Towards In Silico Identification of Genes Contributing to Similarity of Patients’ Multi-Omics Profiles: A Case Study of Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Publicly Available Datasets Used in This Study
2.2. Similarity Network Fusion (SNF)
2.3. Identifying Informative Features Contributing to Cluster Separation
2.4. Other Software Packages Used
3. Results
3.1. SNF Analysis
3.2. Survival Analysis
3.3. Informative Feature Selection
3.4. Enrichment Analysis
3.5. Germline and Somatic Mutations in Immune Genes
3.6. Identification of Hub Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grimwade, D.; Freeman, S.D. Defining minimal residual disease in acute myeloid leukemia: Which platforms are ready for “prime time”? Blood 2014, 124, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Vadakekolathu, J.; Tasian, S.K.; Altmann, H.; Bornhäuser, M.; Pockley, A.G.; Ball, G.R.; Rutella, S. A parsimonious 3-gene signature predicts clinical outcomes in an acute myeloid leukemia multicohort study. Blood Adv. 2019, 3, 1330–1346. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Metzeler, K.H.; Hummel, M.; Bloomfield, C.D.; Spiekermann, K.; Braess, J.; Sauerland, M.-C.; Heinecke, A.; Radmacher, M.; Marcucci, G.; Whitman, S.P.; et al. An 86-probe-set gene-expression signature predicts survival in cytogenetically normal acute myeloid leukemia. Blood 2008, 112, 4193–4201. [Google Scholar] [CrossRef]
- Ng, S.W.K.; Mitchell, A.; Kennedy, J.A.; Chen, W.C.; McLeod, J.; Ibrahimova, N.; Arruda, A.; Popescu, A.; Gupta, V.; Schimmer, A.D.; et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 2016, 540, 433–437. [Google Scholar] [CrossRef]
- Zhu, R.; Tao, H.; Lin, W.; Tang, L.; Hu, Y. Identification of an immune-related gene signature based on immunogenomic landscape analysis to predict the prognosis of adult acute myeloid leukemia patients. Front. Oncol. 2020, 10, 574939. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Lugthart, S.; Li, Y.; Erpelinck-Verschueren, C.; Deng, X.; Christos, P.J.; Schifano, E.; Booth, J.; van Putten, W.; Skrabanek, L.; et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell 2010, 17, 13–27. [Google Scholar] [CrossRef]
- Wang, B.; Mezlini, A.M.; Demir, F.; Fiume, M.; Tu, Z.; Brudno, M.; Haibe-Kains, B.; Goldenberg, A. Similarity network fusion for aggregating data types on a genomic scale. Nat. Methods 2014, 11, 333–337. [Google Scholar] [CrossRef]
- Chierici, M.; Bussola, N.; Marcolini, A.; Francescatto, M.; Zandonà, A.; Trastulla, L.; Agostinelli, C.; Jurman, G.; Furlanello, C. Integrative network fusion: A multi-omics approach in molecular profiling. Front. Oncol. 2020, 10, 1065. [Google Scholar] [CrossRef]
- Guo, D.; Wang, M.; Shen, Z.; Zhu, J. A new immune signature for survival prediction and immune checkpoint molecules in lung adenocarcinoma. J. Transl. Med. 2020, 18, 123. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Liang, Z.; Wang, Y.; Chen, F.; Djekidel, M.N.; Li, G.; Zhang, X.; Xiang, S.; Wang, Z.; et al. Alterations of specific chromatin conformation affect ATRA-induced leukemia cell differentiation. Cell Death Dis. 2018, 9, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.W.; Calvano, S.E.; Xiao, W.; Richards, D.R.; Felciano, R.M.; Baker, H.V.; Cho, R.J.; Chen, R.O.; Brownstein, B.H.; Cobb, J.P.; et al. A network-based analysis of systemic inflammation in humans. Nature 2005, 437, 1032–1037. [Google Scholar] [CrossRef]
- Kelley, J.; de Bono, B.; Trowsdale, J. IRIS: A database surveying known human immune system genes. Genomics 2005, 85, 503–511. [Google Scholar] [CrossRef]
- Ortutay, C.; Vihinen, M. Immunome: A reference set of genes and proteins for systems biology of the human immune system. Cell Immunol. 2006, 244, 87–89. [Google Scholar] [CrossRef]
- Kühn, M.W.M.; Radtke, I.; Bullinger, L.; Goorha, S.; Cheng, J.; Edelmann, J.; Gohlke, J.; Su, X.; Paschka, P.; Pounds, S.; et al. High-resolution genomic profiling of adult and pediatric core-binding factor acute myeloid leukemia reveals new recurrent genomic alterations. Blood 2012, 119, e67–e75. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, M.; Shang, Z.; Li, J.; Zhang, S.; Lian, D.; Zhang, R. Genome-wide haplotype association study identify the FGFR2 gene as a risk gene for acute myeloid leukemia. Oncotarget 2017, 8, 7891–7899. [Google Scholar] [CrossRef]
- Pearl, J. Probabilistic Reasoning in Intelligent Systems, 1st ed.; Morgan Kaufmann: Burlington, MA, USA, 2014. [Google Scholar]
- Tini, G.; Marchetti, L.; Priami, C.; Scott-Boyer, M. Multi-omics integration-a comparison of unsupervised clustering methodologies. Brief. Bioinform. 2019, 20, 1269–1279. [Google Scholar] [CrossRef]
- Shi, J.; Malik, J. Normalized cuts and image segmentation. TPAMI 2000, 22, 888–905. [Google Scholar] [CrossRef]
- Tusher, V.G.; Tibshirani, R.; Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 2001, 98, 5116–5121. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdottir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef] [PubMed]
- Okazuka, K.; Masuko, M.; Seki, Y.; Hama, H.; Honma, N.; Furukawa, T.; Toba, K.; Kishi, K.; Aizawa, Y. Successful all-trans retinoic acid treatment of acute promyelocytic leukemia in a patient with NPM/RAR fusion. Int. J. Hematol. 2007, 86, 246–249. [Google Scholar] [CrossRef]
- Rausch, C.; Rothenberg-Thurley, M.; Dufour, A.; Schneider, S.; Gittinger, H.; Sauerland, C.; Görlich, D.; Krug, U.; Berdel, W.E.; Woermann, B.J.; et al. Validation and refinement of the 2022 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia 2023, 37, 1234–1244. [Google Scholar] [CrossRef]
- Dancik, G.M.; Varisli, L.; Vlahopoulos, S.A. The molecular context of oxidant stress response in cancer establishes ALDH1A1 as a critical target: What this means for acute myeloid leukemia. Int. J. Mol. Sci. 2023, 24, 9372. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, F.; Niu, T. Prediction of prognosis and immunotherapy response of amino acid metabolism genes in acute myeloid leukemia. Front. Nutr. 2022, 9, 1056648. [Google Scholar] [CrossRef]
- Guo, C.; Liu, S.; Wang, J.; Sun, M.-Z.; Greenaway, F.T. ACTB in cancer. Rev. Clin. Chim. Acta 2013, 417, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.H.; Zhang, Q.; Sprung, R.; Day, R.B.; Erdmann-Gilmore, P.; Li, Y.; Xu, Z.; Helton, N.M.; George, D.R.; Mi, Y.; et al. Proteomic and phosphoproteomic landscapes of acute myeloid leukemia. Blood 2022, 140, 1533–1548. [Google Scholar] [CrossRef]
- Abel, H.J.; Oetjen, K.A.; Miller, C.A.; Ramakrishnan, S.M.; Day, R.B.; Helton, N.M.; Fronick, C.C.; Fulton, R.S.; Heath, S.E.; Tarnawsky, S.P.; et al. Genomic landscape of TP53-mutated myeloid malignancies. Blood Adv. 2023, 7, 4586–4598. [Google Scholar] [CrossRef]
- Otálora-Otálora, B.A.; Henríquez, B.; López-Kleine, L.; Rojas, A. RUNX family: Oncogenes or tumor suppressors. Oncol Rep. 2019, 42, 3–19. [Google Scholar] [CrossRef]
- Dao, F.T.; Wang, J.; Yang, L.; Qin, Y.Z. Development of a poor-prognostic-mutations derived immune prognostic model for acute myeloid leukemia. Sci. Rep. 2021, 11, 4856. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G. Two genetic hits (more or less) to cancer. Nat. Rev. Cancer 2001, 1, 157–162. [Google Scholar] [CrossRef] [PubMed]
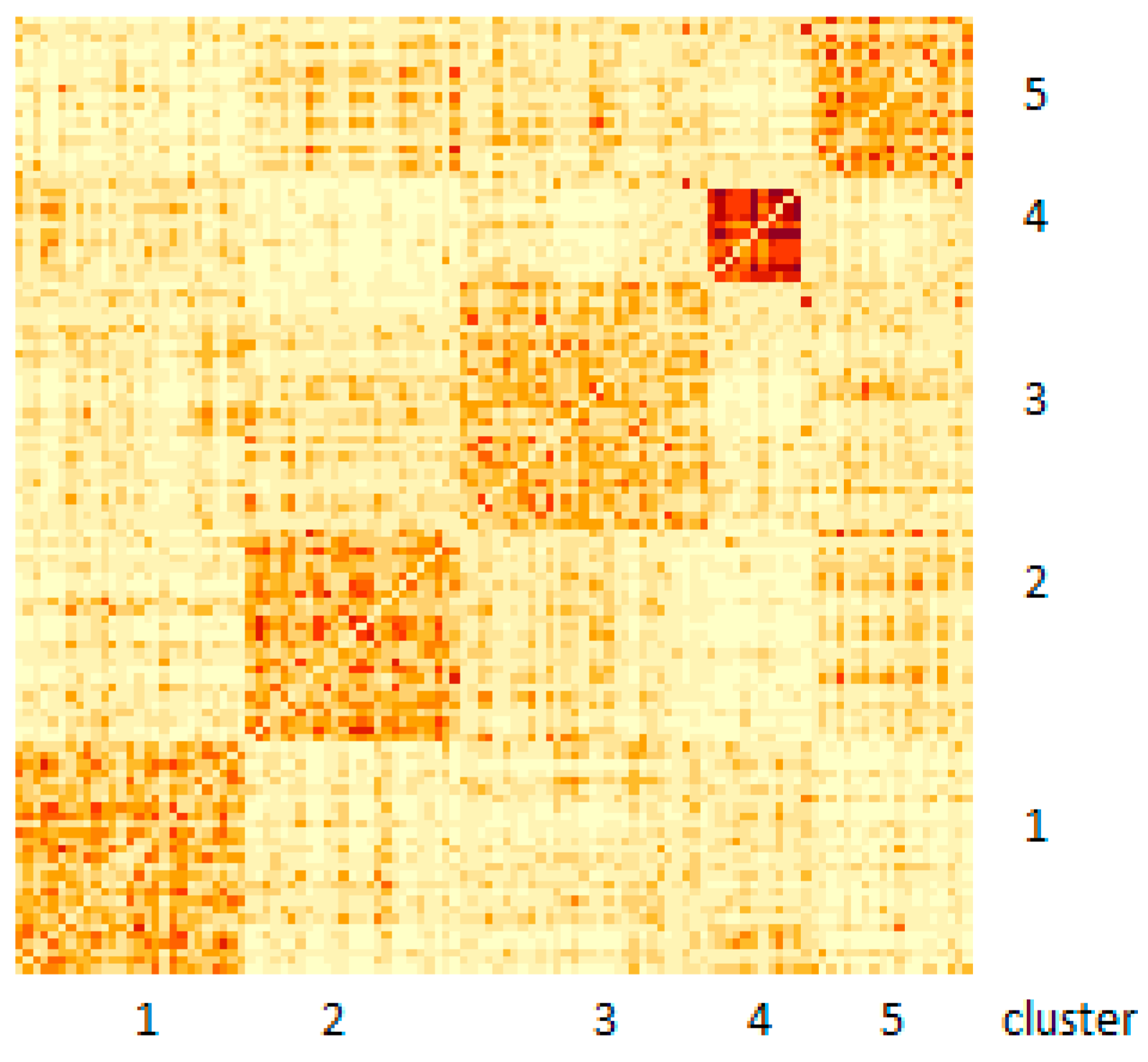
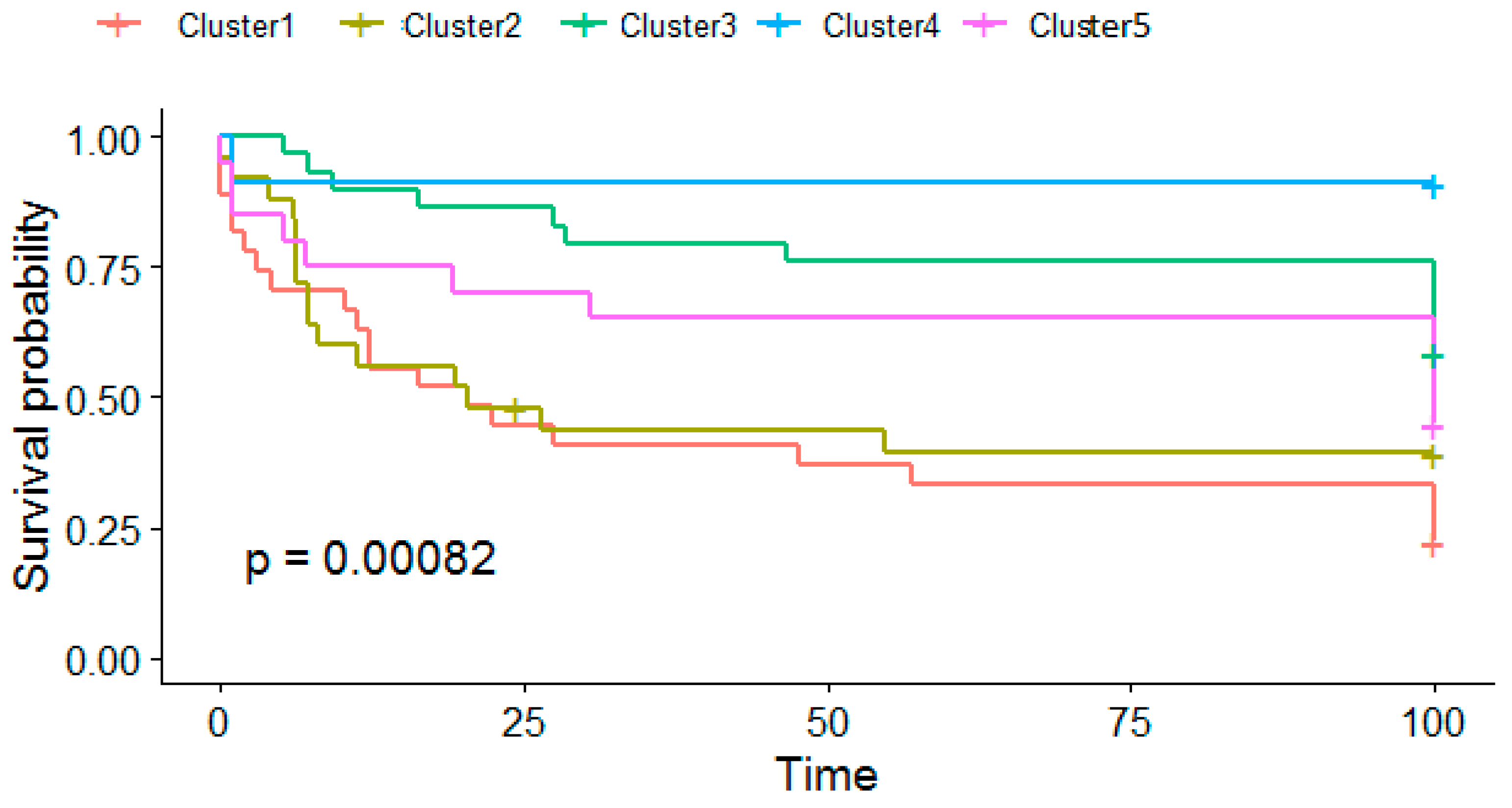
| Characteristics | Category | Cluster | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Number of patients | 27 | 25 | 29 | 11 | 20 | |
| Gender | male | 14 | 13 | 15 | 5 | 11 |
| female | 13 | 12 | 14 | 6 | 9 | |
| Age | mean | 63 | 57 | 46 | 45 | 48 |
| median | 68 | 58 | 48 | 42 | 50 | |
| range | 32–88 | 21–76 | 22–76 | 29–68 | 21–75 | |
| Vital status | dead | 21 | 15 | 12 | 1 | 11 |
| alive | 6 | 10 | 17 | 10 | 9 | |
| FAB classification | M0 | 7 | 0 | 1 | 0 | 3 |
| M1 | 8 | 4 | 9 | 0 | 10 | |
| M2 | 8 | 0 | 13 | 0 | 6 | |
| M3 | 0 | 0 | 0 | 11 | 0 | |
| M4 | 3 | 10 | 6 | 0 | 1 | |
| M5 | 0 | 11 | 0 | 0 | 0 | |
| unclassified | 1 | 0 | 0 | 0 | 0 | |
| ELN risk category | poor | 15 | 3 | 5 | 0 | 6 |
| normal | 12 | 20 | 12 | 1 | 13 | |
| favorable | 0 | 2 | 12 | 10 | 0 | |
| not known | 0 | 0 | 0 | 0 | 1 | |
| Mutations | ||||||
| IDH1_r132 | positive | 2 | 1 | 1 | 0 | 8 |
| negative | 25 | 23 | 28 | 10 | 12 | |
| not known | 1 | 0 | 0 | 1 | 0 | |
| IDH1_r140 | positive | 2 | 2 | 3 | 0 | 1 |
| negative | 24 | 23 | 26 | 11 | 18 | |
| not known | 1 | 0 | 0 | 0 | 1 | |
| IDH1_r172 | positive | 2 | 0 | 0 | 0 | 0 |
| negative | 24 | 25 | 29 | 11 | 19 | |
| not known | 1 | 0 | 0 | 0 | 1 | |
| Activating_ras | positive | 2 | 2 | 1 | 0 | 1 |
| negative | 24 | 23 | 28 | 11 | 19 | |
| not known | 1 | 0 | 0 | 0 | 0 | |
| NPM1c | positive | 1 | 14 | 0 | 0 | 9 |
| negative | 25 | 11 | 29 | 11 | 11 | |
| not known | 1 | 0 | 0 | 0 | 0 | |
| BCR::ABL1 | positive | 0 | 0 | 0 | 0 | 1 |
| negative | 1 | 5 | 2 | 0 | 2 | |
| not known | 26 | 20 | 27 | 11 | 17 | |
| PML::RARA | positive | 0 | 0 | 0 | 3 | 1 |
| negative | 1 | 2 | 0 | 0 | 2 | |
| not known | 26 | 23 | 29 | 8 | 17 | |
| FLT3-ITD | positive | 1 | 9 | 7 | 5 | 10 |
| negative | 23 | 16 | 22 | 6 | 9 | |
| not known | 3 | 0 | 0 | 0 | 1 | |
| Total number of somatic mutations/mutated genes | 339/113 | 519/206 | 689/248 | 33/9 | 181/61 | |
| Number of Genes | Cluster | Total Unique | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| selected in cluster (SIG) | 1542 | 1927 | 1328 | 1720 | 2781 | 4692 |
| enriched in cytokine signaling in immune system | 147 | 185 | 136 | 177 | 291 | 449 |
| enriched in interferon γ response (M5913; 176 genes) | 41 | 55 | 45 | 42 | 84 | 130 |
| enriched in interferon α response (M5911; 97 genes) | 13 | 27 | 21 | 13 | 38 | 56 |
| enriched in inflammatory response (M5932; 170 genes) | 53 | 56 | 42 | 36 | 78 | 128 |
| Type of Interactions | Type of Genes Used | Number of Genes in Interacting Regions | Number of Genes outside Interacting Regions | p-Value |
|---|---|---|---|---|
| intra-chromosomal | immune | 373 | 7437 | 8.97 × 10−4 |
| non-immune | 719 | 17,839 | ||
| inter-chromosomal | immune | 580 | 7230 | 2.19 × 10−6 |
| non-immune | 1088 | 17,470 |
| Number of Immune Genes | Cluster | Total Unique | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| in SIG | 1542 | 1927 | 1328 | 1720 | 2781 | 4692 |
| harboring germline mutations identified in [16] | 56 | 86 | 61 | 79 | 134 | 214 |
| affected by germline mutations via chromatin interactions | 200 | 252 | 205 | 275 | 344 | 583 |
| harboring somatic mutations | 26 | 55 | 37 | 1 | 23 | 138 |
| harboring both germline and somatic mutation | 6 | 9 | 5 | 0 | 4 | 22 |
| affected by both germline and somatic mutation | 1 | 6 | 5 | 0 | 1 | 13 |
| Genes harboring or affected by both germline and somatic mutations | Gene symbols | |||||
| AKR7A2, CNOT6L, CRISPLD2, GALNT2, PEAR1, RUNX1, IKZF3 1 | CELSR3, KRAS, LARS, NRAS, PKHD1, PLXNA2, PRKCZ, PEAR1, RUNX1, AP1M1, CAT, DLG5, PLEC, SMC5, TPI1 | CBL, GLI3, KLHL9, MYLK, NRXN3, ADAM19, FASTKD5, IGF2R, PCNT, TRIB1 | FLT3, PRDM16, RERE, XKR4, CDH23 | |||
| Number of | Cluster | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| genes in SIG | 1542 | 1927 | 1328 | 1720 |
| genes in PPI (% of SIG) | 1483 (96%) | 1866 (97%) | 1271 (96%) | 1670 (97%) |
| connections | 38,768 | 66,333 | 28,387 | 56,629 |
| average connections per gene | 26 | 36 | 22 | 34 |
| genes with top 5% of interactions | 75 | 94 | 67 | 84 |
| genes with top 1% of interactions | 15 | 20 | 14 | 17 |
| Genes with top 1% of interactions | Gene symbols | |||
| ACTB, AKT1, CCND1, ESR1, HIF1A 1, HRAS, IL1B1, IL6, NOTCH1, PTEN, RPS27A, STAT3, TP53, UBC, VEGFA | ACTB, AKT1, CD4, CTNNB1, EGFR, EP300, ESR1, HRAS, HSP90AA1, HSPA8, IL6, JUN, MYC, PTEN, RPS27A, STAT3, TNF, TP53, UBA52, VEGFA | ACTB, AKT1, CASP3, CCND1, CD4, EGFR, EP300, FN1, HSP90AA1, KRAS, MYC, NOTCH1, TP53, VEGFA | ACTB, AKT1, CASP3, CCND1, CD4, EGFR, EP300, FN1, HSP90AA1, KRAS, MYC, NOTCH1, TP53, VEGFA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batten, D.J.; Crofts, J.J.; Chuzhanova, N. Towards In Silico Identification of Genes Contributing to Similarity of Patients’ Multi-Omics Profiles: A Case Study of Acute Myeloid Leukemia. Genes 2023, 14, 1795. https://doi.org/10.3390/genes14091795
Batten DJ, Crofts JJ, Chuzhanova N. Towards In Silico Identification of Genes Contributing to Similarity of Patients’ Multi-Omics Profiles: A Case Study of Acute Myeloid Leukemia. Genes. 2023; 14(9):1795. https://doi.org/10.3390/genes14091795
Chicago/Turabian StyleBatten, Declan J., Jonathan J. Crofts, and Nadia Chuzhanova. 2023. "Towards In Silico Identification of Genes Contributing to Similarity of Patients’ Multi-Omics Profiles: A Case Study of Acute Myeloid Leukemia" Genes 14, no. 9: 1795. https://doi.org/10.3390/genes14091795
APA StyleBatten, D. J., Crofts, J. J., & Chuzhanova, N. (2023). Towards In Silico Identification of Genes Contributing to Similarity of Patients’ Multi-Omics Profiles: A Case Study of Acute Myeloid Leukemia. Genes, 14(9), 1795. https://doi.org/10.3390/genes14091795







