guide; Johns Hopkins University Press: Baltimore, ML, USA, 2004. [Google Scholar]
- Knaus, B.J.; Grünwald, N.J. VCFR: A package to manipulate and visualize variant call format data in R. Mol. Ecol. Resour. 2017, 17, 44–53. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Suzuki, R.; Terada, Y.; Shimodaira, H. Hierarchical Clustering with P-Values via Multiscale Bootstrap Resampling; Kyoto University: Kyoto, Japan, 2019. [Google Scholar]
- Jombart, T.; Ahmed, I. adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef]
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21, 3070–3071. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Use R! Springer International Publishing: Zurique, Switzerland, 2016. [Google Scholar]
- Kassambara, A. ggpubr: “ggplot2” Based Publication Ready Plots. 2020. Available online: https://cran.r-project.org/package=ggpubr (accessed on 9 May 2023).
- Jackson, H.B.; Fahrig, L. What size is a biologically relevant landscape? Landsc. Ecol. 2012, 27, 929–941. [Google Scholar] [CrossRef]
- Causey, O.R.; Kumm, H.W.; Laemmert, H., Jr. Dispersion of Forest Mosquitoes in Brazil: Further Studies. Am. J. Trop. Med. Hyg. 1950, s1-30, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zittra, C.; Vitecek, S.; Obwaller, A.G.; Rossiter, H.; Eigner, B.; Zechmeister, T.; Waringer, J.; Fuehrer, H.-P. Landscape structure affects distribution of potential disease vectors (Diptera: Culicidae). Parasit. Vectors 2017, 10, 205. [Google Scholar] [CrossRef]
- Mayi, M.P.A.; Foncha, D.F.; Kowo, C.; Tchuinkam, T.; Brisco, K.; Anong, D.N.; Ravinder, S.; Cornel, A.J. Impact of deforestation on the abundance, diversity, and richness of Culex mosquitoes in a southwest Cameroon tropical rainforest. J. Vector Ecol. 2019, 44, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Suesdek, L. Microevolution of medically important mosquitoes–A review. Acta Trop. 2019, 191, 162–171. [Google Scholar] [CrossRef]
- Mucci, L.F.; Júnior, R.P.C.; de Paula, M.B.; Scandar, S.A.S.; Pacchioni, M.L.; Fernandes, A.; Consales, C.A. Feeding habits of mosquitoes (Diptera: Culicidae) in an area of sylvatic transmission of yellow fever in the state of São Paulo, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 6. [Google Scholar] [CrossRef][Green Version]
- Gomes, A.d.C.; Torres, M.A.N.; Paula, M.B.d.; Fernandes, A.; Marassá, A.M.; Consales, C.A.; Fonseca, D.F. Ecologia de Haemagogus e Sabethes (Diptera: Culicidae) em áreas epizoóticas do vírus da febre amarela, Rio Grande do Sul, Brasil. Epidemiol. Serviços Saúde 2010, 19, 101–113. [Google Scholar] [CrossRef]
- Bartholomay, L.C.; Waterhouse, R.M.; Mayhew, G.F.; Campbell, C.L.; Michel, K.; Zou, Z.; Ramirez, J.L.; Das, S.; Alvarez, K.; Arensburger, P.; et al. Pathogenomics of Culex quinquefasciatus and Meta-Analysis of Infection Responses to Diverse Pathogens. Science 2010, 330, 88–90. [Google Scholar] [CrossRef]
- Cohuet, A.; Harris, C.; Robert, V.; Fontenille, D. Evolutionary forces on Anopheles: What makes a malaria vector? Trends Parasitol. 2010, 26, 130–136. [Google Scholar] [CrossRef]
- Camargo-Neves, V.L.F.d.; Poletto, D.W.; Rodas, L.A.C.; Pachioli, M.L.; Cardoso, R.P.; Scandar, S.A.S.; Sampaio, S.M.P.; Koyanagui, P.H.; Botti, M.V.; Mucci, L.F.; et al. Entomological investigation of a sylvatic yellow fever area in São Paulo State, Brazil. Cad. Saude Publica 2005, 21, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Saad, L.D.C.; Barata, R.B.; Saad, L.D.C.; Barata, R.B. Surtos de febre amarela no estado de São Paulo, 2000–2010. Epidemiol. Serviços Saúde 2016, 25, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.S.; Rocco, I.M.; Bergo, E.S.; Brasil, R.A.; Siciliano, M.M.; Suzuki, A.; Silveira, V.R.; Bisordi, I.; Souza, R.P.d. Reemergence of yellow fever: Detection of transmission in the State of São Paulo, Brazil, 2008. Rev. Soc. Bras. Med. Trop. 2011, 44, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.S.; Spinola, R.; Tengan, C.H.; Brasil, R.A.; Siciliano, M.M.; Coimbra, T.L.M.; Silveira, V.R.; Rocco, I.M.; Bisordi, I.; Souza, R.P.d. Yellow fever epizootics in non-human primates, São Paulo state, Brazil, 2008–2009. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 45–50. [Google Scholar] [CrossRef]
- Governo do Estado de São Paulo. Boletim Epidemiológico Febre Amarela; Centro de Vigilância Epidemiológica, Secretaria de Saúde do Estado de São Paulo: São Paulo, Brazil, 2018.
- Trevelin, L.C.; Port-Carvalho, M.; Silveira, M.; Morell, E. Abundance, habitat use and diet of Callicebus nigrifrons Spix (Primates, Pitheciidae) in Cantareira State Park, São Paulo, Brazil. Rev. Bras. Zool. 2007, 24, 1071–1077. [Google Scholar] [CrossRef]
- Melliger, R.L.; Braschler, B.; Rusterholz, H.-P.; Baur, B. Diverse effects of degree of urbanisation and forest size on species richness and functional diversity of plants, and ground surface-active ants and spiders. PLoS ONE 2018, 13, e0199245. [Google Scholar] [CrossRef]
- Montagner, F.R.G.; Silva, O.S.; Jahnke, S.M. Mosquito species occurrence in association with landscape composition in green urban areas. Braz. J. Biol. 2018, 78, 233–239. [Google Scholar] [CrossRef]
- Ceretti-Junior, W.; Oliveira-Christe, R.; Wilk-da-Silva, R.; Mucci, L.F.; Duarte, A.M.R.d.C.; Fernandes, A.; Barrio-Nuevo, K.M.; Carvalho, M.P.; Marrelli, M.T.; Medeiros-Sousa, A.R. Diversity analysis and an updated list of mosquitoes (Diptera: Culicidae) found in Cantareira State Park, São Paulo, Brazil. Acta Trop. 2020, 212, 105669. [Google Scholar] [CrossRef]
- Mucci, F.; Medeiros-Sousa, A.; Ceretti-Jr, W.; Fernandes, A.; Camargo, A.; Evangelista, E.; Marrelli, M.T. Haemagogus leucocelaenus and other mosquitoes potentially associated with sylvatic yellow fever in Cantareira State Park in the São Paulo Metropolitan Area, Brazil. J. Am. Mosq. Control. Assoc. 2016, 32, 329–332. [Google Scholar] [CrossRef]
- Petersen, V.; Santana, M.; Alves, J.M.P.; Suesdek, L. Genetic and morphological polymorphisms of Aedes scapularis (Diptera: Culicidae), vector of filariae and arboviruses. Infect. Genet. Evol. 2022, 97, 105193. [Google Scholar] [CrossRef]
- Multini, L.C.; Wilke, A.B.B.; Suesdek, L.; Marrelli, M.T. Population Genetic Structure of Aedes fluviatilis (Diptera: Culicidae). PLoS ONE 2016, 11, e0162328. [Google Scholar] [CrossRef] [PubMed]
- Multini, L.C.; de Souza, A.L.d.S.; Marrelli, M.T.; Wilke, A.B.B. The influence of anthropogenic habitat fragmentation on the genetic structure and diversity of the malaria vector Anopheles cruzii (Diptera: Culicidae). Sci. Rep. 2020, 10, 18018. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Wilk-da-Silva, R.; Marrelli, M.T. Microgeographic population structuring of Aedes aegypti (Diptera: Culicidae). PLoS ONE 2017, 12, e0185150. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, P.F.M.; Besse-lototskaya, A.A. Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica 2014, 45, 69–79. [Google Scholar] [CrossRef]
- Huestis, D.L.; Dao, A.; Diallo, M.; Sanogo, Z.L.; Samake, D.; Yaro, A.S.; Ousman, Y.; Linton, Y.M.; Krishna, A.; Veru, L.; et al. Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature 2019, 574, 404–408. [Google Scholar] [CrossRef]
- Wagner, F.H.; Sanchez, A.; Aidar, M.P.M.; Rochelle, A.L.C.; Tarabalka, Y.; Fonseca, M.G.; Phillips, O.L.; Gloor, E.; Aragão, L.E.O.C. Mapping Atlantic rainforest degradation and regeneration history with indicator species using convolutional network. PLoS ONE 2020, 15, e0229448. [Google Scholar] [CrossRef]
- Fioravanti, C. O Combate à febre Amarela no Estado de São Paulo: História, Desafios e Inovações; Centro de Vigilância Epidemiológica, Secretaria de Saúde do Estado de São Paulo: São Paulo, Brazil, 2018.
- Rašić, G.; Endersby-Harshman, N.; Tantowijoyo, W.; Goundar, A.; White, V.; Yang, Q.; Filipović, I.; Johnson, P.; Hoffmann, A.A.; Arguni, E. Aedes aegypti has spatially structured and seasonally stable populations in Yogyakarta, Indonesia. Parasit. Vectors 2015, 8, 610. [Google Scholar] [CrossRef]
- Rašić, G.; Schama, R.; Powell, R.; Maciel-de Freitas, R.; Endersby-Harshman, N.M.; Filipović, I.; Sylvestre, G.; Máspero, R.C.; Hoffmann, A.A. Contrasting genetic structure between mitochondrial and nuclear markers in the dengue fever mosquito from Rio de Janeiro: Implications for vector control. Evol. Appl. 2015, 8, 901–915. [Google Scholar] [CrossRef]
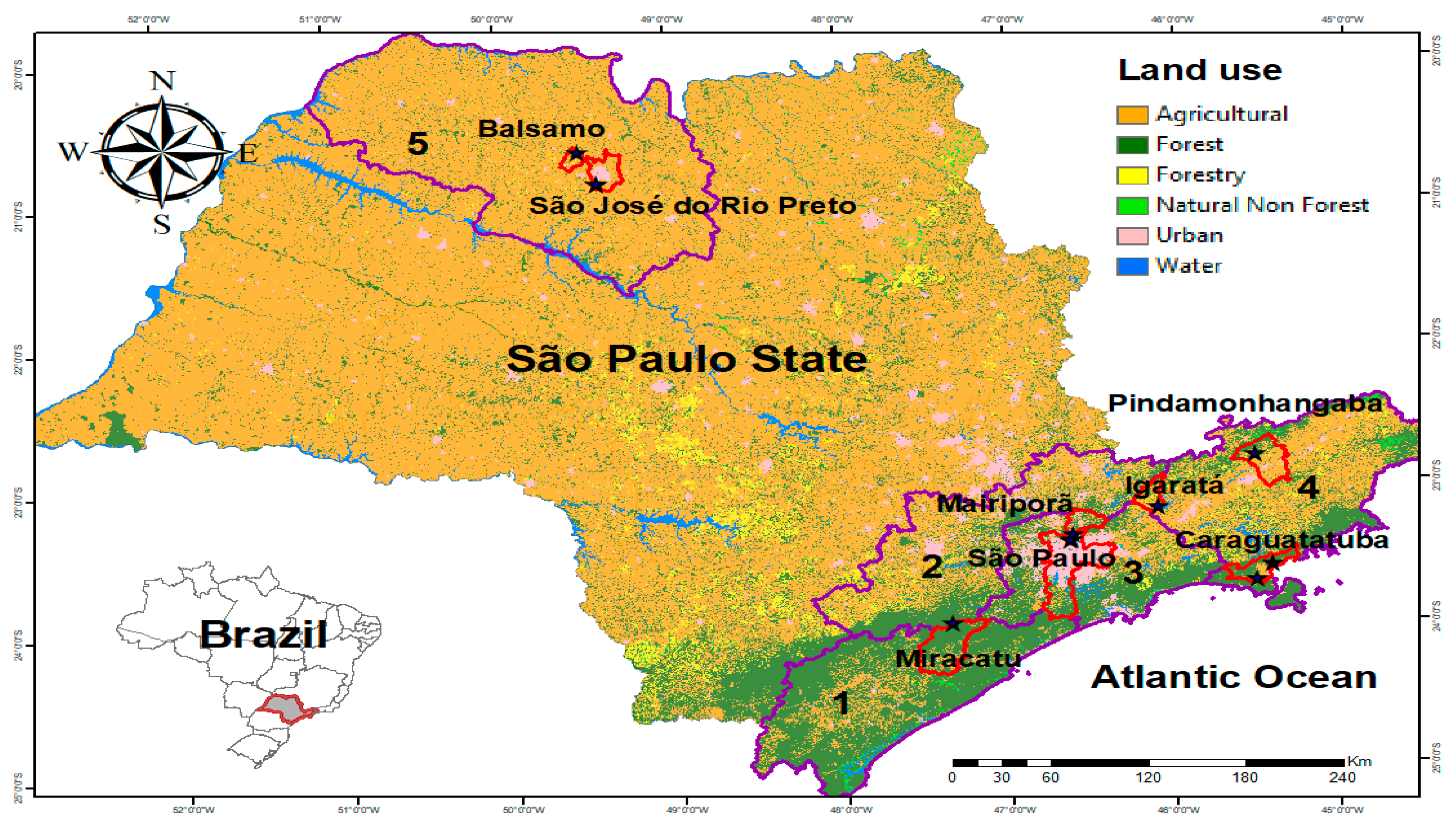

| Municipality | Location | Description | Geographical Coordinates | Number of Specimens | Total | ||
|---|---|---|---|---|---|---|---|
| ♀ | ♂ | * | |||||
| Balsamo | Sítio Madalena | Private rural property | 20°39′46.199″ S 49°31′20.302″ W | 7 | 7 | ||
| Caraguatatuba | Sítio Marisquinho | Private rural property | 23°43′23.099″ S 45°30′41.198″ W | 3 | (1) | 3(1) | |
| Benfica district | Private rural property | 23°36′51.998″ S 45°25′0.998″ W | 4 | 4 | |||
| Igaratá | Pontal das Garças Gated Community | Private property | 23°12′41.000″ S 46°6 21.899″ W | 2 | 2 | ||
| Mairiporã | Cantareira State Park | Pinheirinho trail | 23°24′27.691″ S 46°37′11.010″ W | 6(1) | 6(1) | ||
| Miracatu | “Legado das Águas” Ecological Reserve | Suspended trail | 24°1′54.199″ S 47°21 10.699″ | (4) | (1) | (3) | (8) |
| Rio Preto | Macacos Woods | Vegetation fragment south of the dirt road cutting this fragment | 20°53′4.301″ S 49°24′58.201″ W | 11 | 11 | ||
| Vegetation fragment north of the dirt road cutting this fragment | 20°53′4.898″ S 49°24′51.599″ W | 13 | 13 | ||||
| São Paulo | Cantareira State Park | Bica trail | 23°27′8.600″ S 46°38′8.700″ W | 6(2) | 2(2) | 8(4) | |
| Administration sector | 23°26′49.992″ S 46°37′57.767″ W | 3 | (11) | 3(11) | |||
| Pindamonhangaba | Trabiju Municipal Park | “Water tank” trail | 22°50′23.201″ S 45°31′24.100″ W | 10 | 10 | ||
| Total | 68(7) | 2(12) | (3) | 70(22) | |||
| Neutrality Test | Statistics | SP | MA | IG | RP | CA | BA | PI | MI |
|---|---|---|---|---|---|---|---|---|---|
| Tajima’s D test | Tajima’s D | −1.60937 | −1.62291 | 0 | −0.13906 | −0.85567 | −0.7151 | −0.62518 | 0 |
| Tajima’s D p-value | 0.026 | 0.003 | 1 | 0.535 | 0.222 | 0.265 | 0.308 | 1 | |
| Fu’s FS test | FS | −3.40282 × 1038 | −6.42258 | 0.6932 | −21.3184 | −6.82143 | −2.567 | −3.6223 | 1 × 10−6 |
| FS p-value | <0.001 | <0.001 | 0.367 | <0.001 | <0.001 | 0.031 | 0.021 | 1 |
| Predictor Variable | Intercept | Slope | SE | t-Value | Pr(>|z|) | r2 | F-Statistic | DF | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Distance | −1.87 | 0.5888 | −3.176 | 0.003944 ** | 0.4509 | 20.53 | 25 | 0.000126 | |
| 0.5081 | 0.1121 | 4.531 | 0.000126 *** | ||||||
| Models | Dependent Variable | Predictor Variable | Intercept | Slope | SE | t-Value | Pr(>|z|) | r2 | DF |
|---|---|---|---|---|---|---|---|---|---|
| M1S_2850m | theta S | Water | 4.59829 | 1.46422 | 3.14 | 0.0201 * | 0.09843 | 6 | |
| −0.08244 | 0.10185 | −0.809 | 0.4492 | ||||||
| M2S_2850m | theta S | Agricultural use | 1.99924 | 1.68306 | 1.188 | 0.28 | 0.329 | 6 | |
| 0.06009 | 0.03504 | 1.715 | 0.137 | ||||||
| M3S_2850m | theta S | Urban | 5.04073 | 1.52613 | 3.303 | 0.0163 * | 0.1721 | 6 | |
| −0.11166 | 0.09997 | −1.117 | 0.3067 | ||||||
| M4S_2850m | theta S | Forest | 5.87074 | 2.44473 | 2.401 | 0.0532 | 0.1093 | 6 | |
| −0.03582 | 0.04173 | −0.858 | 0.4237 | ||||||
| M5S_2850m | theta S | Forestry | 2.8586 | 1.4329 | 1.995 | 0.0931 | 0.2864 | 6 | |
| 0.9119 | 0.5877 | 1.552 | 0.1717 | ||||||
| M6S_2850m | theta S | ED | 1.71488 | 3.14653 | 0.545 | 0.605 | 0.1049 | 6 | |
| 0.06902 | 0.0823 | 0.839 | 0.434 | ||||||
| M7S_2850m | theta S | TE | 1.70 × 100 | 3.12 × 100 | 0.545 | 0.605 | 0.108 | 6 | |
| 2.74 × 10−5 | 3.21 × 10−5 | 0.852 | 0.427 | ||||||
| M1S_5700m | theta S | Water | 4.5354 | 1.4739 | 3.077 | 0.0217 * | 0.0787 | 6 | |
| −0.1162 | 0.1623 | −0.716 | 0.501 | ||||||
| M2S_5700m | theta S | Agricultural use | 1.15875 | 1.62083 | 0.715 | 0.5015 | 0.476 | 6 | |
| 0.07723 | 0.03308 | 2.335 | 0.0583 | ||||||
| M3S_5700m | theta S | Urban | 4.92998 | 1.55404 | 3.172 | 0.0193 * | 0.1364 | 6 | |
| −0.07013 | 0.07203 | −0.974 | 0.3678 | ||||||
| M4S_5700m | theta S | Forest | 6.38406 | 2.22982 | 2.863 | 0.0287 * | 0.2026 | 6 | |
| −0.05156 | 0.04177 | −1.235 | 0.2632 | ||||||
| M5S_5700m | theta S | Non-forest formation | 4.629 | 1.508 | 3.07 | 0.0219 * | 0.08719 | 6 | |
| −860.578 | 1136.74 | −0.757 | 0.4777 | ||||||
| M6S_5700m | theta S | Forestry | 3.2103 | 1.5494 | 2.072 | 0.0837 | 0.1544 | 6 | |
| 0.3985 | 0.3808 | 1.047 | 0.3356 | ||||||
| M7S_5700m | theta S | ED | 1.643 | 2.76258 | 0.595 | 0.574 | 0.1454 | 6 | |
| 0.07531 | 0.07454 | 1.01 | 0.351 | ||||||
| M8S_5700m | theta S | TE | 1.66 × 100 | 2.73 × 100 | 0.61 | 0.564 | 0.1477 | 6 | |
| 7.38 × 10−6 | 7.24 × 10−6 | 1.02 | 0.347 | ||||||
| Null Model | theta S | 4.107 | 1.30 × 100 | 3.16 × 100 | 0.0159 * | 7 |
| Models | Dependent Variable | Predictor Variable | Intercept | Slope | SE | t-Value | Pr(>|z|) | r2 | DF |
|---|---|---|---|---|---|---|---|---|---|
| M1pi_2850m | Water | 3.91061 | 1.35692 | 2.882 | 0.028 * | 0.06887 | 6 | ||
| −0.06288 | 0.09439 | −0.666 | 0.53 | ||||||
| M2pi_2850m | theta | Agricultural use | 1.41377 | 1.45007 | 0.975 | 0.3672 | 0.401 | 6 | |
| 0.0605 | 0.03019 | 2.004 | 0.0919 | ||||||
| M3pi_2850m | theta | Urban | 4.52117 | 1.34167 | 3.37 | 0.015 * | 0.2306 | 6 | |
| −0.11784 | 0.08788 | −1.341 | 0.229 | ||||||
| M4pi_2850m | theta | Forest | 5.41905 | 2.17796 | 2.488 | 0.0473 * | 0.1499 | 6 | |
| −0.03824 | 0.03718 | −1.029 | 0.3433 | ||||||
| M5pi_2850m | theta | Forestry | 2.3998 | 1.3077 | 1.835 | 0.116 | 0.2852 | 6 | |
| 0.8298 | 0.5363 | 1.547 | 0.173 | ||||||
| M6pi_2850m | theta | ED | 1.86668 | 2.93816 | 0.635 | 0.549 | 0.06144 | 6 | |
| 0.04816 | 0.07685 | 0.627 | 0.554 | ||||||
| M7pi_2850m | theta | TE | 1.85 × 100 | 2.91 × 100 | 0.634 | 0.55 | 0.06397 | 6 | |
| 3.00 × 10−5 | 3.00 × 10−5 | 0.64 | 0.546 | ||||||
| M1pi_5700m | Water | 3.85752 | 1.36241 | 2.831 | 0.0299 * | 0.05335 | 6 | ||
| −0.08723 | 0.15001 | −0.581 | 0.5821 | ||||||
| M2pi_5700m | Agricultural use | 0.63527 | 1.36349 | 0.466 | 0.6577 | 0.5541 | 6 | ||
| 0.07598 | 0.02783 | 2.73 | 0.0342 * | ||||||
| M3pi_5700m | Urban | 4.40785 | 1.37735 | 3.2 | 0.0186 * | 0.1842 | 6 | ||
| −0.07431 | 0.06384 | −1.164 | 0.2886 | ||||||
| M4pi_5700m | Forest | 5.77105 | 1.99192 | 2.897 | 0.0274 * | 0.2347 | 6 | ||
| −0.05062 | 0.03731 | −1.357 | 0.2237 | ||||||
| M5pi_5700m | Non-forest formation | 4.111 | 1.344 | 3.058 | 0.0223 * | 0.1272 | 6 | ||
| 947.928 | 1013.598 | −0.935 | 0.3858 | ||||||
| M6pi_5700m | Forestry | 2.6975 | 1.4062 | 1.918 | 0.104 | 0.1622 | 6 | ||
| 0.3726 | 0.3456 | 1.078 | 0.322 | ||||||
| M7pi_5700m | ED | 1.66811 | 2.58455 | 0.645 | 0.543 | 0.1005 | 6 | ||
| 0.05709 | 0.06973 | 0.819 | 0.444 | ||||||
| M8pi_5700m | TE | 1.68 × 100 | 2.55 × 100 | 0.657 | 0.535 | 0.1029 | 6 | ||
| 5.61 × 10−6 | 6.77 × 10−6 | 0.829 | 0.439 | ||||||
| Null Model | 3.536 | 1.19 × 100 | 2.984 | 0.0204 * | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilk-da-Silva, R.; Medeiros-Sousa, A.R.; Mucci, L.F.; Alonso, D.P.; Alvarez, M.V.N.; Ribolla, P.E.M.; Marrelli, M.T. Genetic Structuring of One of the Main Vectors of Sylvatic Yellow Fever: Haemagogus (Conopostegus) leucocelaenus (Diptera: Culicidae). Genes 2023, 14, 1671. https://doi.org/10.3390/genes14091671
Wilk-da-Silva R, Medeiros-Sousa AR, Mucci LF, Alonso DP, Alvarez MVN, Ribolla PEM, Marrelli MT. Genetic Structuring of One of the Main Vectors of Sylvatic Yellow Fever: Haemagogus (Conopostegus) leucocelaenus (Diptera: Culicidae). Genes. 2023; 14(9):1671. https://doi.org/10.3390/genes14091671
Chicago/Turabian StyleWilk-da-Silva, Ramon, Antônio Ralph Medeiros-Sousa, Luis Filipe Mucci, Diego Peres Alonso, Marcus Vinicius Niz Alvarez, Paulo Eduardo Martins Ribolla, and Mauro Toledo Marrelli. 2023. "Genetic Structuring of One of the Main Vectors of Sylvatic Yellow Fever: Haemagogus (Conopostegus) leucocelaenus (Diptera: Culicidae)" Genes 14, no. 9: 1671. https://doi.org/10.3390/genes14091671
APA StyleWilk-da-Silva, R., Medeiros-Sousa, A. R., Mucci, L. F., Alonso, D. P., Alvarez, M. V. N., Ribolla, P. E. M., & Marrelli, M. T. (2023). Genetic Structuring of One of the Main Vectors of Sylvatic Yellow Fever: Haemagogus (Conopostegus) leucocelaenus (Diptera: Culicidae). Genes, 14(9), 1671. https://doi.org/10.3390/genes14091671









