Identification and Verification of Candidate miRNA Biomarkers with Application to Infection with Emiliania huxleyi Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. E. huxleyi Strain and Virus Preparation
2.2. miRNA Biomarker Candidate Identification
2.3. Culture System Setup and Sampling
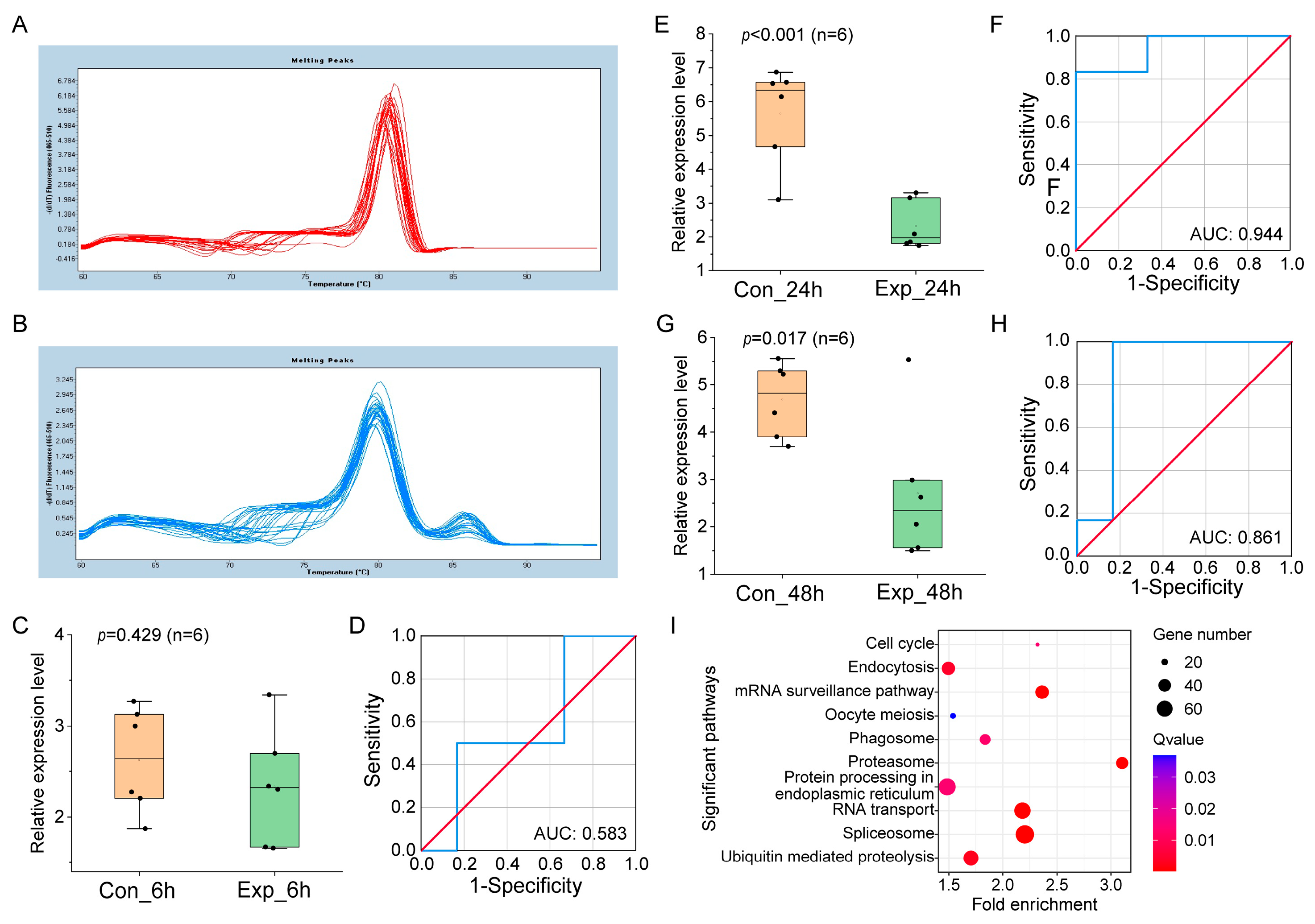
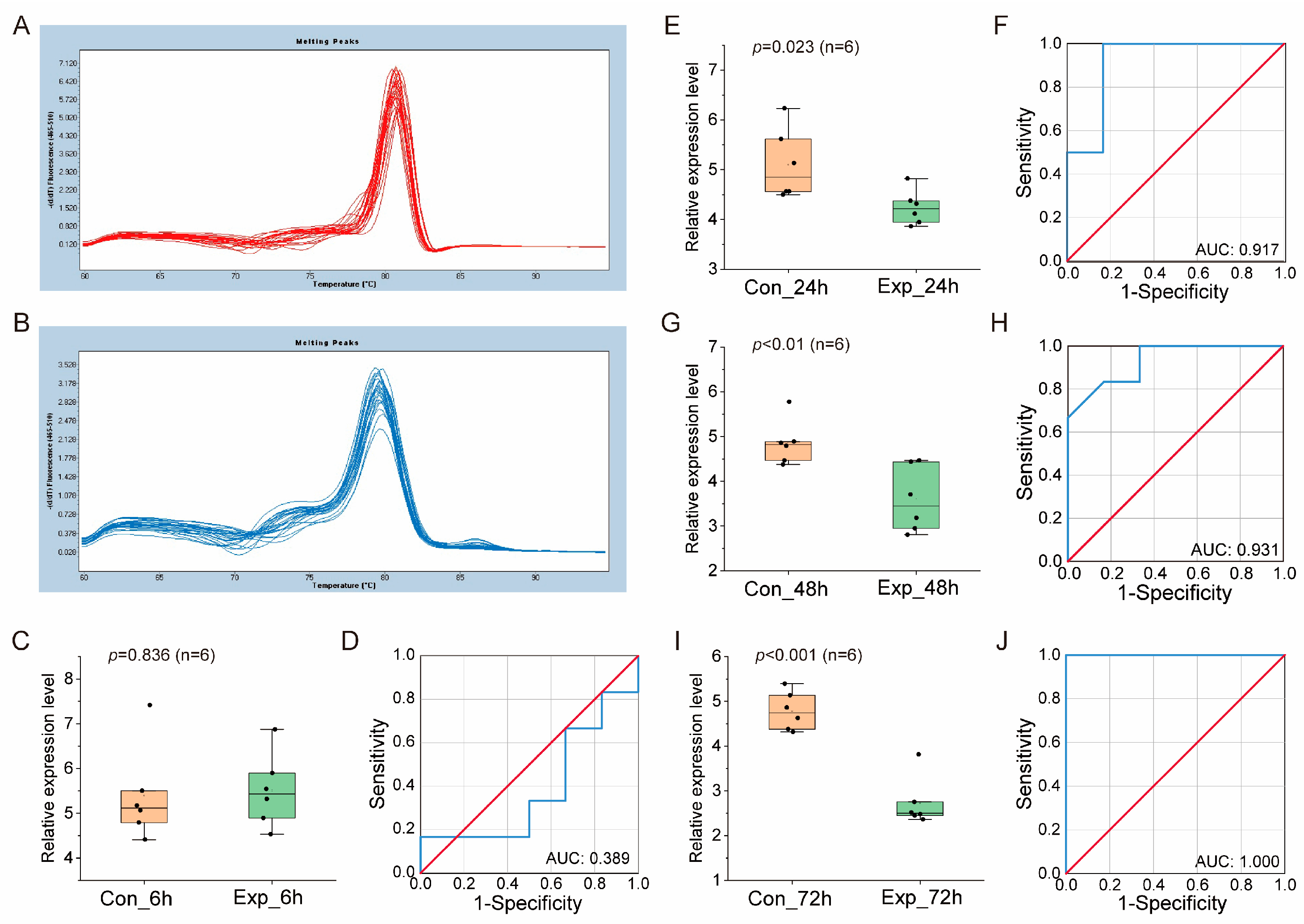
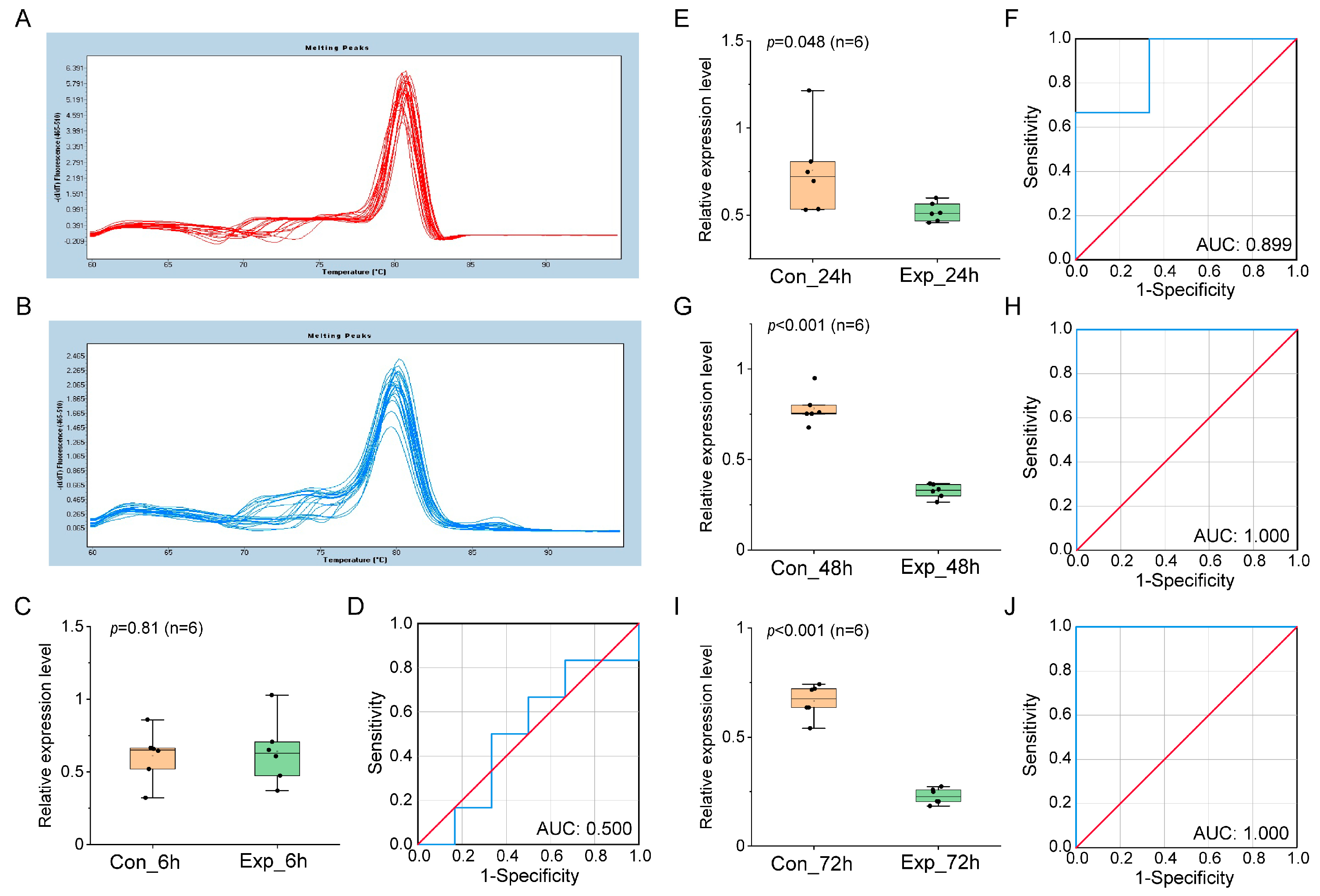
2.4. Quantitative Real-Time PCR and Receiver Operating Characteristic Curve (ROC) Analysis
2.5. Prediction and Function Enrichment Analysis of miRNA Targets
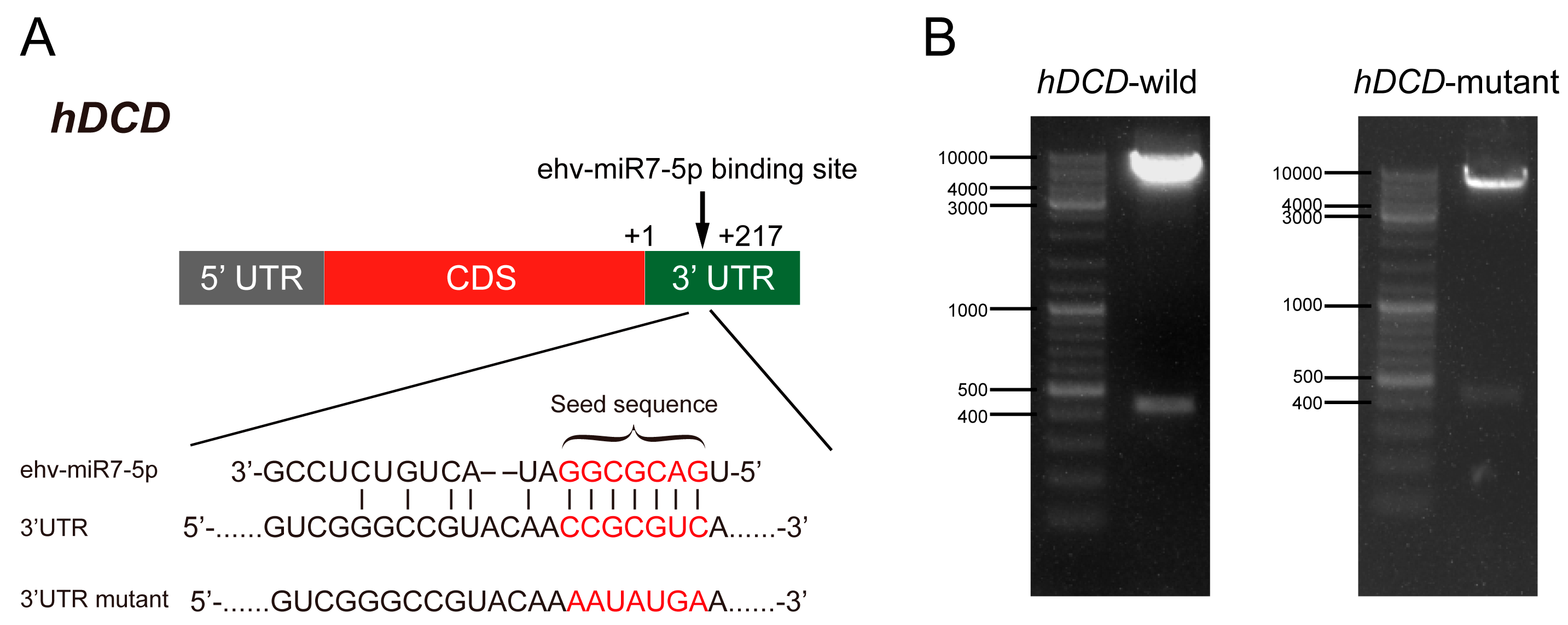
2.6. Dual-Luciferase Reporter Assay
2.7. qPCR of DCD Genes
2.8. Statistical Analysis
3. Results
3.1. The Potential miRNA Biomarker Candidates
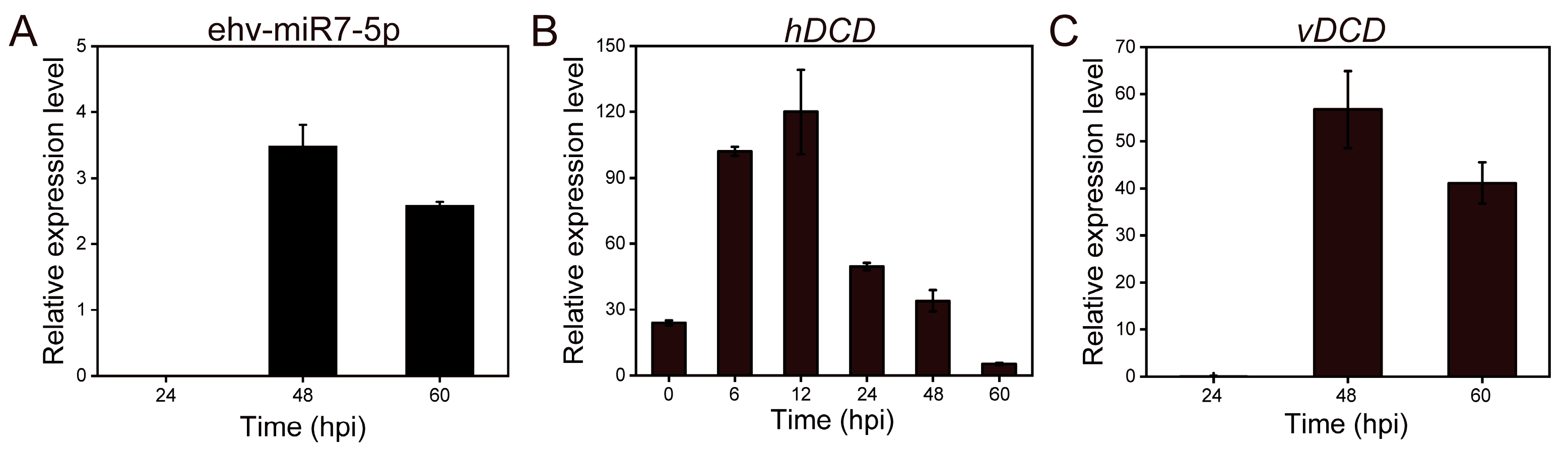
3.2. Verification of miRNA Biomarker Candidates in Different Culture Systems by qRT-PCR
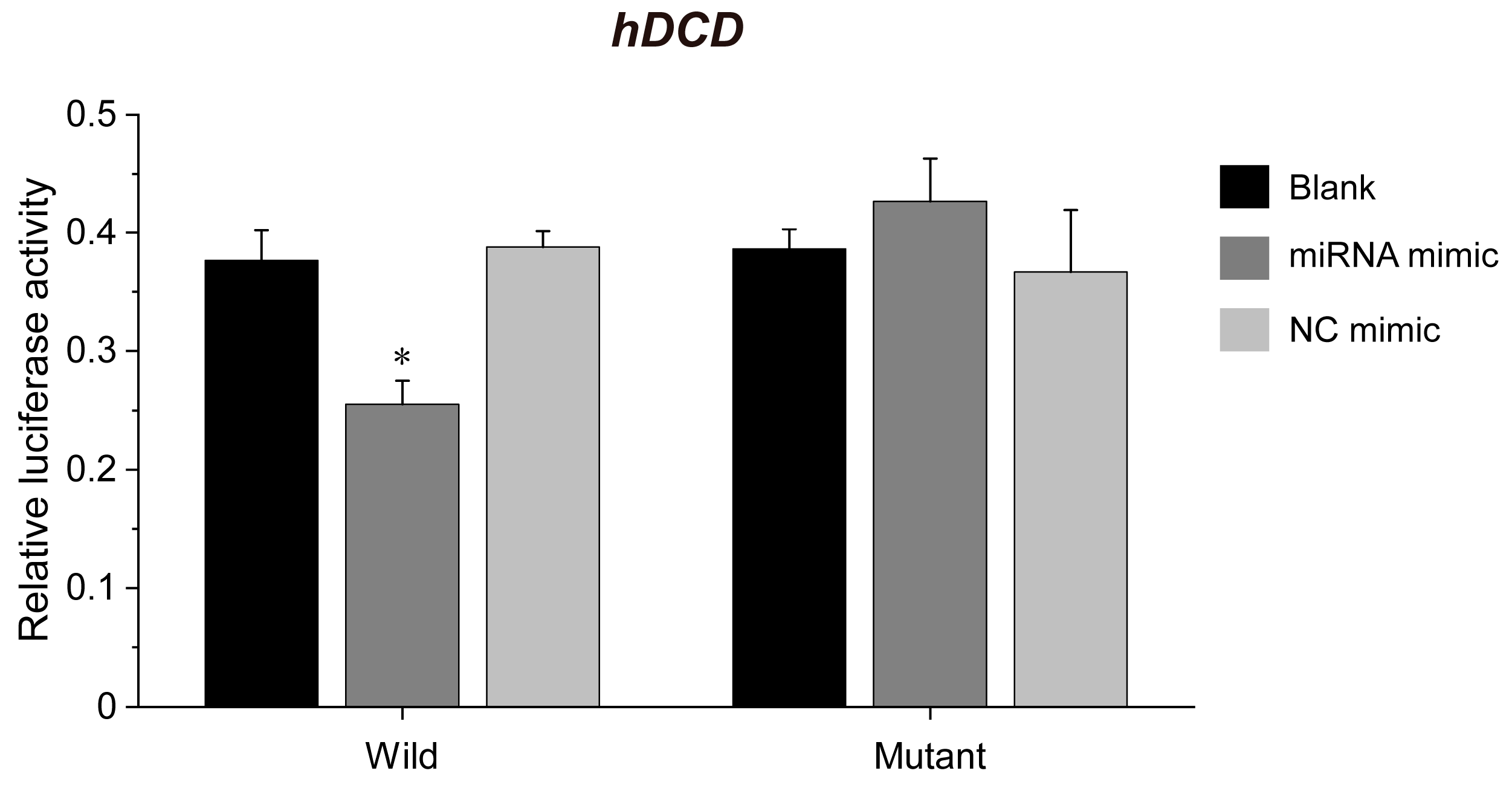
3.3. ehv-miR7-5p Could Target the hDCD Gene
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holligan, P.M.; Fernández, E.; Aiken, J.; Balch, W.M.; Boyd, P.; Burkill, P.H.; Finch, M.; Groom, S.B.; Malin, G.; Muller, K.; et al. A Biogeochemical Study of the Coccolithophore, Emiliania huxleyi, in the North Atlantic. Glob. Biogeochem. Cycles 1993, 7, 879–900. [Google Scholar] [CrossRef]
- Iglesias-Rodriguez, M.D.; Halloran, P.R.; Rickaby, R.E.M.; Hall, I.R.; Colmenero-Hidalgo, E.; Gittins, J.R.; Green, D.R.H.; Tyrrell, T.; Gibbs, S.J.; von Dassow, P.; et al. Phytoplankton Calcification in a High-CO 2 World. Science 2008, 320, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.R.; Brownlee, C.; Wheeler, G. Coccolithophore Cell Biology: Chalking Up Progress. Ann. Rev. Mar. Sci. 2017, 9, 283–310. [Google Scholar] [CrossRef]
- Simó, R. Production of Atmospheric Sulfur by Oceanic Plankton: Biogeochemical, Ecological and Evolutionary Links. Trends Ecol. Evol. 2001, 16, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Ben-Dor, S.; Feldmesser, E.; Levin, Y.; Tawfik, D.S.; Vardi, A. Identification of the Algal Dimethyl Sulfide–Releasing Enzyme: A Missing Link in the Marine Sulfur Cycle. Science 2015, 348, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Bidle, K.D. The Molecular Ecophysiology of Programmed Cell Death in Marine Phytoplankton. Ann. Rev. Mar. Sci. 2015, 7, 341–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cai, W.; Fang, X.; Wang, X.; Li, G. Virus-Induced Apoptosis and Phosphorylation Form of Metacaspase in the Marine Coccolithophorid Emiliania huxleyi. Arch. Microbiol. 2018, 200, 413–422. [Google Scholar] [CrossRef]
- Nissimov, J.I.; Vandzura, R.; Johns, C.T.; Natale, F.; Haramaty, L.; Bidle, K.D. Dynamics of Transparent Exopolymer Particle Production and Aggregation during Viral Infection of the Coccolithophore, Emiliania huxleyi. Environ. Microbiol. 2018, 20, 2880–2897. [Google Scholar] [CrossRef]
- Laber, C.P.; Hunter, J.E.; Carvalho, F.; Collins, J.R.; Hunter, E.J.; Schieler, B.M.; Boss, E.; More, K.; Frada, M.; Thamatrakoln, K.; et al. Coccolithovirus Facilitation of Carbon Export in the North Atlantic. Nat. Microbiol. 2018, 3, 537–547. [Google Scholar] [CrossRef]
- Jover, L.F.; Effler, T.C.; Buchan, A.; Wilhelm, S.W.; Weitz, J.S. The Elemental Composition of Virus Particles: Implications for Marine Biogeochemical Cycles. Nat. Rev. Microbiol. 2014, 12, 519–528. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, W.; Cai, L. The Fate and Biogeochemical Cycling of Viral Elements. Nat. Rev. Microbiol. 2014, 12, 850–851. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, S.; Mausz, M.A.; Schatz, D.; Sheyn, U.; Malitsky, S.; Aharoni, A.; Weinstock, E.; Tzfadia, O.; Ben-Dor, S.; Feldmesser, E.; et al. Rewiring Host Lipid Metabolism by Large Viruses Determines the Fate of Emiliania huxleyi, a Bloom-Forming Alga in the Ocean. Plant Cell 2014, 26, 2689–2707. [Google Scholar] [CrossRef]
- Schleyer, G.; Shahaf, N.; Ziv, C.; Dong, Y.; Meoded, R.A.; Helfrich, E.J.N.; Schatz, D.; Rosenwasser, S.; Rogachev, I.; Aharoni, A.; et al. In Plaque-Mass Spectrometry Imaging of a Bloom-Forming Alga during Viral Infection Reveals a Metabolic Shift towards Odd-Chain Fatty Acid Lipids. Nat. Microbiol. 2019, 4, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Frada, M.J.; Fredricks, H.F.; Vardi, A.; Van Mooy, B.A.S. Targeted and Untargeted Lipidomics of Emiliania huxleyi Viral Infection and Life Cycle Phases Highlights Molecular Biomarkers of Infection, Susceptibility, and Ploidy. Front. Mar. Sci. 2015, 2, 81. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, S.; Cai, W.; Jiang, H.; Lu, X.; Li, G.; Li, J.; Liu, J. Emerging Lipidome Patterns Associated with Marine Emiliania huxleyi-Virus Model System. Sci. Total Environ. 2019, 688, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Kuhlisch, C.; Schleyer, G.; Shahaf, N.; Vincent, F.; Schatz, D.; Vardi, A. Viral Infection of Algal Blooms Leaves a Unique Metabolic Footprint on the Dissolved Organic Matter in the Ocean. Sci. Adv. 2021, 7, eabf4680. [Google Scholar] [CrossRef]
- Zhang, E.; Gao, J.; Wei, Z.; Zeng, J.; Li, J.; Li, G.; Liu, J. MicroRNA-Mediated Regulation of Lipid Metabolism in Virus-Infected Emiliania huxleyi. ISME J. 2022, 16, 2457–2466. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Mockly, S.; Seitz, H. Inconsistencies and Limitations of Current MicroRNA Target Identification Methods; Laganà, A., Ed.; Springer: New York, NY, USA, 2019; pp. 291–314. ISBN 978-1-4939-9207-2. [Google Scholar]
- Pedersen, I.M.; Cheng, G.; Wieland, S.; Volinia, S.; Croce, C.M.; Chisari, F.V.; David, M. Interferon Modulation of Cellular MicroRNAs as an Antiviral Mechanism. Nature 2007, 449, 919–922. [Google Scholar] [CrossRef]
- Tribolet, L.; Kerr, E.; Cowled, C.; Bean, A.G.D.; Stewart, C.R.; Dearnley, M.; Farr, R.J. MicroRNA Biomarkers for Infectious Diseases: From Basic Research to Biosensing. Front. Microbiol. 2020, 11, 1197. [Google Scholar] [CrossRef]
- Kawano, Y.; Iwata, S.; Kawada, J.; Gotoh, K.; Suzuki, M.; Torii, Y.; Kojima, S.; Kimura, H.; Ito, Y. Plasma Viral MicroRNA Profiles Reveal Potential Biomarkers for Chronic Active Epstein–Barr Virus Infection. J. Infect. Dis. 2013, 208, 771–779. [Google Scholar] [CrossRef]
- EL-Abd, N.E.; Fawzy, N.A.; EL-Sheikh, S.M.; Soliman, M.E. Circulating MiRNA-122, MiRNA-199a, and MiRNA-16 as Biomarkers for Early Detection of Hepatocellular Carcinoma in Egyptian Patients with Chronic Hepatitis C Virus Infection. Mol. Diagn. Ther. 2015, 19, 213–220. [Google Scholar] [CrossRef]
- Biswas, S.; Haleyurgirisetty, M.; Lee, S.; Hewlett, I.; Devadas, K. Development and Validation of Plasma MiRNA Biomarker Signature Panel for the Detection of Early HIV-1 Infection. EBioMedicine 2019, 43, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Byun, J.; Guk, K.; Hwang, S.G.; Bae, P.K.; Jung, J.; Kang, T.; Lim, E.-K. Highly Sensitive in Vitro Diagnostic System of Pandemic Influenza A (H1N1) Virus Infection with Specific MicroRNA as a Biomarker. ACS Omega 2019, 4, 14560–14568. [Google Scholar] [CrossRef]
- Pagarete, A.; Lanzén, A.; Puntervoll, P.; Sandaa, R.A.; Larsen, A.; Larsen, J.B.; Allen, M.J.; Bratbak, G. Genomic Sequence and Analysis of EhV-99B1, a New Coccolithovirus from the Norwegian Fjords. Intervirology 2013, 56, 60–66. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA Targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Zhang, E.; Wu, S.; Cai, W.; Zeng, J.; Li, J.; Li, G.; Liu, J. Validation of Superior Reference Genes for QRT-PCR and Western Blot Analyses in Marine Emiliania huxleyi –Virus Model System. J. Appl. Microbiol. 2021, 131, 257–271. [Google Scholar] [CrossRef]
- Frada, M.; Probert, I.; Allen, M.J.; Wilson, W.H.; de Vargas, C. The “Cheshire Cat” Escape Strategy of the Coccolithophore Emiliania huxleyi in Response to Viral Infection. Proc. Natl. Acad. Sci. USA 2008, 105, 15944–15949. [Google Scholar] [CrossRef]
- Drexler, H.C.A. Programmed Cell Death and the Proteasome. Apoptosis 1998, 3, 1–7. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, J.-W.; Jin, U.-H.; Choi, D.; Paek, K.-H.; Pai, H.-S. Activation of the Programmed Cell Death Pathway by Inhibition of Proteasome Function in Plants. J. Biol. Chem. 2003, 278, 19406–19415. [Google Scholar] [CrossRef]
- Sheyn, U.; Rosenwasser, S.; Ben-Dor, S.; Porat, Z.; Vardi, A. Modulation of Host ROS Metabolism Is Essential for Viral Infection of a Bloom-Forming Coccolithophore in the Ocean. ISME J. 2016, 10, 1742–1754. [Google Scholar] [CrossRef]
- Liu, J.; Bratbak, G.; Zheng, T.; Thyrhaug, R. Effects of Virus Infection on Expression of Cell Cycle Regulatory Proteins in the Unicellular Marine Algae Emiliania huxleyi. Acta Oceanol. Sin. 2011, 30, 89–95. [Google Scholar] [CrossRef]
- Malitsky, S.; Ziv, C.; Rosenwasser, S.; Zheng, S.; Schatz, D.; Porat, Z.; Ben-Dor, S.; Aharoni, A.; Vardi, A. Viral Infection of the Marine Alga Emiliania huxleyi Triggers Lipidome Remodeling and Induces the Production of Highly Saturated Triacylglycerol. New Phytol. 2016, 210, 88–96. [Google Scholar] [CrossRef]
- Vincent, F.; Sheyn, U.; Porat, Z.; Schatz, D.; Vardi, A. Visualizing Active Viral Infection Reveals Diverse Cell Fates in Synchronized Algal Bloom Demise. Proc. Natl. Acad. Sci. USA 2021, 118, e2021586118. [Google Scholar] [CrossRef]
- Sheyn, U.; Rosenwasser, S.; Lehahn, Y.; Barak-Gavish, N.; Rotkopf, R.; Bidle, K.D.; Koren, I.; Schatz, D.; Vardi, A. Expression Profiling of Host and Virus during a Coccolithophore Bloom Provides Insights into the Role of Viral Infection in Promoting Carbon Export. ISME J. 2018, 12, 704–713. [Google Scholar] [CrossRef]
- Evans, C.; Malin, G.; Mills, G.P.; Wilson, W.H. Viral Infection Of Emiliania huxleyi (Prymnesiophyceae) Leads To Elevated Production Of Reactive Oxygen Species. J. Phycol. 2006, 42, 1040–1047. [Google Scholar] [CrossRef]
- Schatz, D.; Rosenwasser, S.; Malitsky, S.; Wolf, S.G.; Feldmesser, E.; Vardi, A. Communication via Extracellular Vesicles Enhances Viral Infection of a Cosmopolitan Alga. Nat. Microbiol. 2017, 2, 1485–1492. [Google Scholar] [CrossRef]
- Ziv, C.; Malitsky, S.; Othman, A.; Ben-Dor, S.; Wei, Y.; Zheng, S.; Aharoni, A.; Hornemann, T.; Vardi, A. Viral Serine Palmitoyltransferase Induces Metabolic Switch in Sphingolipid Biosynthesis and Is Required for Infection of a Marine Alga. Proc. Natl. Acad. Sci. USA 2016, 113, E1907-16. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Obeid, L.M. Ceramide and Apoptosis: Exploring the Enigmatic Connections between Sphingolipid Metabolism and Programmed Cell Death. Anticancer Agents Med. Chem. 2012, 12, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, J.; Zhang, E.; Jiang, H.; Li, G.; Li, J.; Zeng, J.; Wu, D. Characterization of the Sphingolipid Profiling of Emiliania huxleyi against Virus Infection. J. Oceanol. Limnol. 2023. [Google Scholar] [CrossRef]


| miRNA ID | Primer Sequences |
|---|---|
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
| ehx-miR20-5p | CGGCGGTAGTCGGCGGTAAA |
| ehv-miR1-3p | GGAATTGGTCGTCACGTTGTTGT |
| ehv-miR2-3p | GGTGAGAGTGCATCGGATTGTGAA |
| ehv-miR3-3p | GGATTTGGGCTGGCCCAAAA |
| ehv-miR4-3p | GGATCTAGGAAAGATTGAGGCCAAA |
| ehv-miR5-3p | GGCGAAGACACTGTGAATCAAGT |
| ehv-miR6-5p | CGGGCGGAAAATATGATTCGTTA |
| ehv-miR7-5p | GCCTGACGCGGATACTGTCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, E.; Zhang, S.; Li, G.; Zhang, Z.; Liu, J. Identification and Verification of Candidate miRNA Biomarkers with Application to Infection with Emiliania huxleyi Virus. Genes 2023, 14, 1716. https://doi.org/10.3390/genes14091716
Zhang E, Zhang S, Li G, Zhang Z, Liu J. Identification and Verification of Candidate miRNA Biomarkers with Application to Infection with Emiliania huxleyi Virus. Genes. 2023; 14(9):1716. https://doi.org/10.3390/genes14091716
Chicago/Turabian StyleZhang, Enquan, Shumiao Zhang, Guiling Li, Zhengxiao Zhang, and Jingwen Liu. 2023. "Identification and Verification of Candidate miRNA Biomarkers with Application to Infection with Emiliania huxleyi Virus" Genes 14, no. 9: 1716. https://doi.org/10.3390/genes14091716





