Comparative Genomics Reveals Novel Species and Insights into the Biotechnological Potential, Virulence, and Resistance of Alcaligenes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset and Genome Curation
2.2. Genomic Features and Phylogeny
2.3. Pangenome Analysis
2.4. Plant-Growth-Promoting Traits Analysis
2.5. Resistome and Virulome Analysis
3. Results and Discussion
3.1. Data Selection and Genus Classification
3.2. Phylogenetic Analysis of Alcaligenes
3.3. Pangenome and Pan-GWAS Analyses of Alcaligenes
3.4. Functional Annotation with Plant-Associated Bacterium Database
3.5. The Plant Growth Promotion Potential of Alcaligenes
3.6. Biodegradation of Xenobiotic Compounds
3.7. Toxic Metal Tolerance
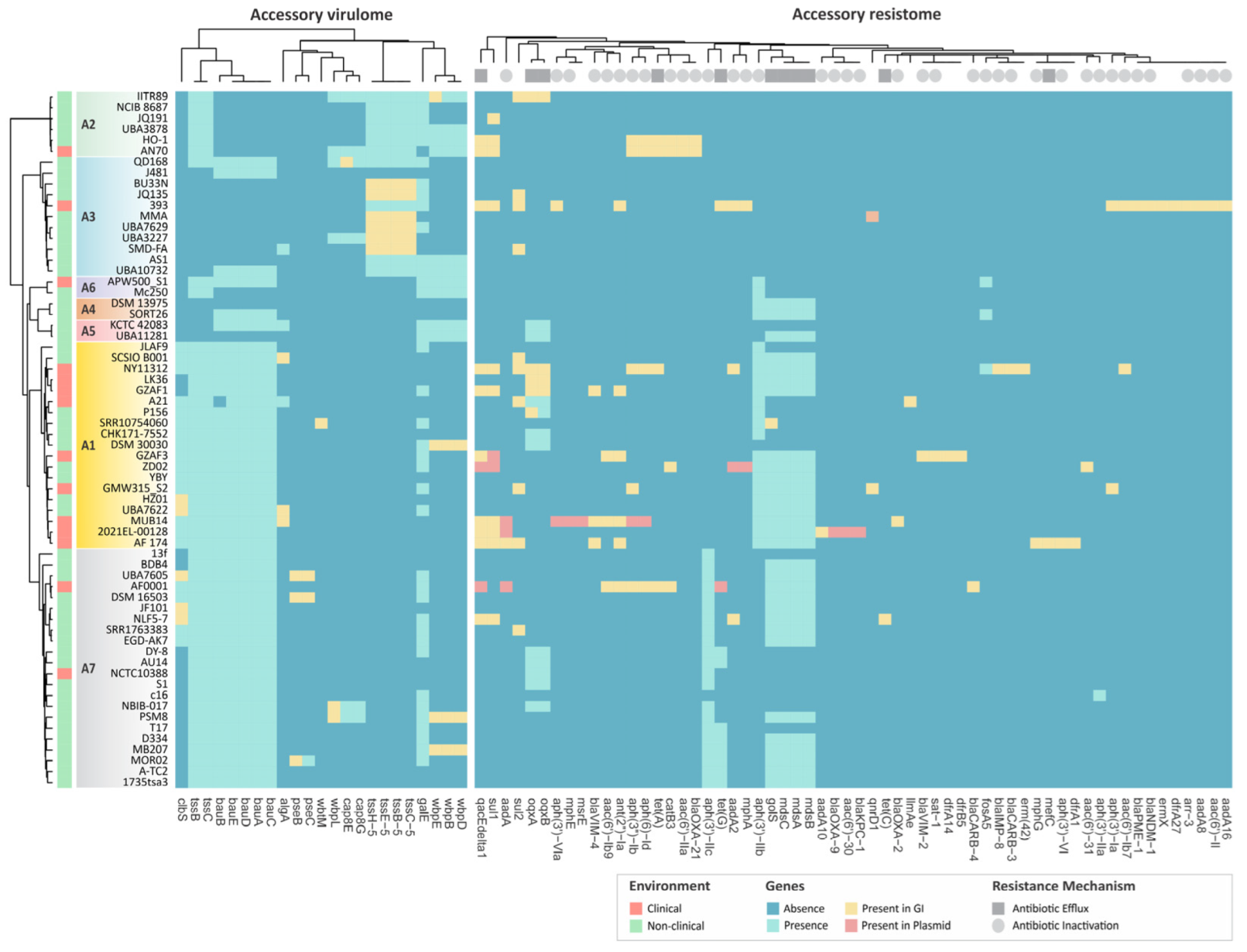
3.8. Virulence Genes and Their Distribution across Alcaligenes Species
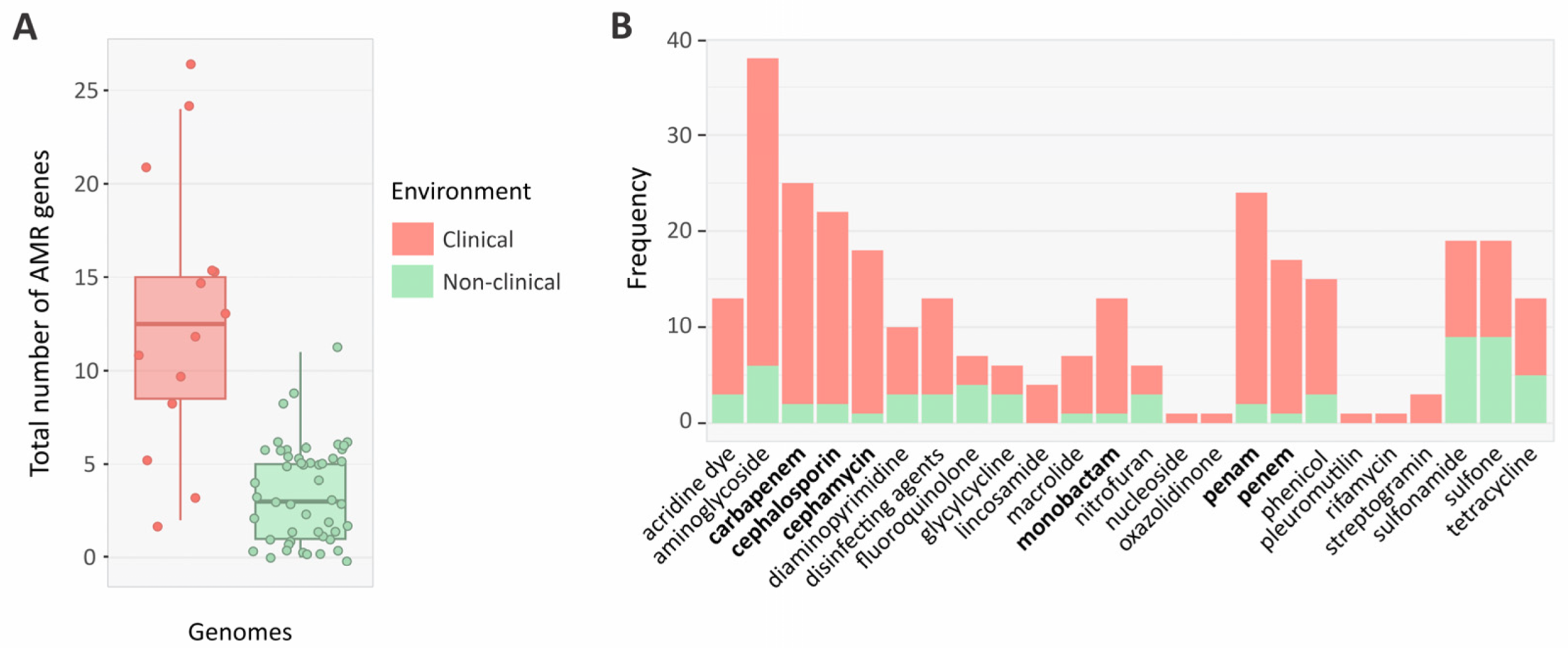
3.9. Resistance Profiles of Clinical and Non-Clinical Genomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ray, S.; Singh, S.; Sarma, B.; Singh, H. Endophytic Alcaligenes isolated from horticultural and medicinal crops promotes growth in okra (Abelmoschus esculentus). J. Plant Growth Regul. 2016, 35, 401–412. [Google Scholar] [CrossRef]
- Sayyed, R.; Chincholkar, S. Growth and siderophores production in Alcaligenes faecalis is regulated by metal ions. Indian J. Microbiol. 2010, 50, 179–182. [Google Scholar] [CrossRef]
- Kumar, A.; Singh Cameotra, S.; Gupta, S. Screening and characterization of potential cadmium biosorbent Alcaligenes strain from industrial effluent. J. Basic Microbiol. 2012, 52, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ndeddy Aka, R.J.; Babalola, O.O. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int. J. Phytoremediat. 2016, 18, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Rehfuss, M.; Urban, J. Alcaligenes faecalis subsp. phenolicus subsp. nov. a phenol-degrading, denitrifying bacterium isolated from a graywater bioprocessor. Syst. Appl. Microbiol. 2005, 28, 421–429. [Google Scholar] [CrossRef]
- Singha, L.P.; Kotoky, R.; Pandey, P. Draft genome sequence of Alcaligenes faecalis BDB4, a polyaromatic hydrocarbon-degrading bacterium isolated from crude oil-contaminated soil. Genome Announc. 2017, 5, e01346-17. [Google Scholar] [CrossRef]
- Sagarkar, S.; Bhardwaj, P.; Yadav, T.C.; Qureshi, A.; Khardenavis, A.; Purohit, H.J.; Kapley, A. Draft genome sequence of atrazine-utilizing bacteria isolated from Indian agricultural soil. Genome Announc. 2014, 2, e01149-13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q.; Ji, J.; Zhao, L.; Zhang, L.; Qiu, J.; He, J. Complete genome sequence of Alcaligenes faecalis strain JQ135, a bacterium capable of efficiently degrading nicotinic acid. Curr. Microbiol. 2018, 75, 1551–1554. [Google Scholar] [CrossRef]
- Yoon, I.-H.; Chang, J.-S.; Lee, J.-H.; Kim, K.-W. Arsenite oxidation by Alcaligenes sp. strain RS-19 isolated from arsenic-contaminated mines in the Republic of Korea. Environ. Geochem. Health 2009, 31, 109–117. [Google Scholar] [CrossRef]
- Adabi, M.; Hashemi, S.H.; Bakhtiyari, S. The First Study of Investigation of Clinical Isolates of Alcaligenes Xylosoxidans and Alcaligenes faecalis by Phenotypic and Genetic Methods in Iran. Iran. J. Med. Microbiol. 2022, 16, 148–154. [Google Scholar] [CrossRef]
- Tan, K.; Conway, S.P.; Brownlee, K.G.; Etherington, C.; Peckham, D.G. Alcaligenes infection in cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 101–104. [Google Scholar] [CrossRef]
- Bizet, J.; Bizet, C. Strains of Alcaligenes faecalis from clinical material. J. Infect. 1997, 35, 167–169. [Google Scholar] [CrossRef]
- Dubois, V.; Arpin, C.; Coulange, L.; Andre, C.; Noury, P.; Quentin, C. TEM-21 extended-spectrum β-lactamase in a clinical isolate of Alcaligenes faecalis from a nursing home. J. Antimicrob. Chemother. 2006, 57, 368–369. [Google Scholar] [CrossRef]
- Huang, C. Extensively drug-resistant Alcaligenes faecalis infection. BMC Infect. Dis. 2020, 20, 833. [Google Scholar] [CrossRef] [PubMed]
- Puah, S.M.; Chua, K.H. First report of TEM-116 and OXA-10 extended-spectrum β-lactamase in clinical isolates of Alcaligenes species from Kuala Lumpur, Malaysia. Jpn. J. Infect. Dis. 2019, 72, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Durán, R.E.; Méndez, V.; Rodríguez-Castro, L.; Barra-Sanhueza, B.; Salvà-Serra, F.; Moore, E.R.; Castro-Nallar, E.; Seeger, M. Genomic and physiological traits of the marine bacterium Alcaligenes aquatilis QD168 isolated from Quintero Bay, Central Chile, reveal a robust adaptive response to environmental stressors. Front. Microbiol. 2019, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Felestrino, É.B.; Sanchez, A.B.; Caneschi, W.L.; Lemes, C.G.d.C.; Assis, R.d.A.B.; Cordeiro, I.F.; Fonseca, N.P.; Villa, M.M.; Vieira, I.T.; Kamino, L.H.Y. Complete genome sequence and analysis of Alcaligenes faecalis strain Mc250, a new potential plant bioinoculant. PLoS ONE 2020, 15, e0241546. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Passarelli-Araujo, H.; Franco, G.R.; Venancio, T.M. Network analysis of ten thousand genomes shed light on Pseudomonas diversity and classification. Microbiol. Res. 2022, 254, 126919. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, L.; Glover, R.H.; Humphris, S.; Elphinstone, J.G.; Toth, I.K. Genomics and taxonomy in diagnostics for food security: Soft-rotting enterobacterial plant pathogens. Anal. Methods 2016, 8, 12–24. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Pradier, L.; Tissot, T.; Fiston-Lavier, A.-S.; Bedhomme, S. PlasForest: A homology-based random forest classifier for plasmid detection in genomic datasets. BMC Bioinform. 2021, 22, 349. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Simon Fraser University Research Computing Group; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Brynildsrud, O.; Bohlin, J.; Scheffer, L.; Eldholm, V. Rapid scoring of genes in microbial pan-genome-wide association studies with Scoary. Genome Biol. 2016, 17, 238. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Patz, S.; Gautam, A.; Matthias, B.; Ruppel, S.; Palenzuela, P.R.; Huson, D.H. PLaBAse: A comprehensive web resource for analyzing the plant growth-promoting potential of plant-associated bacteria. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wu, M.R.; Hou, T.T.; Liu, Y.; Miao, L.L.; Ai, G.M.; Ma, L.; Zhu, H.Z.; Zhu, Y.X.; Gao, X.Y.; Herbold, C.W. Novel Alcaligenes ammonioxydans sp. nov. from wastewater treatment sludge oxidizes ammonia to N2 with a previously unknown pathway. Environ. Microbiol. 2021, 23, 6965–6980. [Google Scholar] [CrossRef]
- Machado, R.A.; Loulou, A.; Bhat, A.H.; Mastore, M.; Terrettaz, C.; Brivio, M.F.; Kallel, S. Acinetobacter nematophilus sp. nov., Alcaligenes nematophilus sp. nov., Enterobacter nematophilus sp. nov., and Kaistia nematophila sp. nov., Isolated from Soil-Borne Nematodes and Proposal for the Elevation of Alcaligenes faecalis subsp. faecalis, Alcaligenes faecalis subsp. parafaecalis, and Alcaligenes faecalis subsp. phenolicus to the Species Level. Taxonomy 2023, 3, 148–168. [Google Scholar]
- Brockhurst, M.A.; Harrison, E.; Hall, J.P.; Richards, T.; McNally, A.; MacLean, C. The ecology and evolution of pangenomes. Curr. Biol. 2019, 29, R1094–R1103. [Google Scholar] [CrossRef]
- Basharat, Z.; Yasmin, A.; He, T.; Tong, Y. Genome sequencing and analysis of Alcaligenes faecalis subsp. phenolicus MB207. Sci. Rep. 2018, 8, 3616. [Google Scholar] [CrossRef] [PubMed]
- Henaut-Jacobs, S.; Passarelli-Araujo, H.; Venancio, T.M. Comparative genomics and phylogenomics of Campylobacter unveil potential novel species and provide insights into niche segregation. Mol. Phylogenet. Evol. 2023, 184, 107786. [Google Scholar] [CrossRef]
- Gruszczyk, J.; Fleurie, A.; Olivares-Illana, V.; Béchet, E.; Zanella-Cleon, I.; Morera, S.; Meyer, P.; Pompidor, G.; Kahn, R.; Grangeasse, C. Structure analysis of the Staphylococcus aureus UDP-N-acetyl-mannosamine dehydrogenase Cap5O involved in capsular polysaccharide biosynthesis. J. Biol. Chem. 2011, 286, 17112–17121. [Google Scholar] [CrossRef]
- Edwards, M.D.; Black, S.; Rasmussen, T.; Rasmussen, A.; Stokes, N.R.; Stephen, T.-L.; Miller, S.; Booth, I.R. Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels 2012, 6, 272–281. [Google Scholar] [CrossRef]
- Akai, M.; Onai, K.; Morishita, M.; Mino, H.; Shijuku, T.; Maruyama, H.; Arai, F.; Itoh, S.; Hazama, A.; Checchetto, V. Aquaporin AqpZ is involved in cell volume regulation and sensitivity to osmotic stress in Synechocystis sp. strain PCC 6803. J. Bacteriol. 2012, 194, 6828–6836. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.-H.; Jeong, K.-W.; Kim, Y.; Heo, Y.-S.; Kim, H.J. Recombinant expression, isotope labeling, and purification of cold shock protein from Colwellia psychrerythraea for NMR study. Bull. Korean Chem. Soc. 2009, 30, 2647–2650. [Google Scholar]
- Sodhi, K.K.; Kumar, M.; Singh, D.K. Multi-metal resistance and potential of Alcaligenes sp. MMA for the removal of heavy metals. SN Appl. Sci. 2020, 2, 1885. [Google Scholar] [CrossRef]
- Fatima, T.; Mishra, I.; Verma, R.; Arora, N.K. Mechanisms of halotolerant plant growth promoting Alcaligenes sp. involved in salt tolerance and enhancement of the growth of rice under salinity stress. 3 Biotech 2020, 10, 361. [Google Scholar] [CrossRef]
- Dixit, V.K.; Misra, S.; Mishra, S.K.; Tewari, S.K.; Joshi, N.; Chauhan, P.S. Characterization of plant growth-promoting alkalotolerant Alcaligenes and Bacillus strains for mitigating the alkaline stress in Zea mays. Antonie Van Leeuwenhoek 2020, 113, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Haouas, A.; El Modafar, C.; Douira, A.; Ibnsouda-Koraichi, S.; Filali-Maltouf, A.; Moukhli, A.; Amir, S. Alcaligenes aquatilis GTE53: Phosphate solubilising and bioremediation bacterium isolated from new biotope “phosphate sludge enriched-compost”. Saudi J. Biol. Sci. 2021, 28, 371–379. [Google Scholar] [CrossRef]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Sethi, B.K.; Mishra, R.R.; Kumari, S.; Thatoi, H. Alkaline phosphatase activity of a phosphate solubilizing Alcaligenes faecalis, isolated from Mangrove soil. Biotechnol. Res. Innov. 2017, 1, 101–111. [Google Scholar] [CrossRef]
- Mishra, P.; Kaur, S.; Sharma, A.N.; Jolly, R.S. Characterization of an indole-3-acetamide hydrolase from Alcaligenes faecalis subsp. parafaecalis and its application in efficient preparation of both enantiomers of chiral building block 2, 3-dihydro-1, 4-benzodioxin-2-carboxylic acid. PLoS ONE 2016, 11, e0159009. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]
- Vyas, P.; Gulati, A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009, 9, 174. [Google Scholar] [CrossRef]
- Lurthy, T.; Cantat, C.; Jeudy, C.; Declerck, P.; Gallardo, K.; Barraud, C.; Leroy, F.; Ourry, A.; Lemanceau, P.; Salon, C. Impact of bacterial siderophores on iron status and ionome in pea. Front. Plant Sci. 2020, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Gehring, A.M.; Mori, I.; Walsh, C.T. Reconstitution and characterization of the Escherichia coli enterobactin synthetase from EntB, EntE, and EntF. Biochemistry 1998, 37, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Khan, A.; Kumar, R.; Ojha, K.K.; Singh, V.K.; Srivastava, A. In silico analysis of comparative affinity of phytosiderophore and bacillibactin for iron uptake by YSL15 and YSL18 receptors of Oryza sativa. J. Biomol. Struct. Dyn. 2023, 41, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Jin, H.M.; Lee, S.H.; Math, R.K.; Madsen, E.L.; Jeon, C.O. Comparative genomic analysis and benzene, toluene, ethylbenzene, and o-, m-, and p-xylene (BTEX) degradation pathways of Pseudoxanthomonas spadix BD-a59. Appl. Environ. Microbiol. 2013, 79, 663–671. [Google Scholar] [CrossRef]
- Rabus, R.; Kube, M.; Beck, A.; Widdel, F.; Reinhardt, R. Genes involved in the anaerobic degradation of ethylbenzene in a denitrifying bacterium, strain EbN1. Arch. Microbiol. 2002, 178, 506–516. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Basu, A. Role of Heavy-Metal Resistant Bacteria Isolated from Rhizosphere in Bioremediation and Plant Development. In Rhizobiology: Molecular Physiology of Plant Roots; Springer: Cham, Switzerland, 2021; pp. 411–435. [Google Scholar]
- Ding, J.; Chen, W.; Zhang, Z.; Qin, F.; Jiang, J.; He, A.; Sheng, G.D. Enhanced removal of cadmium from wastewater with coupled biochar and Bacillus subtilis. Water Sci. Technol. 2021, 83, 2075–2086. [Google Scholar] [CrossRef]
- Nies, D.H. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J. Bacteriol. 1992, 174, 8102–8110. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Perez-Palacios, P.; Delgado-Valverde, M.; Gual-de-Torrella, A.; Oteo-Iglesias, J.; Pascual, Á.; Fernández-Cuenca, F. Co-transfer of plasmid-encoded bla carbapenemases genes and mercury resistance operon in high-risk clones of Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2021, 105, 9231–9242. [Google Scholar] [CrossRef] [PubMed]
- Lal, D.; Lal, R. Evolution of mercuric reductase (merA) gene: A case of horizontal gene transfer. Microbiology 2010, 79, 500–508. [Google Scholar] [CrossRef]
- Schneiker, S.; Keller, M.; Dröge, M.; Lanka, E.; Pühler, A.; Selbitschka, W. The genetic organization and evolution of the broad host range mercury resistance plasmid pSB102 isolated from a microbial population residing in the rhizosphere of alfalfa. Nucleic Acids Res. 2001, 29, 5169–5181. [Google Scholar] [CrossRef]
- Robas, M.; Probanza, A.; González, D.; Jiménez, P.A. Mercury and antibiotic resistance co-selection in Bacillus sp. isolates from the Almadén mining district. Int. J. Environ. Res. Public Health 2021, 18, 8304. [Google Scholar] [CrossRef]
- Hall, J.P.; Harrison, E.; Pärnänen, K.; Virta, M.; Brockhurst, M.A. The impact of mercury selection and conjugative genetic elements on community structure and resistance gene transfer. Front. Microbiol. 2020, 11, 1846. [Google Scholar] [CrossRef]
- Yuan, L.; Li, Z.-H.; Zhang, M.-Q.; Shao, W.; Fan, Y.-Y.; Sheng, G.-P. Mercury/silver resistance genes and their association with antibiotic resistance genes and microbial community in a municipal wastewater treatment plant. Sci. Total Environ. 2019, 657, 1014–1022. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Z.; Xiong, K.; Wang, J.; Rao, X.; Cong, Y. Vi capsular polysaccharide: Synthesis, virulence, and application. Crit. Rev. Microbiol. 2017, 43, 440–452. [Google Scholar] [CrossRef]
- Cabrejos, D.A.L.; Alexandrino, A.V.; Pereira, C.M.; Mendonça, D.C.; Pereira, H.D.M.; Novo-Mansur, M.T.M.; Garratt, R.C.; Goto, L.S. Structural characterization of a pathogenicity-related superoxide dismutase codified by a probably essential gene in Xanthomonas citri subsp. citri. PLoS ONE 2019, 14, e0209988. [Google Scholar] [CrossRef]
- Alquéres, S.; Meneses, C.; Rouws, L.; Rothballer, M.; Baldani, I.; Schmid, M.; Hartmann, A. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol. Plant-Microbe Interact. 2013, 26, 937–945. [Google Scholar] [CrossRef]
- Cavinato, L.; Genise, E.; Luly, F.R.; Di Domenico, E.G.; Del Porto, P.; Ascenzioni, F. Escaping the phagocytic oxidative burst: The role of SODB in the survival of Pseudomonas aeruginosa within macrophages. Front. Microbiol. 2020, 11, 326. [Google Scholar] [CrossRef]
- Uzzau, S.; Bossi, L.; Figueroa-Bossi, N. Differential accumulation of Salmonella [Cu, Zn] superoxide dismutases SodCI and SodCII in intracellular bacteria: Correlation with their relative contribution to pathogenicity. Mol. Microbiol. 2002, 46, 147–156. [Google Scholar] [CrossRef]
- Sheldon, J.R.; Skaar, E.P. Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence. PLoS Pathog. 2020, 16, e1008995. [Google Scholar] [CrossRef] [PubMed]
- Coulthurst, S. The Type VI secretion system: A versatile bacterial weapon. Microbiology 2019, 165, 503–515. [Google Scholar] [CrossRef]
- Poirel, L.; Corvec, S.; Rapoport, M.; Mugnier, P.; Petroni, A.; Pasteran, F.; Faccone, D.; Galas, M.; Drugeon, H.; Cattoir, V. Identification of the novel narrow-spectrum β-lactamase SCO-1 in Acinetobacter spp. from Argentina. Antimicrob. Agents Chemother. 2007, 51, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Papagiannitsis, C.; Loli, A.; Tzouvelekis, L.; Tzelepi, E.; Arlet, G.; Miriagou, V. SCO-1, a novel plasmid-mediated class A β-lactamase with carbenicillinase characteristics from Escherichia coli. Antimicrob. Agents Chemother. 2007, 51, 2185–2188. [Google Scholar] [CrossRef]
- Papagiannitsis, C.; Tzouvelekis, L.; Kotsakis, S.; Tzelepi, E.; Miriagou, V. Sequence of pR3521, an IncB plasmid from Escherichia coli encoding ACC-4, SCO-1, and TEM-1 β-lactamases. Antimicrob. Agents Chemother. 2011, 55, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Venditti, C.; Butera, O.; Proia, A.; Rigacci, L.; Mariani, B.; Parisi, G.; Messina, F.; Capone, A.; Nisii, C.; Di Caro, A. Reduced susceptibility to carbapenems in a Klebsiella pneumoniae clinical isolate producing SCO-1 and CTX-M-15 β-lactamases together with OmpK35 and OmpK36 Porin deficiency. Antimicrob. Agents Chemother. 2020, 64, e00556-20. [Google Scholar] [CrossRef]
- Chiou, J.; Li, R.; Chen, S. CARB-17 family of β-lactamases mediates intrinsic resistance to penicillins in Vibrio parahaemolyticus. Antimicrob. Agents Chemother. 2015, 59, 3593–3595. [Google Scholar] [CrossRef]
- Choury, D.; Szajnert, M.-F.; Joly-Guillou, M.-L.; Azibi, K.; Delpech, M.; Paul, G. Nucleotide sequence of the bla RTG-2 (CARB-5) gene and phylogeny of a new group of carbenicillinases. Antimicrob. Agents Chemother. 2000, 44, 1070–1074. [Google Scholar] [CrossRef]
- Nishino, K.; Latifi, T.; Groisman, E.A. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2006, 59, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; Penstoft, L.N.; Nørskov-Lauritsen, N. Motility, biofilm formation and antimicrobial efflux of sessile and planktonic cells of Achromobacter xylosoxidans. Pathogens 2019, 8, 14. [Google Scholar] [CrossRef]
- Magallon, A.; Roussel, M.; Neuwirth, C.; Tetu, J.; Cheiakh, A.-C.; Boulet, B.; Varin, V.; Urbain, V.; Bador, J.; Amoureux, L. Fluoroquinolone resistance in Achromobacter spp.: Substitutions in QRDRs of GyrA, GyrB, ParC and ParE and implication of the RND efflux system AxyEF-OprN. J. Antimicrob. Chemother. 2021, 76, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bador, J.; Amoureux, L.; Blanc, E.; Neuwirth, C. Innate aminoglycoside resistance of Achromobacter xylosoxidans is due to AxyXY-OprZ, an RND-type multidrug efflux pump. Antimicrob. Agents Chemother. 2013, 57, 603–605. [Google Scholar] [CrossRef]
- Conroy, O.; Kim, E.-H.; McEvoy, M.M.; Rensing, C. Differing ability to transport nonmetal substrates by two RND-type metal exporters. FEMS Microbiol. Lett. 2010, 308, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pontel, L.B.; Audero, M.E.P.; Espariz, M.; Checa, S.K.; Soncini, F.C. GolS controls the response to gold by the hierarchical induction of Salmonella-specific genes that include a CBA efflux-coding operon. Mol. Microbiol. 2007, 66, 814–825. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Ning, J.; Sajid, A.; Cheng, G.; Yuan, Z.; Hao, H. The nature and epidemiology of OqxAB, a multidrug efflux pump. Antimicrob. Resist. Infect. Control 2019, 8, 44. [Google Scholar] [CrossRef]
- Kazama, H.; Hamashima, H.; Sasatsu, M.; Arai, T. Characterization of the antiseptic-resistance gene qacE Δ 1 isolated from clinical and environmental isolates of Vibrio parahaemolyticus and Vibrio cholerae non-O1. FEMS Microbiol. Lett. 1999, 174, 379–384. [Google Scholar]
- Chiu, C.-H.; Chen, H.-L.; Kao, L.-S.; Yang, C.-Y.; Chu, C.; Doublet, B.; Praud, K.; Cloeckaert, A. Variant Salmonella genomic island 1 antibiotic resistance gene clusters in Salmonella enterica serovar Derby isolates from humans in Taiwan. J. Antimicrob. Chemother. 2007, 59, 325–326. [Google Scholar] [CrossRef]
- Okade, H.; Nakagawa, S.; Sakagami, T.; Hisada, H.; Nomura, N.; Mitsuyama, J.; Yamagishi, Y.; Mikamo, H. Characterization of plasmid-mediated quinolone resistance determinants in Klebsiella pneumoniae and Escherichia coli from Tokai, Japan. J. Infect. Chemother. 2014, 20, 778–783. [Google Scholar] [CrossRef] [PubMed]
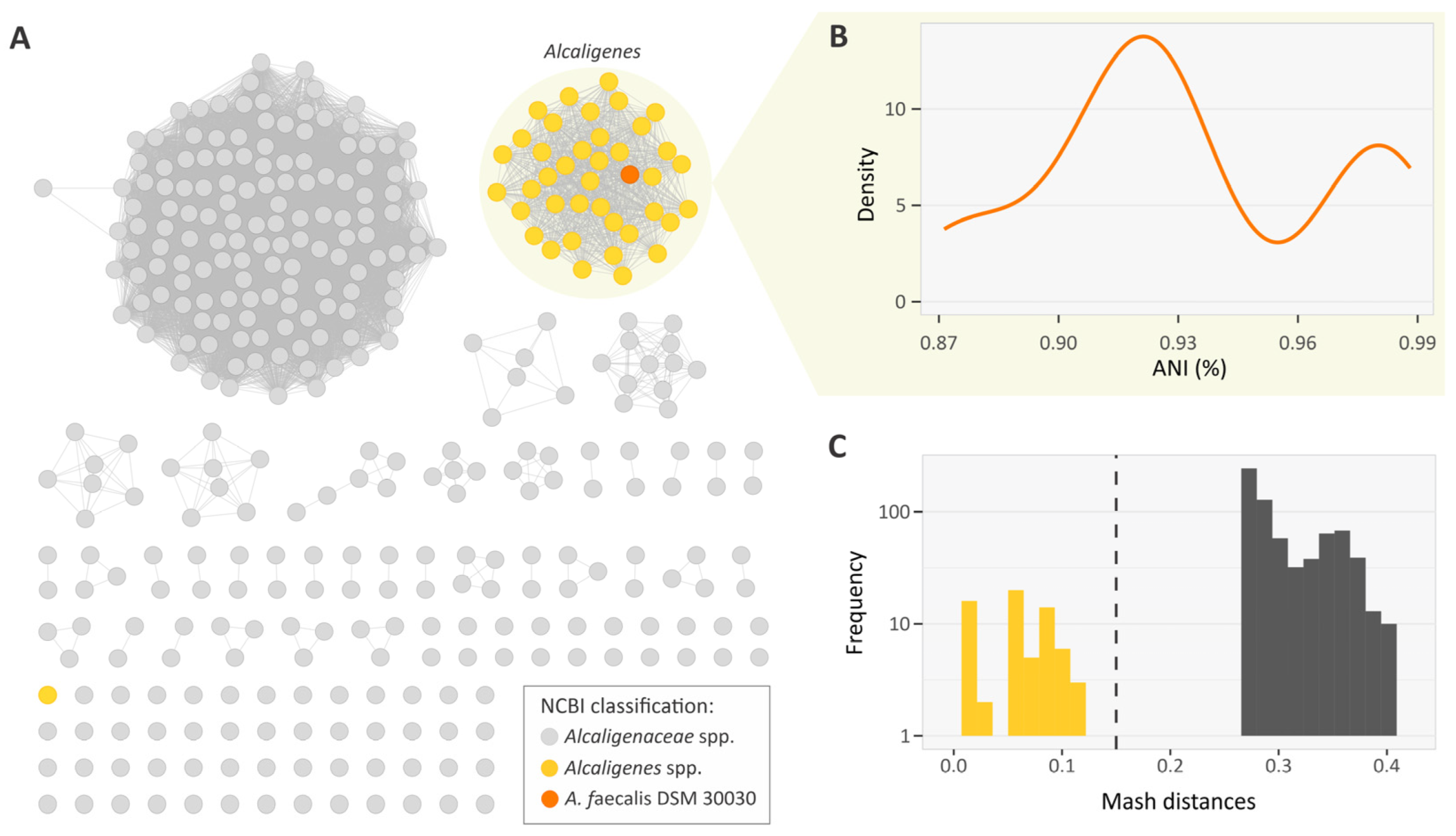
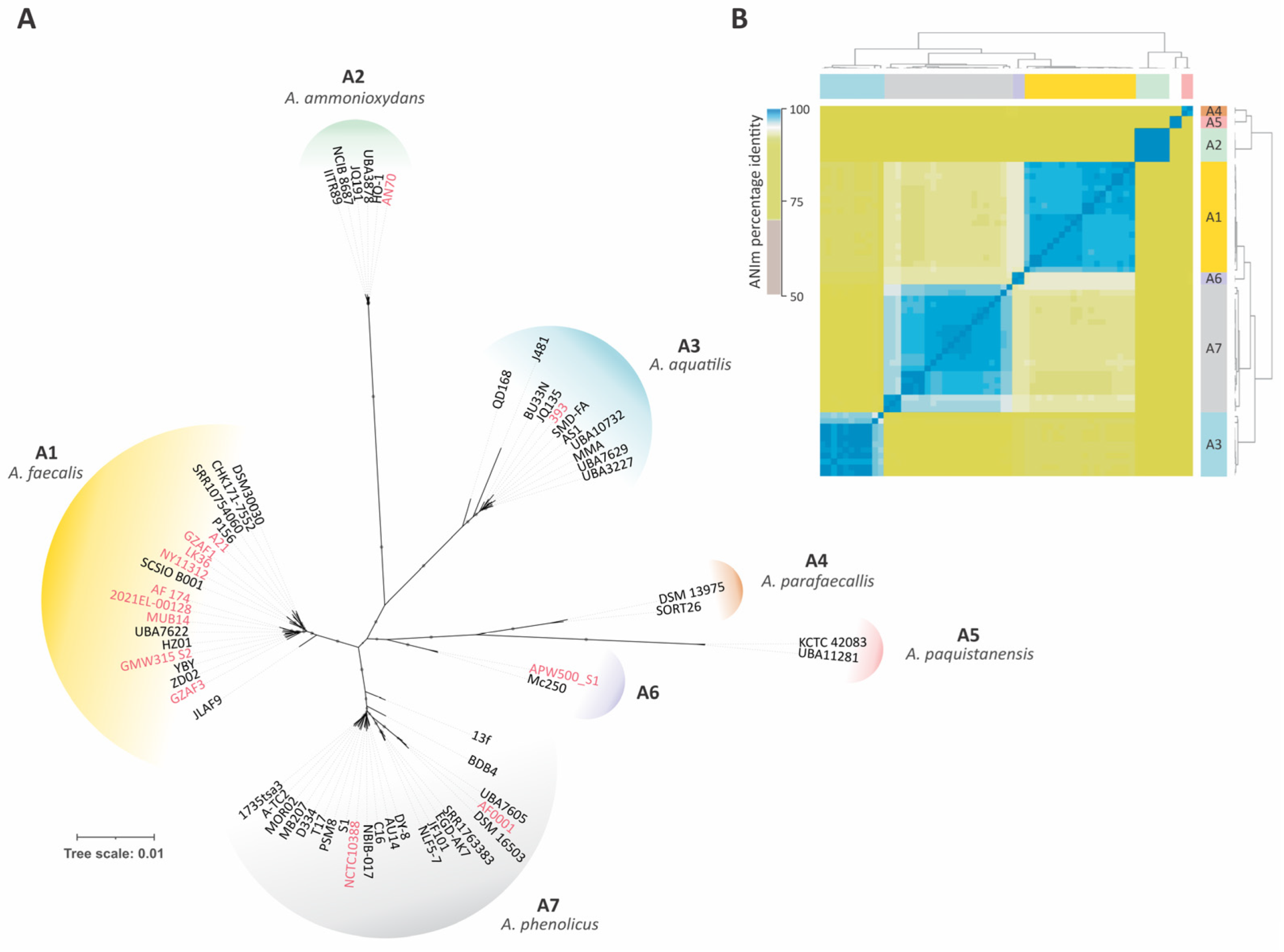
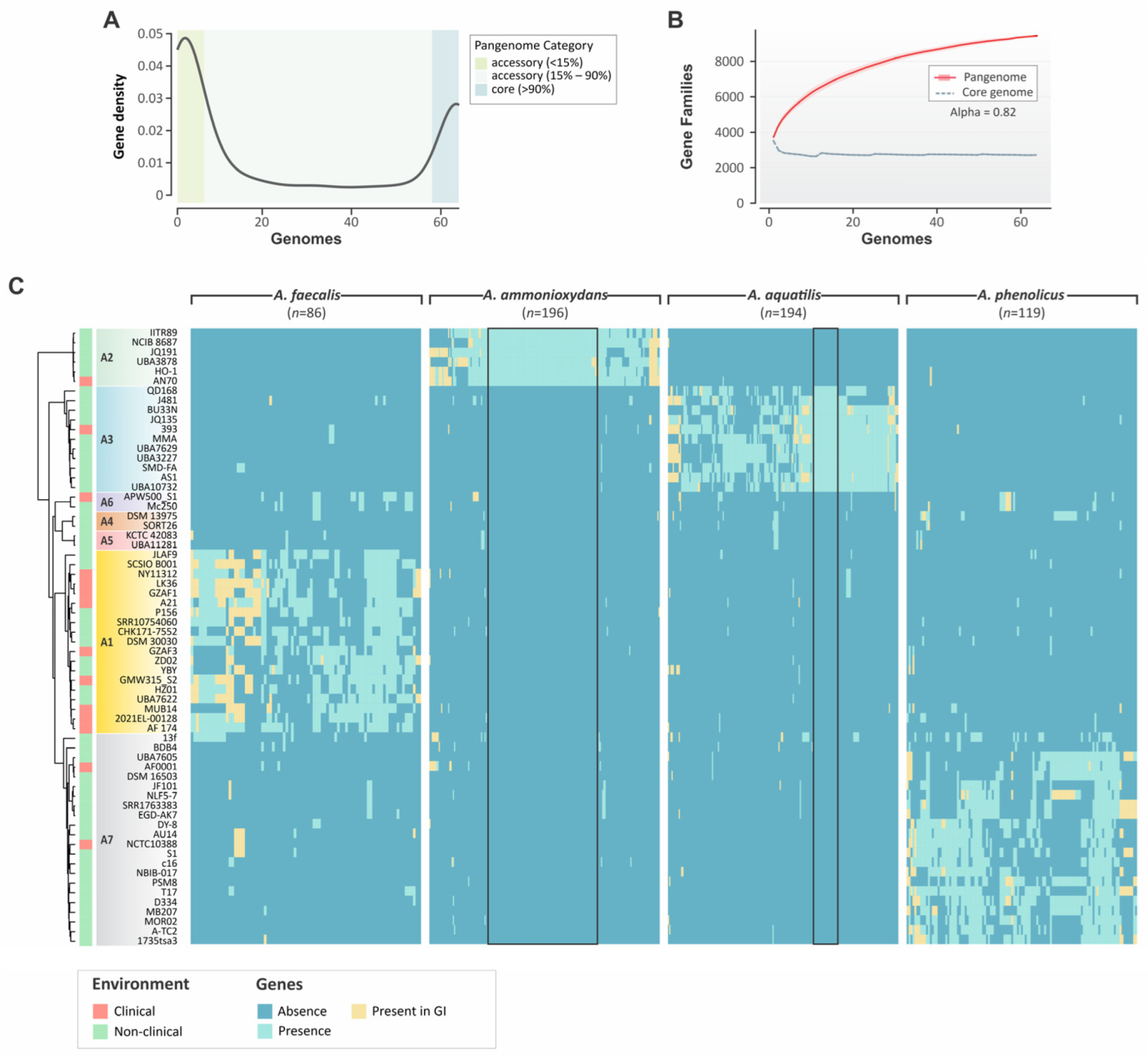
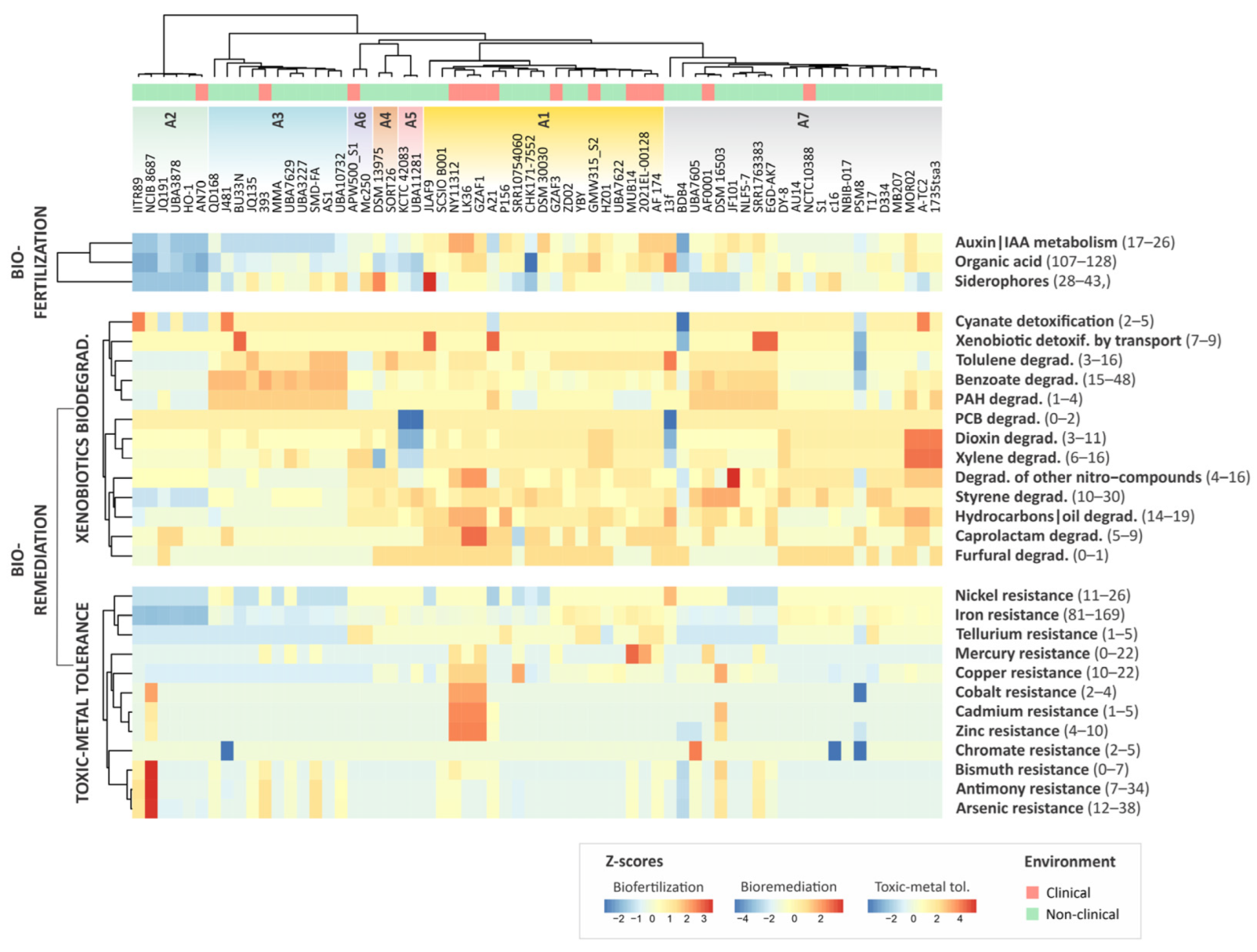
| Accession | Strain | NCBI Classification | Reclassified as | Source | Group | Type |
|---|---|---|---|---|---|---|
| GCA_000967305.2 | ZD02 | A. faecalis | - | Nematode | A1 | Non-clinical |
| GCA_001641975.2 | P156 | A. faecalis | - | Soil | A1 | Non-clinical |
| GCA_002119995.1 | GZAF3 | A. faecalis | - | Homo sapiens | A1 | Clinical |
| GCA_002120075.1 | GZAF1 | A. faecalis | - | Homo sapiens | A1 | Clinical |
| GCA_002443155.1 | DSM 30030 | A. faecalis | - | Activated sludge | A1 | Non-clinical |
| GCA_002484125.1 | UBA7622 | A. faecalis | - | Wood | A1 | Non-clinical |
| GCA_003122065.1 | YBY | A. faecalis | - | Activated sludge | A1 | Non-clinical |
| GCA_003939865.1 | AF_174 | A. faecalis | - | Hospital washroom sink | A1 | Clinical |
| GCA_008373885.1 | LK36 | A. faecalis | - | Homo sapiens | A1 | Clinical |
| GCA_010092625.1 | MUB14 | A. faecalis | - | Homo sapiens | A1 | Clinical |
| GCA_015905185.1 | A21 | A. faecalis | - | Homo sapiens | A1 | Clinical |
| GCA_016446305.1 | SCSIO B001 | A. faecalis | - | Fungi | A1 | Non-clinical |
| GCA_018066525.1 | 2021EL-00128 | A. faecalis | - | Homo sapiens | A1 | Clinical |
| GCA_018682645.1 | HZ01 | A. faecalis subsp. faecalis | A. faecalis | Contaminated culture of M. chubuense | A1 | Non-clinical |
| GCA_019836945.1 | GMW315_S2 | A. faecalis | - | Hospital effluent | A1 | Clinical |
| GCA_020741625.1 | CHK171-7552 | A. faecalis | - | Poultry feces | A1 | Non-clinical |
| GCA_023921285.1 | JLAF9 | A. faecalis | - | Chicken manure | A1 | Non-clinical |
| GCA_027595045.1 | NY11312 | A. faecalis | - | Homo sapiens | A1 | Clinical |
| GCA_946479345.1 | SRR10754060 | A. faecalis | - | Plant | A1 | Non-clinical |
| GCA_000275465.1 | NCIB 8687 | A. faecalis subsp. faecalis | A. ammonioxydans | - | A2 | Non-clinical |
| GCA_001516865.1 | IITR89 | A. faecalis subsp. phenolicus | A. ammonioxydans | River water | A2 | Non-clinical |
| GCA_002392125.1 | UBA3878 | A. faecalis | A. ammonioxydans | Wood | A2 | Non-clinical |
| GCA_004319585.1 | AN70 | A. faecalis | A. ammonioxydans | Homo sapiens | A2 | Clinical |
| GCA_019343455.1 | HO-1 | A. ammonioxydans | - | Wastewater | A2 | Non-clinical |
| GCA_022436505.1 | JQ191 | A. ammonioxydans | - | Soil | A2 | Non-clinical |
| GCA_002242175.1 | JQ135 | A. faecalis | A. aquatilis | Wastewater | A3 | Non-clinical |
| GCA_002362965.1 | UBA3227 | A. faecalis | A. aquatilis | Metal/plastic | A3 | Non-clinical |
| GCA_002484005.1 | UBA7629 | A. faecalis | A. aquatilis | Metal | A3 | Non-clinical |
| GCA_003076515.1 | BU33N | A. aquatilis | - | Sediment | A3 | Non-clinical |
| GCA_003511485.1 | UBA10732 | A. faecalis | A. aquatilis | - | A3 | Non-clinical |
| GCA_003671915.1 | QD168 | A. aquatilis | - | Marine sediment | A3 | Non-clinical |
| GCA_003716855.1 | J481 | A. faecalis | A. aquatilis | Salt marsh sediment | A3 | Non-clinical |
| GCA_003938225.2 | 393 | A. aquatilis | - | Hospital washroom sink | A3 | Clinical |
| GCA_020907215.1 | MMA | Alcaligenes sp. | A. aquatilis | River water | A3 | Non-clinical |
| GCA_023373785.1 | AS1 | A. aquatilis | - | Activated sludge | A3 | Non-clinical |
| GCA_025960365.1 | SMD-FA | Alcaligenes sp. | A. aquatilis | Sludge | A3 | Non-clinical |
| GCA_017377875.1 | SORT26 | Alcaligenes sp. | A. parafaecalis | Residential yard | A4 | Non-clinical |
| GCA_026344135.1 | DSM 13975 | A. faecalis subsp. parafaecalis | A. parafaecalis | Water | A4 | Non-clinical |
| GCA_003521065.1 | UBA11281 | A. faecalis | A. pakistanensis | - | A5 | Non-clinical |
| GCA_014652815.1 | KCTC 42083 | A. pakistanensis | - | Industrial wastewater | A5 | Non-clinical |
| GCA_009497775.1 | Mc250 | A. faecalis | Alcaligenes sp. | Plant | A6 | Non-clinical |
| GCA_019693795.1 | APW500_S1 | A. faecalis | Alcaligenes sp. | Hospital effluent | A6 | Clinical |
| GCA_000429385.1 | DSM 16503 | A. faecalis subsp. phenolicus | A. phenolicus | Wastewater bioprocessor | A7 | Non-clinical |
| GCA_000465875.3 | EGD-AK7 | Alcaligenes sp. | A. phenolicus | Soil | A7 | Non-clinical |
| GCA_000770015.1 | MOR02 | A. faecalis | A. phenolicus | Nematode | A7 | Non-clinical |
| GCA_001530325.1 | NBIB-017 | A. faecalis | A. phenolicus | Soil | A7 | Non-clinical |
| GCA_002082085.1 | MB207 | A. faecalis subsp. phenolicus | A. phenolicus | Tannery effluent | A7 | Non-clinical |
| GCA_002205415.1 | BDB4 | A. faecalis | A. phenolicus | Soil | A7 | Non-clinical |
| GCA_002476455.1 | UBA7605 | A. faecalis | A. phenolicus | Wood | A7 | Non-clinical |
| GCA_005311025.1 | AU14 | A. faecalis | A. phenolicus | Plant | A7 | Non-clinical |
| GCA_016807785.1 | c16 | A. faecalis | A. phenolicus | Wastewater | A7 | Non-clinical |
| GCA_020496585.1 | 13f | Alcaligenes sp. | A. phenolicus | Soil | A7 | Non-clinical |
| GCA_022343965.1 | T17 | A. faecalis | A. phenolicus | Sediment | A7 | Non-clinical |
| GCA_023101245.1 | D334 | A. faecalis | A. phenolicus | Mangrove | A7 | Non-clinical |
| GCA_023702805.1 | PSM8 | A. faecalis | A. phenolicus | Dumpsite | A7 | Non-clinical |
| GCA_024134565.1 | 1735tsa3 | Alcaligenes sp. | A. phenolicus | Clean room | A7 | Non-clinical |
| GCA_024266725.1 | NLF5-7 | Alcaligenes sp. | A. phenolicus | Wastewater | A7 | Non-clinical |
| GCA_024584725.1 | DY-8 | A. faecalis | A. phenolicus | Soil | A7 | Non-clinical |
| GCA_024654895.1 | AF0001 | A. faecalis | A. phenolicus | Homo sapiens | A7 | Clinical |
| GCA_026344155.1 | A-TC2 | Alcaligenes sp. | A. phenolicus | Nematode | A7 | Non-clinical |
| GCA_026799675.1 | S1 | A. faecalis | A. phenolicus | Soil | A7 | Non-clinical |
| GCA_029000325.1 | JF101 | A. faecalis | A. phenolicus | Undersea mud | A7 | Non-clinical |
| GCA_900445215.1 | NCTC10388 | A. faecalis subsp. faecalis | A. phenolicus | Homo sapiens | A7 | Clinical |
| GCA_937863365.1 | SRR1763383 | A. faecalis | A. phenolicus | Wastewater | A7 | Non-clinical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa-Silva, F.; Venancio, T.M. Comparative Genomics Reveals Novel Species and Insights into the Biotechnological Potential, Virulence, and Resistance of Alcaligenes. Genes 2023, 14, 1783. https://doi.org/10.3390/genes14091783
Pedrosa-Silva F, Venancio TM. Comparative Genomics Reveals Novel Species and Insights into the Biotechnological Potential, Virulence, and Resistance of Alcaligenes. Genes. 2023; 14(9):1783. https://doi.org/10.3390/genes14091783
Chicago/Turabian StylePedrosa-Silva, Francisnei, and Thiago M. Venancio. 2023. "Comparative Genomics Reveals Novel Species and Insights into the Biotechnological Potential, Virulence, and Resistance of Alcaligenes" Genes 14, no. 9: 1783. https://doi.org/10.3390/genes14091783






