Quantitative Evaluation of CFTR Gene Expression: A Comparison between Relative Quantification by Real-Time PCR and Absolute Quantification by Droplet Digital PCR
Abstract
:1. Introduction
2. Materials and Methods
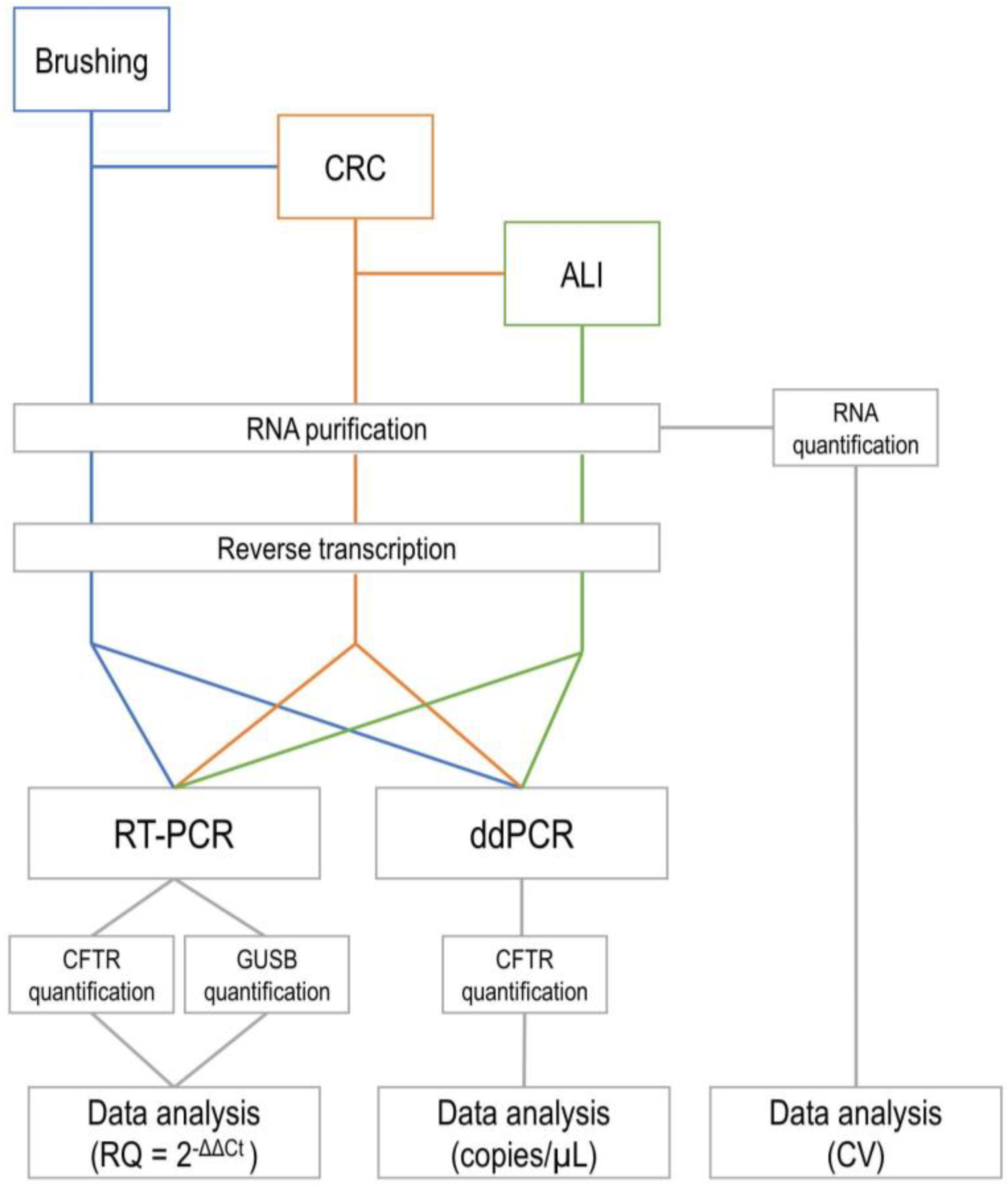
2.1. Case Series, Nasal Brushing and Cell Cultures
2.2. DNA Purification, Quantification, and CFTR Mutational Analysis
2.3. RNA Purification, Quantification and Reverse Transcription
2.4. CFTR Expression Analysis by RT-PCR
2.5. CFTR Expression Analysis by ddPCR
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogan, M.P.; Stoltz, D.A.; Hornick, D.B. Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation-specific treatment. Chest 2011, 139, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Vankeerberghen, A.; Cuppens, H.; Cassiman, J.J. The cystic fibrosis transmembrane conductance regulator: An intriguing protein with pleiotropic functions. J. Cyst. Fibros. 2002, 1, 13–29. [Google Scholar] [PubMed]
- Lucarelli, M.; Pierandrei, S.; Bruno, S.M.; Strom, R. The genetics of CFTR: Genotype-phenotype relationship, diagnostic challenge and therapeutic implications. In Cystic Fibrosis-Renewed Hopes Through Research; Sriramulu, D., Ed.; Intech-Open access company: Rijeka, Croatia, 2012; pp. 91–122. [Google Scholar]
- Terlizzi, V.; Castaldo, G.; Salvatore, D.; Lucarelli, M.; Raia, V.; Angioni, A.; Carnovale, V.; Cirilli, N.; Casciaro, R.; Colombo, C.; et al. Genotype-phenotype correlation and functional studies in patients with cystic fibrosis bearing CFTR complex alleles. J. Med. Genet. 2017, 54, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, M.; Bruno, S.M.; Pierandrei, S.; Ferraguti, G.; Stamato, A.; Narzi, F.; Amato, A.; Cimino, G.; Bertasi, S.; Quattrucci, S.; et al. A Genotypic-Oriented View of CFTR Genetics Highlights Specific Mutational Patterns Underlying Clinical Macrocategories of Cystic Fibrosis. Mol. Med. 2015, 21, 257–275. [Google Scholar]
- Lucarelli, M.; Porcaro, L.; Biffignandi, A.; Costantino, L.; Giannone, V.; Alberti, L.; Bruno, S.M.; Corbetta, C.; Torresani, E.; Colombo, C.; et al. A New Targeted CFTR Mutation Panel Based on Next-Generation Sequencing Technology. J. Mol. Diagn. 2017, 19, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, M.; Bruno, S.M.; Pierandrei, S.; Ferraguti, G.; Testino, G.; Truglio, G.; Strom, R.; Quattrucci, S. The Impact on Genetic Testing of Mutational Patterns of CFTR Gene in Different Clinical Macrocategories of Cystic Fibrosis. J. Mol. Diagn. 2016, 18, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Tsui, L.C.; Dorfman, R. The cystic fibrosis gene: A molecular genetic perspective. Cold Spring Harb. Perspect. Med. 2013, 3, a009472. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Terlizzi, V.; Lucarelli, M.; Salvatore, D.; Angioni, A.; Bisogno, A.; Braggion, C.; Buzzetti, R.; Carnovale, V.; Casciaro, R.; Castaldo, G.; et al. Clinical expression of cystic fibrosis in a large cohort of Italian siblings. BMC Pulm. Med. 2018, 18, 196. [Google Scholar] [CrossRef]
- Terlizzi, V.; Carnovale, V.; Castaldo, G.; Castellani, C.; Cirilli, N.; Colombo, C.; Corti, F.; Cresta, F.; D’Adda, A.; Lucarelli, M.; et al. Clinical expression of patients with the D1152H CFTR mutation. J. Cyst. Fibros. 2015, 14, 447–452. [Google Scholar] [CrossRef]
- Narzi, L.; Ferraguti, G.; Stamato, A.; Narzi, F.; Valentini, S.B.; Lelli, A.; Delaroche, I.; Lucarelli, M.; Strom, R.; Quattrucci, S. Does cystic fibrosis neonatal screening detect atypical CF forms? Extended genetic characterization and 4-year clinical follow-up. Clin. Genet. 2007, 72, 39–46. [Google Scholar]
- Bareil, C.; Guittard, C.; Altieri, J.P.; Templin, C.; Claustres, M.; des Georges, M. Comprehensive and rapid genotyping of mutations and haplotypes in congenital bilateral absence of the vas deferens and other cystic fibrosis transmembrane conductance regulator-related disorders. J. Mol. Diagn. 2007, 9, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.V.; Aveta, A.; Comegna, M.; Cernera, G.; Iacotucci, P.; Carnovale, V.; Taccetti, G.; Terlizzi, V.; Castaldo, G. Extensive CFTR Gene Analysis Revealed a Higher Occurrence of Cystic Fibrosis Transmembrane Regulator-Related Disorders (CFTR-RD) among CF Carriers. J. Clin. Med. 2020, 9, 3853. [Google Scholar] [CrossRef]
- Bear, C.E. A Therapy for Most with Cystic Fibrosis. Cell 2020, 180, 211. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.D. Novel personalized therapies for cystic fibrosis: Treating the basic defect in all patients. J. Intern. Med. 2015, 277, 155–166. [Google Scholar] [PubMed]
- Bacalhau, M.; Camargo, M.; Magalhaes-Ghiotto, G.A.V.; Drumond, S.; Castelletti, C.H.M.; Lopes-Pacheco, M. Elexacaftor-Tezacaftor-Ivacaftor: A Life-Changing Triple Combination of CFTR Modulator Drugs for Cystic Fibrosis. Pharmaceuticals 2023, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Straniero, L.; Solda, G.; Costantino, L.; Seia, M.; Melotti, P.; Colombo, C.; Asselta, R.; Duga, S. Whole-gene CFTR sequencing combined with digital RT-PCR improves genetic diagnosis of cystic fibrosis. J. Hum. Genet. 2016, 61, 977–984. [Google Scholar] [CrossRef]
- Sette, G.; Lo Cicero, S.; Blacona, G.; Pierandrei, S.; Bruno, S.M.; Salvati, V.; Castelli, G.; Falchi, M.; Fabrizzi, B.; Cimino, G.; et al. Theratyping cystic fibrosis in vitro in ALI culture and organoid models generated from patient-derived nasal epithelial conditionally reprogrammed stem cells. Eur. Respir. J. 2021, 58, 2100908. [Google Scholar] [CrossRef]
- Sette, G.; Salvati, V.; Giordani, I.; Pilozzi, E.; Quacquarini, D.; Duranti, E.; De Nicola, F.; Pallocca, M.; Fanciulli, M.; Falchi, M.; et al. Conditionally reprogrammed cells (CRC) methodology does not allow the in vitro expansion of patient-derived primary and metastatic lung cancer cells. Int. J. Cancer 2018, 143, 88–99. [Google Scholar] [CrossRef]
- Lucarelli, M.; Narzi, L.; Piergentili, R.; Ferraguti, G.; Grandoni, F.; Quattrucci, S.; Strom, R. A 96-well formatted method for exon and exon/intron boundary full sequencing of the CFTR gene. Anal. Biochem. 2006, 353, 226–235. [Google Scholar] [CrossRef]
- Ferraguti, G.; Pierandrei, S.; Bruno, S.M.; Ceci, F.; Strom, R.; Lucarelli, M. A template for mutational data analysis of the CFTR gene. Clin. Chem. Lab Med. 2011, 49, 1447–1451. [Google Scholar] [CrossRef]
- Lucarelli, M.; Grandoni, F.; Rossi, T.; Mazzilli, F.; Antonelli, M.; Strom, R. Simultaneous cycle sequencing assessment of (TG)m and Tn tract length in CFTR gene. Biotechniques 2002, 32, 540–547. [Google Scholar] [CrossRef]
- Artika, I.M.; Dewi, Y.P.; Nainggolan, I.M.; Siregar, J.E.; Antonjaya, U. Real-Time Polymerase Chain Reaction: Current Techniques, Applications, and Role in COVID-19 Diagnosis. Genes 2022, 13, 2387. [Google Scholar] [CrossRef]
- Sterrantino, M.; Fuso, A.; Pierandrei, S.; Bruno, S.M.; Testino, G.; Cimino, G.; Angeloni, A.; Lucarelli, M. Quantitative Evaluation of CFTR Pre-mRNA Splicing Dependent on the (TG)mTn Poly-Variant Tract. Diagnostics 2021, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, S.; Balducci, S.; Guerrini, F.; Del Re, M.; Cacciola, R. Digital Droplet PCR in Hematologic Malignancies: A New Useful Molecular Tool. Diagnostics 2022, 12, 1305. [Google Scholar] [CrossRef]
- Bergougnoux, A.; Taulan-Cadars, M.; Claustres, M.; Raynal, C. Current and future molecular approaches in the diagnosis of cystic fibrosis. Expert Rev. Respir. Med. 2018, 12, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Ishak, A.; AlRawashdeh, M.M.; Esagian, S.M.; Nikas, I.P. Diagnostic, Prognostic, and Therapeutic Value of Droplet Digital PCR (ddPCR) in COVID-19 Patients: A Systematic Review. J. Clin. Med. 2021, 10, 5712. [Google Scholar] [CrossRef]
- Kojabad, A.A.; Farzanehpour, M.; Galeh, H.E.G.; Dorostkar, R.; Jafarpour, A.; Bolandian, M.; Nodooshan, M.M. Droplet digital PCR of viral DNA/RNA, current progress, challenges, and future perspectives. J. Med. Virol. 2021, 93, 4182–4197. [Google Scholar] [CrossRef]
- Li, H.; Bai, R.; Zhao, Z.; Tao, L.; Ma, M.; Ji, Z.; Jian, M.; Ding, Z.; Dai, X.; Bao, F.; et al. Application of droplet digital PCR to detect the pathogens of infectious diseases. Biosci. Rep. 2018, 38, BSR20181170. [Google Scholar] [CrossRef]
- Olmedillas-Lopez, S.; Olivera-Salazar, R.; Garcia-Arranz, M.; Garcia-Olmo, D. Current and Emerging Applications of Droplet Digital PCR in Oncology: An Updated Review. Mol. Diagn. Ther. 2022, 26, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Tosco, A.; Castaldo, A.; Colombo, C.; Claut, L.; Carnovale, V.; Iacotucci, P.; Lucarelli, M.; Cimino, G.; Fabrizzi, B.; Caporelli, N.; et al. Clinical outcomes of a large cohort of individuals with the F508del/5T;TG12 CFTR genotype. J. Cyst. Fibros. 2022, 21, 850–855. [Google Scholar] [CrossRef] [PubMed]



| Protocol | Level of RNA Concentration or CFTR Expression | Average Coefficient of Variation of the RNA or CFTR Quantification (CV% ± S.D.) |
|---|---|---|
| RNA purification | RNA concentration ≤ 10 ng/μL (N = 11) | 12.4 ± 7.5 |
| RNA concentration > 10 ng/μL (N = 32) | 1.3 ± 0.6 | |
| RT-PCR | Total CFTR expression (N = 53) | 11.2 ± 7.0 |
| Low CFTR expression (RQ ≤ 1; N = 15) (undifferentiated CF-CRC-AESC) | 14.9 ± 8.8 | |
| High CFTR expression (RQ > 1; N = 38) (brushing and differentiated CF-CRC-AESC) | 9.8 ± 5.7 | |
| ddPCR | Total CFTR expression (N = 55) | 9.0 ± 7.4 |
| Low CFTR expression (copies/μL ≤ 26; N = 17) (undifferentiated CF-CRC-AESC) | 12.8 ± 8.7 | |
| High CFTR expression (copies/μL > 26; N = 38) (brushing and differentiated CF-CRC-AESC) | 7.3 ± 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, S.M.; Blaconà, G.; Lo Cicero, S.; Castelli, G.; Virgulti, M.; Testino, G.; Pierandrei, S.; Fuso, A.; Cimino, G.; Ferraguti, G.; et al. Quantitative Evaluation of CFTR Gene Expression: A Comparison between Relative Quantification by Real-Time PCR and Absolute Quantification by Droplet Digital PCR. Genes 2023, 14, 1781. https://doi.org/10.3390/genes14091781
Bruno SM, Blaconà G, Lo Cicero S, Castelli G, Virgulti M, Testino G, Pierandrei S, Fuso A, Cimino G, Ferraguti G, et al. Quantitative Evaluation of CFTR Gene Expression: A Comparison between Relative Quantification by Real-Time PCR and Absolute Quantification by Droplet Digital PCR. Genes. 2023; 14(9):1781. https://doi.org/10.3390/genes14091781
Chicago/Turabian StyleBruno, Sabina Maria, Giovanna Blaconà, Stefania Lo Cicero, Germana Castelli, Mariarita Virgulti, Giancarlo Testino, Silvia Pierandrei, Andrea Fuso, Giuseppe Cimino, Giampiero Ferraguti, and et al. 2023. "Quantitative Evaluation of CFTR Gene Expression: A Comparison between Relative Quantification by Real-Time PCR and Absolute Quantification by Droplet Digital PCR" Genes 14, no. 9: 1781. https://doi.org/10.3390/genes14091781








