Behavioral and Cognitive Performance Following Exposure to Second-Hand Smoke (SHS) from Tobacco Products Associated with Oxidative-Stress-Induced DNA Damage and Repair and Disruption of the Gut Microbiome
Abstract
:1. Introduction
2. Materials and Methods
2.1. SHS Exposure and Behavioral and Cognitive Data
2.2. 16S Gut Microbiome Analysis
2.3. Immunohistochemistry
2.4. Histochemical Staining
2.5. Statistical Analyses
3. Results
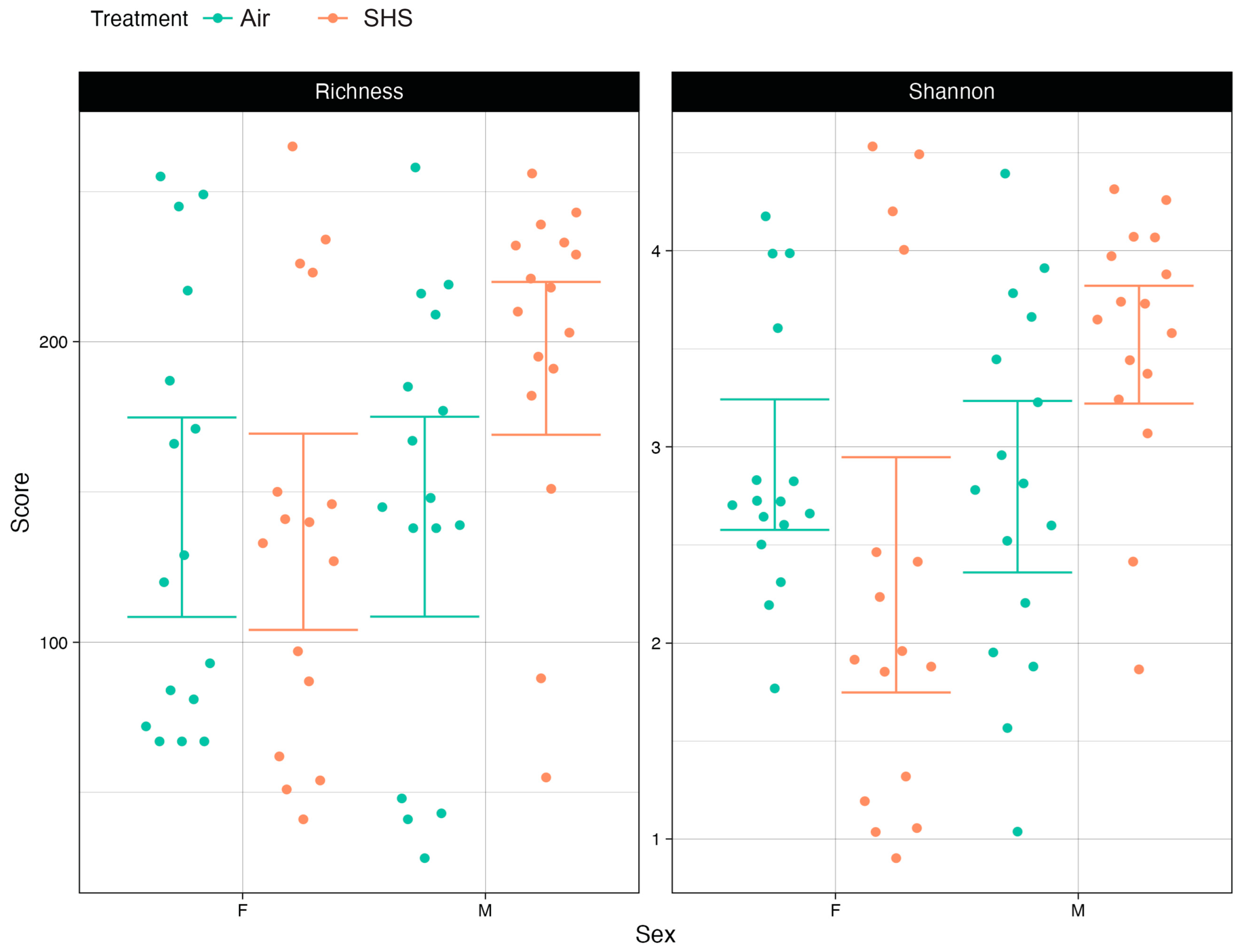
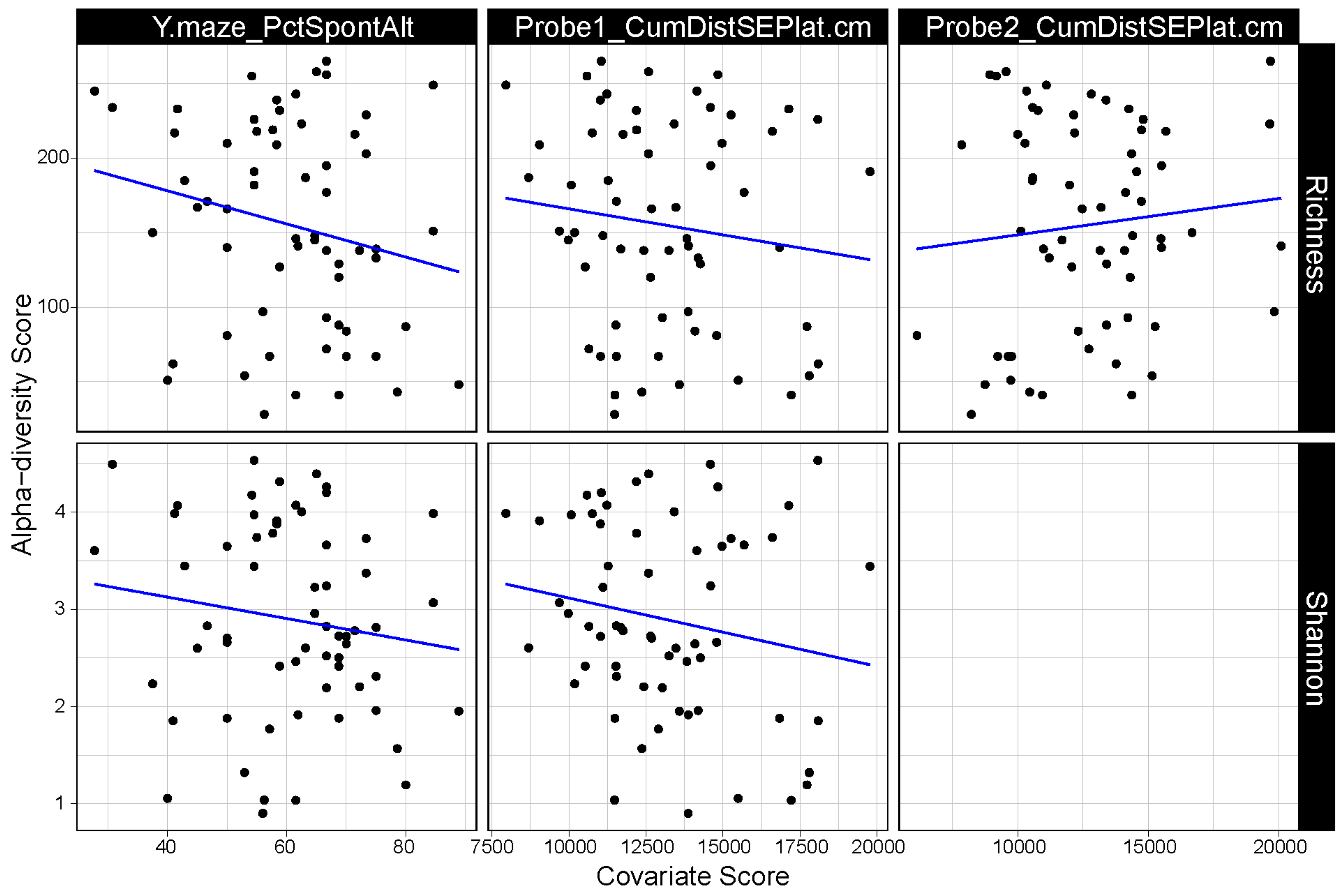
3.1. α Diversity of the Gut Microbiome Correlates with Cognitive Performance
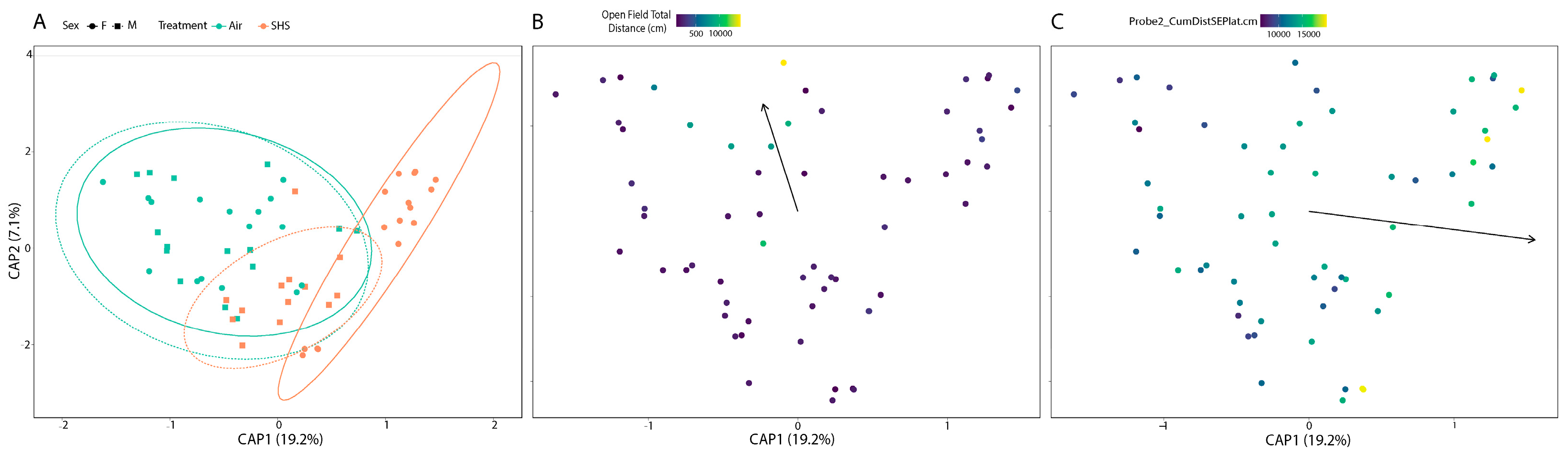
3.2. β-Diversity of the Gut Microbiome Correlates with Cognitive Performance
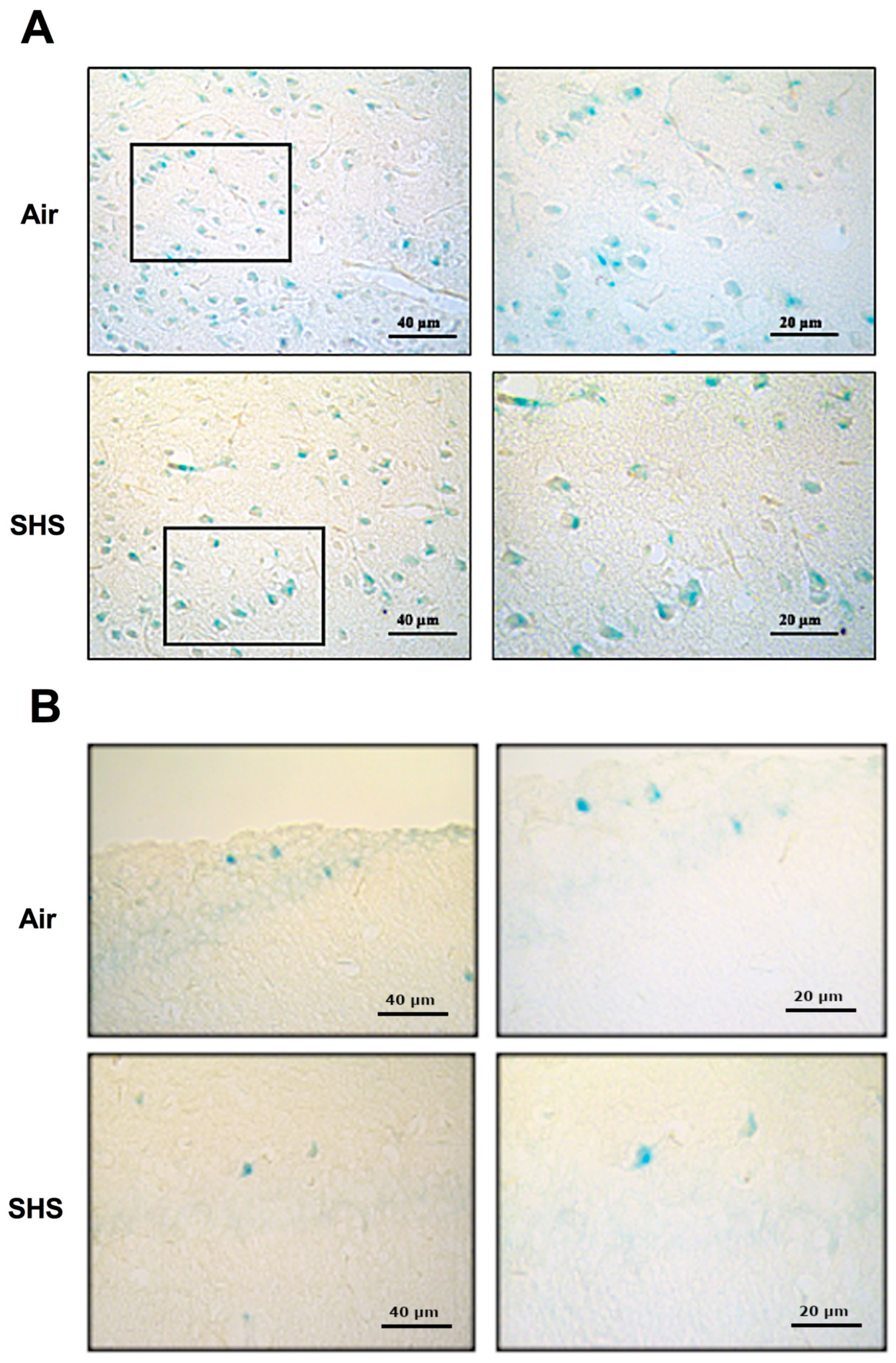
3.3. β-Galactosidase
3.4. Relationship between Percentages of p21-Labeled Cells in the CA1 Region of the Hippocampus and the Prefrontal Cortex
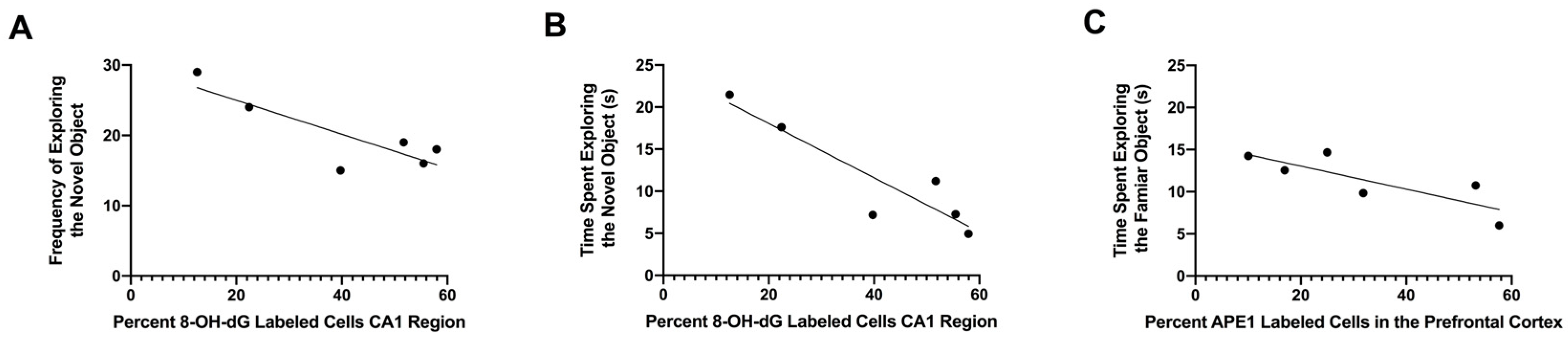
3.5. Relationship between Percentages of 8-OH-dG-Labeled Cells in the CA1 Region of the Hippocampus and APE1-Labeled Cells in the Prefrontal Cortex with Performance in the Object Recognition Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousuf, H.; Hofstra, M.; Tijssen, J.; Leenen, B.; Lindemans, J.W.; van Rossum, A.; Narula, J.; Hofstra, L. Estimated worldwide mortality attributed to secondhand tobacco smoke exposure. JAMA Netw. Open 2020, 3, e201177. [Google Scholar] [CrossRef]
- Akhtar, W.Z.; Andresen, E.M.; Cannell, M.B.; Xu, X. Association of blood cotinine level with cognitive and physical performance in non-smoking older adults. Environ. Res. 2013, 121, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, T.M.; O’Neill, T.S. Everyday prospective memory and executive function deficits associated with exposure to second-hand smoke. J. Addict. 2013, 2013, 160486. [Google Scholar] [CrossRef]
- Heffernan, T.M.; O’Neill, T.S. Exposure to second-hand smoke damages everyday prospective memory. Addiction 2013, 108, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Orsitto, G.; Turi, V.; Venezia, A.; Fulvio, F.; Manca, C. Relation of secondhand smoking to mild cognitive impairment in older inpatients. Sci. World J. 2012, 2012, 726948. [Google Scholar] [CrossRef]
- Barnes, D.E.; Haight, T.J.; Mehta, K.M.; Carlson, M.C.; Kuller, L.H.; Tager, I.B. Tager Secondhand smoke, vascular disease, and dementia incidence: Findings from the cardiovascular health cognition study. Am. J. Epidemiol. 2010, 171, 292–302. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Naughton, F.; Matthews, F.E. Matthews Exposure to secondhand smoke and cognitive impairment in non-smokers: National cross sectional study with cotinine measurement. BMJ 2009, 338, b462. [Google Scholar] [CrossRef]
- Cataldo, J.K.; Prochaska, J.J.; Glantz, S.A. Cigarette smoking is a risk factor for Alzheimer’s Disease: An analysis controlling for tobacco industry affiliation. J. Alzheimer’s Dis. 2010, 19, 465–480. [Google Scholar] [CrossRef]
- Chen, R. Association of environmental tobacco smoke with dementia and Alzheimer’s disease among never smokers. Alzheimer’s Dement. 2012, 8, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Schick, S.; Glantz, S. Philip Morris toxicological experiments with fresh sidestream smoke: More toxic than mainstream smoke. Tob. Control. 2005, 14, 396–404. [Google Scholar] [CrossRef]
- Mappin-Kasirer, B.; Pan, H.; Lewington, S.; Kizza, J.; Gray, R.; Clarke, R.; Peto, R. Tobacco smoking and the risk of Parkinson disease. Neurology 2020, 94, e2132–e2138. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, B.; Xiao, Y.; Wang, D.; Chen, W. Secondhand smoking and neurological disease: A meta-analysis of cohort studies. Rev. Environ. Health 2021, 36, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.L.; Cooke, A.C.; Johnson, C.N.; Quinn, J.L. The gut microbiome as a driver of individual variation in cognition and functional behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170286. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M. Cognitive function and the microbiome. Int. Rev. Neurobiol. 2016, 131, 227–246. [Google Scholar]
- Foster, J.A.; McVey Neufeld, K.-A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Allen, A.P.; Dinan, T.G.; Clarke, G.; Cryan, J.F. A psychology of the human brain-gut-microbiome axis. Soc. Personal. Psychol. Compass 2017, 11, e12309. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut miocrobiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Lynch, J.; Hsiao, E. Microbiomes as sources of emergent host phenotypes. Science 2019, 365, 1405–1409. [Google Scholar] [CrossRef]
- Vazquez-Villasenor, I.; Garwood, C.J.; Simpson, J.E.; Heath, P.R.; Mortiboys, H.; Wharton, S.B. The microbbiome and host behavior. Annu. Rev. Neurosci. 2017, 40, 21–49. [Google Scholar]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbbila colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Huang, S.Y.; Chen, S.D.; Zhang, Y.R.; Huang, Y.Y.; Yu, J.T. Investigating Casual Associations Among Gut Microbiota, Metabolites, and Neurodegenerative Diseases: A Mendelian Randomization Study. J. Alzheimer’s Dis. 2023, 87, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Trans. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhou, Y.; Meng, R.; Tang, J.; Zhu, J.; Aldrich, M.C.; Cox, N.J.; Zhu, Y.; Li, Y.; Zhou, D. Cross-talks between gut microbiota and tobacco smoking: A two-sample Mendelian randomization study. BMC Med. 2023, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Perez, R.; Torres, E.; Krenik, D.; Boutros, S.; Patel, E.; Chlebowski, A.; Torres, E.R.; Perveen, Z.; Penn, A.; et al. Effects of chronic second-hand smoke (SHS) exposure on cognitive performance and metabolic pathways in the hippocampus of wild-type and human tau mice. Environ. Health Perspect. 2021, 129, 57009. [Google Scholar] [CrossRef]
- Dujardin, S.; Commins, C.; Lathuiliere, A.; Beerepoot, P.; Fernandes, A.R.; Kamath, T.V.; De Los Santos, M.B.; Klickstein, N.; Corjuc, D.L.; Corjuc, B.T.; et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 2020, 26, 1256–1263. [Google Scholar] [CrossRef]
- Mufson, E.; Ward, S.; Binder, L. Prefribillar tau oligomers in mild cogntive impairments and Alzheimer’sdisease. Neuro-Degener. Dis. 2014, 13, 151–153. [Google Scholar] [CrossRef]
- Polydoro, M.; Acker, C.; Duff, K.; Casitillo, P.; Davies, P. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J. Neurosci. 2010, 29, 10741–10749. [Google Scholar] [CrossRef]
- Valanvanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Lopes, L.; Davenport, C.; Torrres, E.; Chlebowski, A.; Mikami, A.; Raber, J.; Kisby, G. Neuropathological Examination of Mice Chronically Exposed to Secondhand Smoke. Mil. Med. 2023; in press. [Google Scholar]
- Valieva, Y.; Ivanova, E.; Fayzullin, A.; Kurkov, A.; Igrunkova, A. Senescence-Associated-Galactosidase Detection in Pathology. Diagnostics 2022, 12, 2309. [Google Scholar] [CrossRef]
- de Mera-Rodríguez, J.A.; Álvarez-Hernán, G.; Gañán, Y.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Is Senescence-Associated β-Galactosidase a Reliable in vivo Marker of Cellular Senescence During Embryonic Development? Front. Cell Dev. Biol. 2021, 9, 623175. [Google Scholar] [CrossRef]
- Martínez-Zamudio, R.I.; Dewald, H.K.; Vasilopoulos, T.; Gittens-Williams, L.; Fitzgerald-Bocarsly, P.; Herbig, U. Senescence-associated β-galactosidase reveals the abundance of senescent CD8+ T cells in aging humans. Aging Cell 2021, 20, e13344. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Panth, N.; Manandhar, B.; Singh, S.K.; Gupta, G.; Wich, P.R.; Nammi, S.; MacLoughlin, R.; Adams, J.; Warkiani, M.E.; et al. Attenuation of Cigarette-Smoke-Induced Oxidative Stress, Senescence, and Inflammation by Berberine-Loaded Liquid Crystalline Nanoparticles: In Vitro Study in 16HBE and RAW264.7 Cells. Antioxidants 2022, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Stagaman, K.; Kasschau, K.; Holden, S.; Shulzhenko, N.; Sharpton, T.J.; Raber, J. Fecal implants from AppNL-G-F and AppNL-G-F/E4 donor mice sufficient to induce behavioral phenotypes in germ-free mice. Front. Behav. Neurosci. 2022, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Shimada, R.; Okada, Y.; Kawmata, T.; Kibayashi, K. Senescence-associated-β-galactosidase staining following traumatic brain injury in the mouse cerebrum. PLoS ONE 2019, 14, e0213673. [Google Scholar] [CrossRef]
- Kundu, P.; Torres, E.R.S.; Stagaman, K.; Kasschau, K.; Okhovat, M.; Holden, S.; Ward, K.; Nevonen, B.; Davis, T.; Saito, T.; et al. Integrated analysis of behavioral, epigenetic, and gut microbiome analyses in AppNL-GF, AppNL-F, and wild type mice. Sci. Rep. 2021, 11, 4678. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Anaya, A.F.; Torres, E.; Lee, J.; Boutros, S.; Grygoryev, D.; Hammer, A.; Kasschau, K.; Sharpton, T.; Turker, M.; et al. Effects of Six Sequential Charged Particle Beams on Behavioral and Cognitive Performance in B6D2F1 Female and Male Mice. Front. Physiol. 2020, 11, 959. [Google Scholar] [CrossRef]
- Rachmian, N.; Krizhanovsky, V. Senescent cells in the brain and where to find them. FEBS J. 2023, 290, 1256–1266. [Google Scholar] [CrossRef]
- Lee, M.Y.; Ojeda-Britez, S.; Ehrbar, D.; Samwer, A.; Begley, T.J.; Melendez, J.A. Selenoproteins and the senescence-associated epitranscriptome. Exp. Biol. Med. 2022, 247, 2090–2102. [Google Scholar] [CrossRef]
- Dong, L.; Wu, J.; Li, A.; Bi, W.; Liu, T.; Cao, L.; Liu, Y.; Liu, A. The inhibitory mechanism of Cordyceps sinensis on cigarette smoke extract-induced senescence in human bronchial epithelial cells. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Akinyeke, T.; Stagaman, K.; Duvoisin, R.; Meshul, C.K.; Sharpton, T.J.; Raber, J. Effects of sub-chronic MPTP exposure on behavioral and cognitive performance and the microbiome of wild-type and mGlu8 knockout female and male mice. Front. Behav. Neurosci. 2018, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.A.; Tiznado, V. Hippocampal CA1 Subregion as a Context Decoder. J. Neurosci. 2016, 36, 6602–6604. [Google Scholar] [CrossRef] [PubMed]
- Graille, M.; Wild, P.; Sauvain, J.J.; Hemmendinger, M.; Guseva Canu, I.; Hopf, N.B. Urinary 8-OHdG as a Biomarker for Oxidative Stress: A Systematic Literature Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3743. [Google Scholar] [CrossRef]
- Gao, X.; Lai, C.Q.; Scott, T.; Shen, J.; Cai, T.; Ordovas, J.M.; Tucker, K.L. Urinary 8-Hydroxy-2-deoxyguanosine and Cognitive Function in Puerto Rican Adults. Am. J. Epidemiol. 2010, 172, 271–278. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Tirle, T.; López-Nogueroles, M.; Vento, M.; Baquero, M.; Cháfer-Pericás, C. Chafer-Pericas Oxidative Damage of DNA as Early Marker of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 6136. [Google Scholar] [CrossRef]
- Abou-Elela, D.H.; El-Edel, R.H.; Shalaby, A.S.; Fouaad, M.A.; Sonbol, A.A. Sonbol Telomere length and 8-hydroxy-2-deoxyguanosine as markers for early prediction of Alzheimer disease. Indian J. Psychiatr. 2020, 62, 678–683. [Google Scholar]
- Liu, Z.; Liu, Y.; Tu, X.; Shen, H.; Qiu, H.; Chen, H.; He, J. High Serum Levels of Malondialdehyde and 8-OHdG are both Associated with Early Cognitive Impairment in Patients with Acute Ischaemic Stroke. Sci. Rep. 2017, 7, 9493. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, Y.; He, J. High serum levels of 8-OHdG are an independent predictor of post-stroke depression in Chinese stroke survivors. Neuropsychiatr. Dis. Treat. 2018, 14, 587–596. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Jha, N.; Kesari, K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022, 38, 223–244. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbă, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxid. Med. Cell Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Chen, W.-Y.; Mukda, S.; Yang, Y.-R.; Sun, S.-F.; Chen, S.-D. Oxidative DNA damage is concurrently repaired by base excision repair (BER) and apyrimidinic endonuclease 1 (APE1)-initiated nonhomologous end joining (NHEJ) in cortical neurons. Neuropathol. Appl. Neurobiol. 2020, 46, 375–390. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Gao, H.; Kuang, X.; Xiao, H.; Yang, C.; Li, M. APE1 promotes non-homologous end joining by initiating DNA double-strand break formation and decreasing ubiquitination of artemis following oxidative genotoxic stress. J. Trans. Med. 2023, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Villasenor, I.; Garwood, C.J.; Simpson, J.E.; Heath, P.R.; Mortiboys, H.; Wharton, S.B. Persistent DNA damage alters the neuronal transcriptome suggesting cell cycle dysregulation and altered mitochondrial function. Eur. J. Neurosci. 2021, 54, 6987–7005. [Google Scholar] [CrossRef]
- Sah, E.; Krishnamurthy, S.; Ahmidouch, M.Y.; Gillispie, G.J.; Milligan, C.; Orr, M.E. The Cellular Senescence Stress Response in Post-Mitotic Brain Cells: Cell Survival at the Expense of Tissue Degeneration. Life 2021, 11, 229. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]

| Marker | Region and Description (SHS vs. Air) | % Labelled Cells Air; SHS |
|---|---|---|
| 8-OHdG | PFC Darker and more punctate staining throughout | 32.7 ± 11.3; 61.5 ± 5.7 * |
| HIPP-CA1 Darker and more punctate staining throughout | 30.2 ± 15.8; 55.1 ± 3.1 | |
| HIPP-CA3 Darker and more punctate staining throughout | 29.2 ± 3.5; 57.1 ± 6.3 | |
| OGG1 | HIPP-CA4 Darker and more punctate staining throughout | 40.8 ± 10.1; 58.9 ± 2.9 |
| PFC Darker and more punctate staining in air exposed | - | |
| APE1 | HIPP Darker staining of CA1 neurons. Less dark staining in CA2 and CA3 neurons. | - |
| PFC Darker and more punctate staining throughout | 17.4 ± 7.5; 47.6 ± 13.8 * | |
| HIPP-CA1 Darker and more punctate staining throughout | 30.2 ± 15.8; 56.3 ± 4.5 | |
| HIPP-CA3 Darker and more punctate staining throughout | 30.4 ± 9.1; 58.9 ± 8.7 * | |
| HIPP-CA4 Darker and more punctate staining throughout | 32.7 ± 10.1; 66.3 ± 7.8 * | |
| - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raber, J.; Stagaman, K.; Kasschau, K.D.; Davenport, C.; Lopes, L.; Nguyen, D.; Torres, E.R.; Sharpton, T.J.; Kisby, G. Behavioral and Cognitive Performance Following Exposure to Second-Hand Smoke (SHS) from Tobacco Products Associated with Oxidative-Stress-Induced DNA Damage and Repair and Disruption of the Gut Microbiome. Genes 2023, 14, 1702. https://doi.org/10.3390/genes14091702
Raber J, Stagaman K, Kasschau KD, Davenport C, Lopes L, Nguyen D, Torres ER, Sharpton TJ, Kisby G. Behavioral and Cognitive Performance Following Exposure to Second-Hand Smoke (SHS) from Tobacco Products Associated with Oxidative-Stress-Induced DNA Damage and Repair and Disruption of the Gut Microbiome. Genes. 2023; 14(9):1702. https://doi.org/10.3390/genes14091702
Chicago/Turabian StyleRaber, Jacob, Keaton Stagaman, Kristin D. Kasschau, Conor Davenport, Leilani Lopes, Dennis Nguyen, Eileen Ruth Torres, Thomas J. Sharpton, and Glen Kisby. 2023. "Behavioral and Cognitive Performance Following Exposure to Second-Hand Smoke (SHS) from Tobacco Products Associated with Oxidative-Stress-Induced DNA Damage and Repair and Disruption of the Gut Microbiome" Genes 14, no. 9: 1702. https://doi.org/10.3390/genes14091702
APA StyleRaber, J., Stagaman, K., Kasschau, K. D., Davenport, C., Lopes, L., Nguyen, D., Torres, E. R., Sharpton, T. J., & Kisby, G. (2023). Behavioral and Cognitive Performance Following Exposure to Second-Hand Smoke (SHS) from Tobacco Products Associated with Oxidative-Stress-Induced DNA Damage and Repair and Disruption of the Gut Microbiome. Genes, 14(9), 1702. https://doi.org/10.3390/genes14091702









