Translational Relevance of Advanced Age and Atherosclerosis in Preclinical Trials of Biotherapies for Peripheral Artery Disease
Abstract
1. Introduction
2. Literature Review
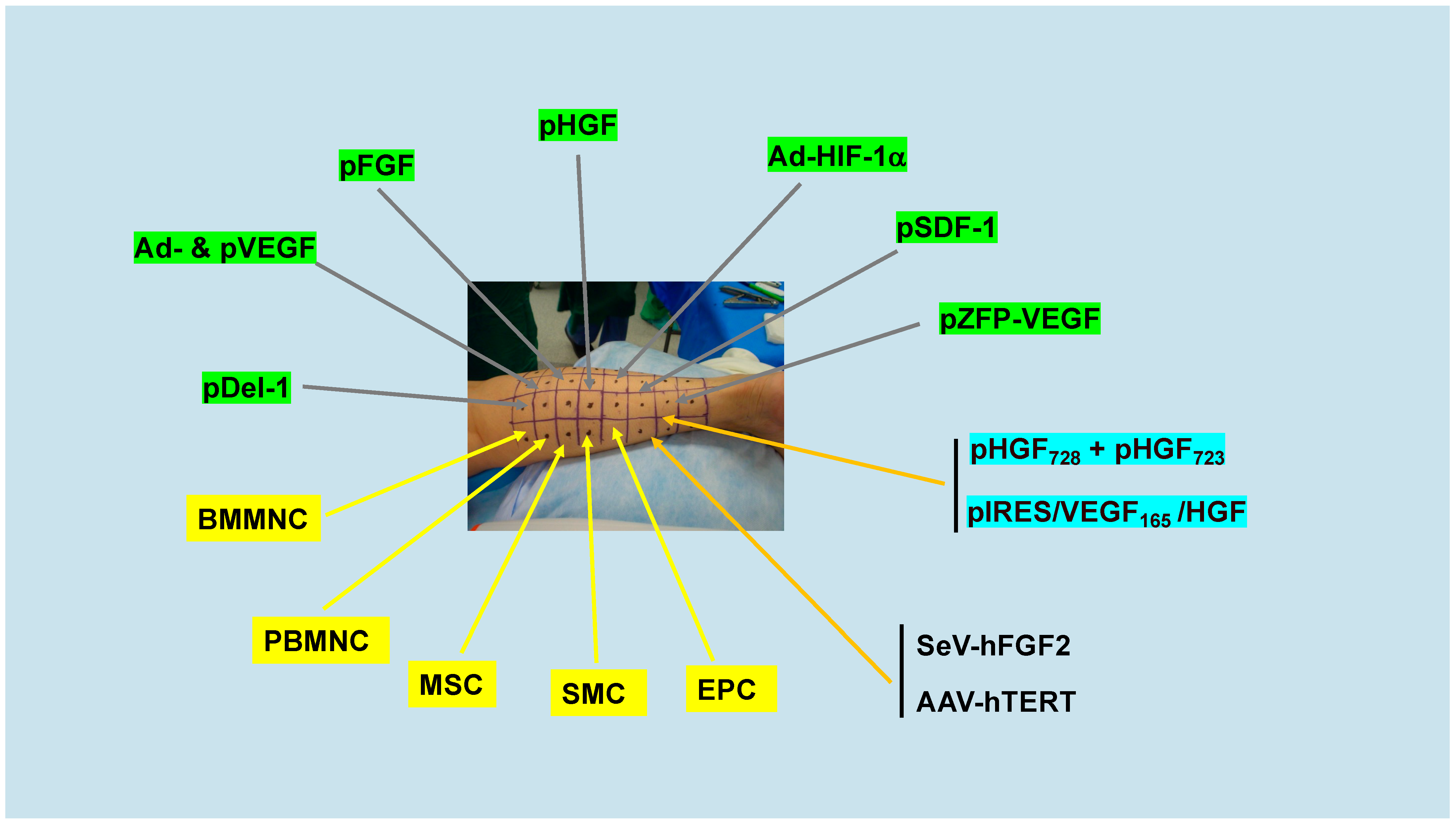
2.1. Meta-Analyses of Early Clinical Trials of Genes and Cells
2.2. Recent and Ongoing Clinical Trials of Genes and Cells II
2.3. Predictive Value of Preclinical Models
2.4. Age and Atherosclerosis in Mouse CLI Models
2.5. Recent and Ongoing Preclinical Trials of Genes, MiRs, and NO-Donors
3. Summary and Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Annex, B.H.; Cooke, J.P. New Directions in Therapeutic Angiogenesis and Arteriogenesis in Peripheral Arterial Disease. Circ. Res. 2021, 128, 1944–1957. [Google Scholar] [CrossRef] [PubMed]
- Kuppuswamy, S.; Annex, B.H.; Ganta, V.C. Targeting Anti-Angiogenic VEGF165b-VEGFR1 Signalling Promotes Nitric Oxide Independent Therapeutic Angiogenesis in Preclinical Peripheral Artery Disease Models. Cells 2022, 11, 2676. [Google Scholar] [CrossRef] [PubMed]
- Ganta, V.C.; Annex, B.H. Peripheral vascular disease: Preclinical models and emerging therapeutic targeting of the vascular endothelial growth factor ligand-receptor system. Expert Opin. Ther. Targets 2021, 25, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Rooke, T.W.; Hirsch, A.T.; Misra, S.; Sidawy, A.N.; Beckman, J.A.; Findeiss, L.; Golzarian, J.; Gornik, H.L.; Jaff, M.R.; Moneta, G.L.; et al. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, 1555–1570. [Google Scholar] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. Guidelines for the Management of Patients with Peripheral Arterial Disease. Circulation 2006, 113, 463–654. [Google Scholar]
- Iyer, S.R.; Annex, B.H. Therapeutic Angiogenesis for Peripheral Artery Disease: Lessons Learned in Translational Science. JACC Basic Transl. Sci. 2017, 2, 503–512. [Google Scholar] [CrossRef]
- Mohler, E.R., 3rd; Hiatt, W.R.; Creager, M.A. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 2003, 108, 1481–1486. [Google Scholar] [CrossRef]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Lawall, H.; Bramlage, P.; Amann, B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb. Haemost. 2010, 103, 696–709. [Google Scholar] [PubMed]
- De Haro, J.; Acin, F.; Lopez-Quintana, A.; Florez, A.; Martinez-Aguilar, E.; Varela, C. Meta-analysis of randomized, controlled clinical trials in angiogenesis: Gene and cell therapy in peripheral arterial disease. Heart Vessels 2009, 24, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Lazarous, D.F.; Unger, E.F.; Epstein, S.E.; Stine, A.; Arevalo, J.L.; Chew, E.Y.; Quyyumi, A.A. Basic fibroblast growth factor in patients with intermittent claudication. J. Am. Coll. Cardiol. 2000, 36, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Mohler, E.R., 3rd; Lederman, R.J.; Mendelsohn, F.O.; Saucedo, J.F.; Goldman, C.K.; Blebea, J.; Macko, J.; Kessler, P.D.; Rasmussen, H.S.; et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: A phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 2003, 108, 1933–1938. [Google Scholar] [PubMed]
- Lederman, R.J.; Mendelsohn, F.O.; Anderson, R.D.; Saucedo, J.F.; Tenaglia, A.N.; Hermiller, J.B.; Hillegass, W.B.; Rocha-Singh, K.; Moon, T.E.; Whitehouse, M.J.; et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): A randomised trial. Lancet 2002, 359, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, K.; Manninen, H.; Hedman, M.; Matsi, P.; Mussalo, H.; Alhava, E.; Ylä-Herttuala, S. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: A randomized, placebo-controlled, double-blinded phase II study. Mol. Ther. 2002, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Grossman, P.M.; Mendelsohn, F.; Henry, T.D.; Hermiller, J.B.; Litt, M.; Saucedo, J.F.; Weiss, R.J.; Kandzari, D.E.; Kleiman, N.; Anderson, R.D.; et al. Results from a phase II multicenter, double-blind placebo-controlled study of Del-1 (VLTS-589) for intermittent claudication in subjects with peripheral arterial disease. Am. Heart J. 2007, 153, 874–880. [Google Scholar] [CrossRef]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Hammer, A.; Steiner, S. Gene therapy for therapeutic angiogenesis in peripheral arterial disease—A systematic review and meta-analysis of randomized, controlled trials. Vasa 2013, 42, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Belch, J.; Hiatt, W.R.; Baumgartner, I.; Driver, I.V.; Nikol, S.; Norgren, L.; Van Belle, E. Effect of fibroblast growth factor NV1FGF on amputation and death: A randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet 2011, 377, 1929–1937. [Google Scholar] [CrossRef]
- Duong Van Huyen, J.P.; Smadja, D.M.; Bruneval, P.; Gaussem, P.; Dal-Cortivo, L.; Julia, P.; Fiessinger, J.N.; Cavazzana-Calvo, M.; Aiach, M.; Emmerich, J. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod. Pathol. 2008, 21, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.J.; Simons, M.; Mendelsohn, F.O.; Daniel, G.; Henry, T.D.; Koga, M.; Morishita, R.; Annex, B.H. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation 2008, 118, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Nikol, S.; Baumgartner, I.; Van Belle, E.; Diehm, C.; Visoná, A.; Capogrossi, M.C.; Ferreira-Maldent, N.; Gallino, A.; Wyatt, M.G.; Wijesinghe, L.D.; et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol. Ther. 2008, 16, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Yasuda, K.; Iwai, T.; Sasajima, T.; Ishimaru, S.; Ohashi, Y.; Yamaguchi, T.; Ogihara, T.; Morishita, R. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010, 17, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Olin, J.W.; Belch, J.J.; Moneta, G.L.; Henry, T.D.; Rajagopalan, S.; Annex, B.H.; Hiatt, W.R. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation 2011, 124, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Krankenberg, H.; Balzer, J.O.; Kalka, C.; Baumgartner, I.; Schlüter, M.; Tonn, T.; Seeger, F.; Dimmeler, S.; Lindhoff-Last, E.; et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: A randomized-start, placebo-controlled pilot trial (PROVASA). Circ. Cardiovasc. Interv. 2011, 4, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Teraa, M.; Sprengers, R.W.; Schutgens, R.E.; Slaper-Cortenbach, I.C.; van der Graaf, Y.; Algra, A.; van der Tweel, I.; Doevendans, P.A.; Mali, W.P.; Moll, F.L.; et al. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: The randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial. Circulation 2015, 131, 851–860. [Google Scholar] [PubMed]
- Fowkes, F.G.; Price, J.F. Gene therapy for critical limb ischaemia: The TAMARIS trial. Lancet 2011, 377, 1894–1896. [Google Scholar] [CrossRef]
- Forster, R.; Liew, A.; Bhattacharya, V.; Shaw, J.; Stansby, G. Gene therapy for peripheral arterial disease. Cochrane Database Syst. Rev. 2018, 10, CD012058. [Google Scholar] [CrossRef]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ. Res. 2017, 120, 1326–1340. [Google Scholar] [CrossRef]
- Xie, B.; Luo, H.; Zhang, Y.; Wang, Q.; Zhou, C.; Xu, D. Autologous Stem Cell Therapy in Critical Limb Ischemia: A Meta-Analysis of Randomized Controlled Trials. Stem Cells Int. 2018, 2018, 7528464. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, D.; Liu, G.; Ran, X. Autologous stem cell therapy for peripheral arterial disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Huang, Q.; Zhang, X.; Wu, Z.; Qiu, P.; Jiang, Y.; Wang, R.; Zhao, Z.; Xu, Z.; Qin, J.; et al. A meta-analysis of randomized controlled trials on therapeutic efficacy and safety of autologous cell therapy for atherosclerosis obliterans. J. Vasc. Surg. 2022, 75, 1440–1449.e5. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Camacho, L.; Rojas-Torres, M.; Durán-Ruiz, M.C. Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia. Int. J. Mol. Sci. 2021, 22, 2335. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Salmeron, R.; de la Cuesta-Diaz, A.; Constantino-Bermejo, M.; Pérez-Camacho, I.; Marcos-Sánchez, F.; Hmadcha, A.; Soria, B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transpl. 2011, 20, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, B.; Gentile, P.; Orlandi, F.; Bocchini, I.; Di Pasquali, C.; Agovino, A.; Gizzi, C.; Patrizi, F.; Scioli, M.G.; Orlandi, A.; et al. Limb rescue: A new autologous-peripheral blood mononuclear cells technology in critical limb ischemia and chronic ulcers. Tissue Eng. Part C Methods 2015, 21, 423–435. [Google Scholar] [CrossRef]
- O’Neill, K.M.; Campbell, D.C.; Edgar, K.S.; Gill, E.K.; Moez, A.; McLoughlin, K.J.; O’Neill, C.L.; Dellett, M.; Hargey, C.J.; Abudalo, R.A.; et al. NOX4 is a major regulator of cord blood-derived endothelial colony-forming cells which promotes post-ischaemic revascularization. Cardiovasc. Res. 2020, 116, 393–405. [Google Scholar] [CrossRef]
- Hu, X.; Wu, R.; Jiang, Z.; Wang, L.; Chen, P.; Zhang, L.; Yang, L.; Wu, Y.; Chen, H.; Chen, H.; et al. Leptin signalling is required for augmented therapeutic properties of mesenchymal stem cells conferred by hypoxia preconditioning. Stem Cells 2014, 32, 2702–2713. [Google Scholar] [CrossRef]
- Ma, Q.; Xia, X.; Tao, Q.; Lu, K.; Shen, J.; Xu, Q.; Hu, X.; Tang, Y.; Block, N.L.; Webster, K.A.; et al. Profound Actions of an Agonist of Growth Hormone-Releasing Hormone on Angiogenic Therapy by Mesenchymal Stem Cells. Arter Thromb. Vasc. Biol. 2016, 36, 663–672. [Google Scholar] [CrossRef]
- Deev, R.V.; Bozo, I.Y.; Mzhavanadze, N.D.; Voronov, D.A.; Gavrilenko, A.V.; Chervyakov, Y.V.; Staroverov, I.N.; Kalinin, R.E.; Shvalb, P.G.; Isaev, A.A. pCMV-vegf165 Intramuscular Gene Transfer is an Effective Method of Treatment for Patients with Chronic Lower Limb Ischemia. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 473–482. [Google Scholar] [CrossRef]
- Deev, R.; Plaksa, I.; Bozo, I.; Isaev, A. Results of an International Postmarketing Surveillance Study of pl-VEGF165 Safety and Efficacy in 210 Patients with Peripheral Arterial Disease. Am. J. Cardiovasc. Drugs 2017, 17, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Deev, R.; Plaksa, I.; Bozo, I.; Mzhavanadze, N.; Suchkov, I.; Chervyakov, Y.; Staroverov, I.; Kalinin, R.; Isaev, A. Results of 5-year follow-up study in patients with peripheral artery disease treated with PL-VEGF165 for intermittent claudication. Ther. Adv. Cardiovasc. Dis. 2018, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Cui, S.; Wang, Q.; Liu, C.; Jin, B.; Guo, W.; Liu, C.; Chu, T.; Shu, C.; Zhang, F.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Study of Hepatocyte Growth Factor in the Treatment of Critical Limb Ischemia. Mol. Ther. 2019, 27, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S. Gene Therapy of Critical Limb Ischemia Enters Clinical Use. Mol. Ther. 2019, 27, 2053. [Google Scholar] [CrossRef] [PubMed]
- Morishita, R.; Shimamura, M.; Takeya, Y.; Nakagami, H.; Chujo, M.; Ishihama, T.; Yamada, E.; Rakugi, H. Combined Analysis of Clinical Data on HGF Gene Therapy to Treat Critical Limb Ischemia in Japan. Curr. Gene Ther. 2020, 20, 25–35. [Google Scholar] [PubMed]
- Sanada, F.; Fujikawa, T.; Shibata, K.; Taniyama, Y.; Rakugi, H.; Morishita, R. Therapeutic Angiogenesis Using HGF Plasmid. Ann. Vasc. Dis. 2020, 13, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.A.; Shaibani, A.; Sang, C.N.; Christiansen, M.; Kudrow, D.; Vinik, A.; Shin, N. Gene therapy for diabetic peripheral neuropathy: A randomized, placebo-controlled phase III study of VM202, a plasmid DNA encoding human hepatocyte growth factor. Clin. Transl. Sci. 2021, 14, 1176–1184. [Google Scholar] [CrossRef]
- Barć, P.; Antkiewicz, M.; Śliwa, B.; Frączkowska, K.; Guziński, M.; Dawiskiba, T.; Małodobra-Mazur, M.; Witkiewicz, W.; Kupczyńska, D.; Strzelec, B.; et al. Double VEGF/HGF Gene Therapy in Critical Limb Ischemia Complicated by Diabetes Mellitus. J. Cardiovasc. Transl. Res. 2021, 14, 409–415. [Google Scholar] [CrossRef]
- Shishehbor, M.H.; Rundback, J.; Bunte, M.; Hammad, T.A.; Miller, L.; Patel, P.D.; Sadanandan, S.; Fitzgerald, M.; Pastore, J.; Kashyap, V.; et al. SDF-1 plasmid treatment for patients with peripheral artery disease (STOP-PAD): Randomized, double-blind, placebo-controlled clinical trial. Vasc. Med. 2019, 24, 200–207. [Google Scholar] [CrossRef]
- Zaccagnini, G.; Gaetano, C.; Della Pietra, L.; Nanni, S.; Grasselli, A.; Mangoni, A.; Benvenuto, R.; Fabrizi, M.; Truffa, S.; Germani, A.; et al. Telomerase mediates vascular endothelial growth factor-dependent responsiveness in a rat model of hind limb ischemia. J. Biol. Chem. 2005, 280, 14790–14798. [Google Scholar] [CrossRef]
- Golledge, J. Update on the pathophysiology and medical treatment of peripheral artery disease. Nat. Rev. Cardiol. 2022, 19, 456–474. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Luo, L.; Marcelina, O.; Kasim, V.; Wu, S. Therapeutic angiogenesis-based strategy for peripheral artery disease. Theranostics 2022, 12, 5015–5033. [Google Scholar] [CrossRef] [PubMed]
- Rebar, E.J.; Huang, Y.; Hickey, R.; Nath, A.K.; Meoli, D.; Nath, S.; Chen, B.; Xu, L.; Liang, Y.; Jamieson, A.C.; et al. Induction of angiogenesis in a mouse model using engineered transcription factors. Nat. Med. 2002, 8, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Campia, U.; Gerhard-Herman, M.; Piazza, G.; Goldhaber, S.Z. Peripheral Artery Disease: Past, Present, and Future. Am. J. Med. 2019, 132, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Firnhaber, J.M.; Powell, C.S. Lower Extremity Peripheral Artery Disease: Diagnosis and Treatment. Am. Fam. Physician 2019, 99, 362–369. [Google Scholar] [PubMed]
- Boden, J.; Lassance-Soares, R.M.; Wang, H.; Wei, Y.; Spiga, M.G.; Adi, J.; Layman, H.; Yu, H.; Vazquez-Padron, R.I.; Andreopoulos, F.; et al. Vascular Regeneration in Ischemic Hindlimb by Adeno-Associated Virus Expressing Conditionally Silenced Vascular Endothelial Growth Factor. J. Am. Heart Assoc. 2016, 5, e001815. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Omer, S.M.; Golledge, J. Evaluation of the clinical relevance and limitations of current pre-clinical models of peripheral artery disease. Clin. Sci. 2016, 130, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Omer, S.M.; Li, J.; Morton, S.K.; Jose, R.J.; Golledge, J. Development of a two-stage limb ischemia model to better simulate human peripheral artery disease. Sci. Rep. 2020, 10, 3449. [Google Scholar] [CrossRef]
- Golledge, J.; Fernando, M.E.; Armstrong, D.G. Current Management of Peripheral Artery Disease: Focus on Pharmacotherapy. Drugs 2022, 82, 1165–1177. [Google Scholar] [CrossRef]
- Annex, B.H. Therapeutic angiogenesis for critical limb ischaemia. Nat. Rev. Cardiol. 2013, 10, 387–396. [Google Scholar] [CrossRef]
- Peck, M.A.; Crawford, R.S.; Abularrage, C.J.; Patel, V.I.; Conrad, M.F.; Yoo, J.H.; Watkins, M.T.; Albadawi, H. A functional murine model of hindlimb demand ischemia. Ann. Vasc. Surg. 2010, 24, 532–537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, J.B.; Phillips, E.H.; Riggins, T.A.; Sangha, G.S.; Chakraborty, S.; Lee, J.Y.; Lycke, R.J.; Hernandez, C.L.; Soepriatna, A.H.; Thorne, B.R.; et al. Imaging of small animal peripheral artery disease models: Recent advancements and translational potential. Int. J. Mol. Sci. 2015, 16, 11131–11177. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, S.; Patel, A.S.; Mattock, K.; Egginton, S.; Smith, A.; Modarai, B. Towards a more relevant hind limb model of muscle ischaemia. Atherosclerosis 2013, 227, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Zheng, J.; Zöllner, F.G.; Schwenke, K.; Pallavi, P.; Keese, M. A Modified Surgical Model of Hind Limb Ischemia in ApoE-/- Mice using a Miniature Incision. J. Vis. Exp. 2021, 13, 171. [Google Scholar]
- Aref, Z.; de Vries, M.R.; Quax, P.H.A. Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment. Int. J. Mol. Sci. 2019, 20, 3704. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y.; Bertolini, F.; Man, S.; Rogers, M.S.; Cervi, D.; Foutz, T.; Rawn, K.; Voskas, D.; Dumont, D.J.; Ben-David, Y.; et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005, 7, 101–111. [Google Scholar] [PubMed]
- Bosch-Marce, M.; Okuyama, H.; Wesley, J.B.; Sarkar, K.; Kimura, H.; Liu, Y.V.; Zhang, H.; Strazza, M.; Rey, S.; Savino, L.; et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ. Res. 2007, 101, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Westvik, T.S.; Fitzgerald, T.N.; Muto, A.; Maloney, S.P.; Pimiento, J.M.; Fancher, T.T.; Magri, D.; Westvik, H.H.; Nishibe, T.; Velazquez, O.C.; et al. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J. Vasc. Surg. 2009, 49, 464–473. [Google Scholar] [CrossRef]
- Wang, J.; Peng, X.; Lassance-Soares, R.M.; Najafi, A.H.; Alderman, L.O.; Sood, S.; Xue, Z.; Chan, R.; Faber, J.E.; Epstein, S.E.; et al. Aging-induced collateral dysfunction: Impaired responsiveness of collaterals and susceptibility to apoptosis via dysfunctional eNOS signaling. J. Cardiovasc. Transl. Res. 2011, 4, 779–789. [Google Scholar] [CrossRef][Green Version]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Fukino, K.; Suzuki, T.; Saito, Y.; Shindo, T.; Amaki, T.; Kurabayashi, M.; Nagai, R. Regulation of angiogenesis by the aging suppressor gene klotho. Biochem. Biophys. Res. Commun. 2002, 293, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.R.; Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res Rev. 2022, 82, 101766. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Takeshita, Y.; Murohara, T.; Sasaki, K.; Egami, K.; Shintani, S.; Katsuda, Y.; Ikeda, H.; Nabeshima, Y.; Imaizumi, T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation 2004, 110, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Martín-Núñez, E.; Pérez-Castro, A.; Tagua, V.G.; Hernández-Carballo, C.; Ferri, C.; Pérez-Delgado, N.; Rodríguez-Ramos, S.; Cerro-López, P.; López-Castillo, Á.; Delgado-Molinos, A.; et al. Klotho expression in peripheral blood circulating cells is associated with vascular and systemic inflammation in atherosclerotic vascular disease. Sci Rep. 2022, 12, 8422. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Poledniczek, M.; Neumayer, C.; Kopp, C.W.; Schlager, O.; Gremmel, T.; Jozkowicz, A.; Gschwandtner, M.E.; Koppensteiner, R.; Wadowski, P.P. Micro- and Macrovascular Effects of Inflammation in Peripheral Artery Disease-Pathophysiology and Translational Therapeutic Approaches. Biomedicines 2023, 11, 2284. [Google Scholar] [CrossRef]
- Krishna, S.M.; Moxon, J.V.; Golledge, J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int. J. Mol. Sci. 2015, 16, 11294–11322. [Google Scholar] [CrossRef]
- McDermott, M.M.; Ferrucci, L.; Gonzalez-Freire, M.; Kosmac, K.; Leeuwenburgh, C.; Peterson, C.A.; Saini, S.; Sufit, R. Skeletal Muscle Pathology in Peripheral Artery Disease: A Brief Review. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2577–2585. [Google Scholar] [CrossRef]
- Burgmaier, M.; Schutters, K.; Willems, B.; van der Vorst, E.P.; Kusters, D.; Chatrou, M.; Norling, L.; Biessen, E.A.; Cleutjens, J.; Perretti, M.; et al. AnxA5 reduces plaque inflammation of advanced atherosclerotic lesions in apoE(-/-) mice. J. Cell Mol. Med. 2014, 18, 2117–2124. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, X.; Dai, D.; Zhang, B.; Lu, L.; Tao, R. The anti-inflammatory vasostatin-2 attenuates atherosclerosis in ApoE-/- mice and inhibits monocyte/macrophage recruitment. Thromb. Haemost. 2017, 117, 401–414. [Google Scholar] [CrossRef]
- Kahles, F.; Liberman, A.; Halim, C.; Rau, M.; Möllmann, J.; Mertens, R.W.; Rückbeil, M.; Diepolder, I.; Walla, B.; Diebold, S.; et al. The incretin hormone GIP is upregulated in patients with atherosclerosis and stabilizes plaques in ApoE-/- mice by blocking monocyte/macrophage activation. Mol. Metab. 2018, 14, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, J.; He, J.; Zhou, M.; Adi, J.; Webster, K.A.; Yu, H. Impaired CXCR4 expression and cell engraftment of bone marrow-derived cells from aged atherogenic mice. Atherosclerosis 2011, 219, 92–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, H.; Liu, P.; Lin, S. Effects of Astragaloside IV on the SDF-1/CXCR4 Expression in Atherosclerosis of apoE(-/-) Mice Induced by Hyperlipaemia. Evid. Based Complement. Altern. Med. 2015, 2015, 385154. [Google Scholar] [CrossRef]
- Heidt, T.; Sager, H.B.; Courties, G.; Dutta, P.; Iwamoto, Y.; Zaltsman, A.; von Zur Muhlen, C.; Bode, C.; Fricchione, G.L.; Denninger, J.; et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014, 20, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.M.; Megens, R.T.; Zernecke, A.; Bidzhekov, K.; van den Akker, N.M.; Rademakers, T.; van Zandvoort, M.A.; Hackeng, T.M.; Koenen, R.R.; Weber, C. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation 2014, 129, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Lombardi, D.M.; Polinsky, P.; Powell-Braxton, L.; Bunting, S.; Schwartz, S.M.; Rosenfeld, M.E. Peripheral vascular stenosis in apolipoprotein E-deficient mice. Potential roles of lipid deposition, medial atrophy, and adventitial inflammation. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3593–3601. [Google Scholar] [CrossRef]
- Rodriguez-Menocal, L.; Wei, Y.; Pham, S.M.; St-Pierre, M.; Li, S.; Webster, K.A.; Goldschmidt-Clermont, P.; Vazquez-Padron, R.I. A novel mouse model of in-stent restenosis. Atherosclerosis 2010, 209, 359–366. [Google Scholar] [CrossRef][Green Version]
- Cesar, L.; Suarez, S.V.; Adi, J.; Adi, N.; Vazquez-Padron, R.; Yu, H.; Ma, Q.; Goldschmidt-Clermont, P.J.; Agatston, A.; Kurlansky, P.; et al. An essential role for diet in exercise-mediated protection against dyslipidemia, inflammation, and atherosclerosis in ApoE-/-mice. PLoS ONE 2011, 6, e17263. [Google Scholar] [CrossRef]
- Balestrieri, M.L.; Lu, S.J.; de Nigris, F.; Giovane, A.; Williams-Ignarro, S.; D’Armiento, F.P.; Napoli, C. Therapeutic angiogenesis in diabetic apolipoprotein E-deficient mice using bone marrow cells, functional hemangioblasts and metabolic intervention. Atherosclerosis 2010, 209, 403–414. [Google Scholar] [CrossRef]
- Kang, J.; Albadawi, H.; Patel, V.I.; Abbruzzese, T.A.; Yoo, J.H.; Austen WGJr Watkins, M.T. Apolipoprotein E-/- mice have delayed skeletal muscle healing after hind limb ischemia-reperfusion. J. Vasc. Surg. 2008, 48, 701–708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Couffinhal, T.; Silver, M.; Kearney, M.; Sullivan, A.; Witzenbichler, B.; Magner, M.; Annex, B.; Peters, K.; Isner, J.M. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation 1999, 99, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, Y.; Reed, E.A.; Odronic, S.I.; Kontos, C.D.; Annex, B.H. An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia. J. Vasc. Surg. 2006, 44, 166–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lejay, A.; Charles, A.L.; Georg, I.; Goupilleau, F.; Delay, C.; Talha, S.; Thaveau, F.; Chakfé, N.; Geny, B. Critical Limb Ischaemia Exacerbates Mitochondrial Dysfunction in ApoE-/- Mice Compared with ApoE+/+ Mice, but N-acetyl Cysteine still Confers Protection. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M.W.; Moore, K.J. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Yao, C.; Li, Z.L.; Teng, Y.; Li, W.; Wang, J.S.; Ye, C.S.; Chang, G.Q.; Huang, X.L.; Li, X.X.; et al. Differentially expressed microRNAs at different stages of atherosclerosis in ApoE-deficient mice. Chin. Med. J. 2013, 126, 515–520. [Google Scholar] [CrossRef]
- Vogiatzi, G.; Oikonomou, E.; Deftereos, S.; Siasos, G.; Tousoulis, D. Peripheral artery disease: A micro-RNA-related condition? Curr. Opin. Pharmacol. 2018, 39, 105–112. [Google Scholar] [CrossRef]
- Pérez-Cremades, D.; Cheng, H.S.; Feinberg, M.W. Noncoding RNAs in Critical Limb Ischemia. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 523–533. [Google Scholar] [CrossRef]
- Gao, L.; Zeng, H.; Zhang, T.; Mao, C.; Wang, Y.; Han, Z.; Chen, K.; Zhang, J.; Fan, Y.; Gu, J.; et al. MicroRNA-21 deficiency attenuated atherogenesis and decreased macrophage infiltration by targeting Dusp-8. Atherosclerosis 2019, 291, 78–86. [Google Scholar] [CrossRef]
- Rotllan, N.; Price, N.; Pati, P.; Goedeke, L.; Fernández-Hernando, C. microRNAs in lipoprotein metabolism and cardiometabolic disorders. Atherosclerosis 2016, 246, 352–360. [Google Scholar] [CrossRef]
- Desjarlais, M.; Dussault, S.; Rivard, F.; Harel, S.; Sanchez, V.; Hussain, S.N.A.; Rivard, A. Forced expression of microRNA-146b reduces TRAF6-dependent inflammation and improves ischemia-induced neovascularization in hypercholesterolemic conditions. Atherosclerosis 2019, 289, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Qun, L.; Wenda, X.; Weihong, S.; Jianyang, M.; Wei, C.; Fangzhou, L.; Zhenyao, X.; Pingjin, G. miRNA-27b modulates endothelial cell angiogenesis by directly targeting Naa15 in atherogenesis. Atherosclerosis 2016, 254, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, H.; Timur, A.A.; Pasupuleti, V.; Yao, Y.; Zhang, T.; You, S.A.; Fan, C.; Yu, Y.; Jia, X.; et al. Receptor and Molecular Mechanism of AGGF1 Signaling in Endothelial Cell Functions and Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2756–2769. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, D.; Li, J.; Wang, X.; Wang, N.; Xu, C.; Wang, Q.K. Aggf1 acts at the top of the genetic regulatory hierarchy in specification of hemangioblasts in zebrafish. Blood 2014, 123, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chen, Q.; Wang, Q.K. Functional role of transcriptional factor TBX5 in pre-mRNA splicing and Holt-Oram syndrome via association with SC35. J. Biol. Chem. 2009, 284, 25653–25663. [Google Scholar] [CrossRef]
- Zhang, T.; Yao, Y.; Wang, J.; Li, Y.; He, P.; Pasupuleti, V.; Hu, Z.; Jia, X.; Song, Q.; Tian, X.L.; et al. Haploinsufficiency of Klippel-Trenaunay syndrome gene Aggf1 inhibits developmental and pathological angiogenesis by inactivating PI3K and AKT and disrupts vascular integrity by activating VE-cadherin. Hum. Mol. Genet. 2016, 25, 5094–5110. [Google Scholar] [PubMed]
- Fan, C.; Ouyang, P.; Timur, A.A.; He, P.; You, S.A.; Hu, Y.; Ke, T.; Driscoll, D.J.; Chen, Q.; Wang, Q.K. Novel roles of GATA1 in regulation of angiogenic factor AGGF1 and endothelial cell function. J. Biol. Chem. 2009, 284, 23331–23343. [Google Scholar] [CrossRef]
- Tian, X.L.; Kadaba, R.; You, S.A.; Liu, M.; Timur, A.A.; Yang, L.; Chen, Q.; Szafranski, P.; Rao, S.; Wu, L.; et al. Identification of an angiogenic factor that when mutated causes susceptibility to Klippel-Trenaunay syndrome. Nature 2004, 427, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yao, Y.; Yao, Y.; Liu, S.; Huang, Y.; Lu, S.; Bai, Y.; Zhou, B.; Xu, Y.; Li, L.; et al. Angiogenic factor AGGF1 promotes therapeutic angiogenesis in a mouse limb ischemia model. PLoS ONE 2012, 7, e46998. [Google Scholar] [CrossRef] [PubMed]
- Mac Gabhann, F.; Annex, B.H. AGGF1 Shows the α5β1 Integrin to Be Another Akt-or in a Common Angiogenesis Scene. Arterioscler Thromb. Vasc. Biol. 2021, 41, 2770–2772. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, Y.; Song, Q.; Hu, C.; Xie, W.; Xu, C.; Chen, Q.; Wang, Q.K. Angiogenic Factor AGGF1-Primed Endothelial Progenitor Cells Repair Vascular Defect in Diabetic Mice. Diabetes 2019, 68, 1635–1648. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Peng, H.; Song, Q.; Da, X.; Li, H.; He, Z.; Ren, X.; Xu, C.; Yao, Y.; et al. Angiogenic factor AGGF1 blocks neointimal formation after vascular injury via interaction with integrin α7 on vascular smooth muscle cells. J. Biol. Chem. 2022, 298, 101759. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.J.; Bates, D.O. VEGF-A splicing: The key to anti-angiogenic therapeutics? Nat. Rev. Cancer 2008, 8, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Rennel, E.S.; Varey, A.H.; Churchill, A.J.; Wheatley, E.R.; Stewart, L.; Mather, S.; Bates, D.O.; Harper, S.J. VEGF(121)b, a new member of the VEGF(xxx)b family of VEGF-A splice isoforms, inhibits neovascularisation and tumour growth in vivo. Br. J. Cancer 2009, 101, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Catena, R.; Larzabal, L.; Larrayoz, M.; Molina, E.; Hermida, J.; Agorreta, J.; Montes, R.; Pio, R.; Montuenga, L.M.; Calvo, A. VEGF121b and VEGF165b are weakly angiogenic isoforms of VEGF-A. Mol. Cancer 2010, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Li, X.; Harper, S.J.; Bates, D.O.; Claesson-Welsh, L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008, 68, 4683–4692. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Jorganes, A.; Araldi, E.; Penalva, L.O.; Sandhu, D.; Fernández-Hernando, C.; Suárez, Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2595–2606. [Google Scholar] [CrossRef]
- Yue, J.; Tigyi, G. Conservation of miR-15a/16-1 and miR-15b/16-2 clusters. Mamm. Genome 2010, 21, 88–94. [Google Scholar] [CrossRef]
- Spinetti, G.; Fortunato, O.; Caporali, A.; Shantikumar, S.; Marchetti, M.; Meloni, M.; Descamps, B.; Floris, I.; Sangalli, E.; Vono, R.; et al. MicroRNA-15a and microRNA-16 impair human circulating proangiogenic cell functions and are increased in the proangiogenic cells and serum of patients with critical limb ischemia. Circ. Res. 2013, 112, 335–346. [Google Scholar] [CrossRef]
- Yin, K.J.; Olsen, K.; Hamblin, M.; Zhang, J.; Schwendeman, S.P.; Chen, Y.E. Vascular endothelial cell-specific microRNA-15a inhibits angiogenesis in hindlimb ischemia. J. Biol. Chem. 2012, 287, 27055–27064. [Google Scholar] [CrossRef]
- Desjarlais, M.; Dussault, S.; Dhahri, W.; Mathieu, R.; Rivard, A. MicroRNA-150 Modulates Ischemia-Induced Neovascularization in Atherosclerotic Conditions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 900–908. [Google Scholar] [CrossRef]
- Ryan, T.E.; Schmidt, C.A.; Tarpey, M.D.; Amorese, A.J.; Yamaguchi, D.J.; Goldberg, E.J.; Iñigo, M.M.; Karnekar, R.; O’Rourke, A.; Ervasti, J.M.; et al. PFKFB3-mediated glycolysis rescues myopathic outcomes in the ischemic limb. JCI Insight 2020, 5, e139628. [Google Scholar] [CrossRef]
- Gomes de Almeida Schirmer, B.; Crucet, M.; Stivala, S.; Vucicevic, G.; da Silva Barcelos, L.; Vanhoutte, P.M.; Pellegrini, G.; Camici, G.G.; Seebeck, P.; Pfundstein, S.; et al. The NO-donor MPC-1011 stimulates angiogenesis and arteriogenesis and improves hindlimb ischemia via a cGMP-dependent pathway involving VEGF and SDF-1α. Atherosclerosis 2020, 304, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Pignatelli, P.; Cangemi, R.; Andreozzi, P.; Panico, M.A.; Meloni, V.; Violi, F. Imbalance between nitric oxide generation and oxidative stress in patients with peripheral arterial disease: Effect of an antioxidant treatment. J. Vasc. Surg. 2006, 44, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Giordano, T.; Kevil, C.G. Nitrite and nitric oxide metabolism in peripheral artery disease. Nitric Oxide 2012, 26, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.F.; Liang, X.Y.; Liu, W.; Lv, S.; He, S.J.; Kuang, H.B.; Yang, S.L. Roles of eNOS in atherosclerosis treatment. Inflamm. Res. 2019, 68, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ismaeel, A.; Papoutsi, E.; Miserlis, D.; Lavado, R.; Haynatzki, G.; Casale, G.P.; Bohannon, W.T.; Smith, R.S.; Eidson, J.L.; Brumberg, R.; et al. The Nitric Oxide System in Peripheral Artery Disease: Connection with Oxidative Stress and Biopterins. Antioxidants 2020, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Woessner, M.; VanBruggen, M.D.; Pieper, C.F.; Sloane, R.; Kraus, W.E.; Gow, A.J.; Allen, J.D. Beet the Best? Circ. Res. 2018, 123, 654–659. [Google Scholar] [CrossRef]
- Kenjale, A.A.; Ham, K.L.; Stabler, T.; Robbins, J.L.; Johnson, J.L.; Vanbruggen, M.; Privette, G.; Yim, E.; Kraus, W.E.; Allen, J.D. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J. Appl. Physiol. 2011, 110, 1582–1591. [Google Scholar] [CrossRef]
- Loffredo, L.; Perri, L.; Catasca, E.; Pignatelli, P.; Brancorsini, M.; Nocella, C.; De Falco, E.; Bartimoccia, S.; Frati, G.; Carnevale, R.; et al. Dark chocolate acutely improves walking autonomy in patients with peripheral artery disease. J. Am. Heart Assoc. 2014, 3, e001072. [Google Scholar] [CrossRef]
- Loffredo, L.; Perri, L.; Catasca, E.; Pignatelli, P.; Brancorsini, M.; Nocella, C.; De Falco, E.; Bartimoccia, S.; Frati, G.; Carnevale, R.; et al. Cocoa to Improve Walking Performance in Older People with Peripheral Artery Disease: The COCOA-PAD Pilot Randomized Clinical Trial. Circ. Res. 2020, 126, 589–599. [Google Scholar]
- Park, S.Y.; Pekas, E.J.; Headid, R.J., 3rd; Son, W.M.; Wooden, T.K.; Song, J.; Layec, G.; Yadav, S.K.; Mishra, P.K.; Pipinos, I.I. Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H456–H467. [Google Scholar] [CrossRef] [PubMed]
- Kotalczyk, A.; Vallabhaneni, S.R.; Lip, G.Y.H. Review new concepts in pharmacotherapy for peripheral arterial disease. Curr. Opin. Cardiol. 2021, 36, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Omarjee, L.; Le Pabic, E.; Custaud, M.A.; Fontaine, C.; Locher, C.; Renault, A.; Jaquinandi, V.; Azzola, V.; Barbeau-Terrier, C.; Laporte, I.; et al. Effects of sildenafil on maximum walking time in patients with arterial claudication: The ARTERIOFIL study. Vasc. Pharmacol 2019, 118–119, 106563. [Google Scholar] [CrossRef] [PubMed]
- Kokkinidis, D.G.; Arfaras-Melainis, A.; Giannopoulos, S.; Katsaros, I.; Jawaid, O.; Jonnalagadda, A.K.; Parikh, S.A.; Secemsky, E.A.; Giri, J.; Kumbhani, D.J.; et al. Statin therapy for reduction of cardiovascular and limb-related events in critical limb ischemia: A systematic review and meta-analysis. Vasc Med. 2020, 25, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.K.; Jorge, D.W.; Bortolanza, G.; da Rocha, J.B.T. Effects of statin use on primary patency, mortality, and limb loss in patients undergoing lower-limb arterial angioplasty: A systematic review and meta-analysis. Int. J. Clin. Pharm. 2023, 45, 17–25. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, E.M.; Brewer, H.B.; Sipahi, I.; Nicholls, S.J.; Ganz, P.; Schoenhagen, P.; Waters, D.D.; Pepine, C.J.; Crowe, T.D.; et al. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N. Engl. J. Med. 2006, 354, 1253–1263. [Google Scholar] [CrossRef]
- Hai, Q.; Smith, J.D. Acyl-Coenzyme A: Cholesterol Acyltransferase (ACAT) in Cholesterol Metabolism: From Its Discovery to Clinical Trials and the Genomics Era. Metabolites 2021, 11, 543. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Zheng, Y.; Yu, Q.; Zeng, M.; Bai, L.; Yang, L.; Guo, M.; Jiang, X.; Gan, J. Inhibitors of the NLRP3 inflammasome pathway as promising therapeutic candidates for inflammatory diseases (Review). Int. J. Mol. Med. 2023, 51, 35. [Google Scholar] [CrossRef]
- Borrego, A.; Colombo, F.; de Souza, J.G.; Jensen, J.R.; Dassano, A.; Piazza, R.; Rodrigues Dos Santos, B.A.; Ribeiro, O.G.; De Franco, M.; Cabrera, W.H.K.; et al. Pycard and BC017158 Candidate Genes of Irm1 Locus Modulate Inflammasome Activation for IL-1β Production. Front. Immunol. 2022, 13, 899569. [Google Scholar] [CrossRef]
- Ritchey, B.; Hai, Q.; Han, J.; Barnard, J.; Smith, J.D. Genetic variant in 3′ untranslated region of the mouse pycard gene regulates inflammasome activity. eLife 2021, 10, e68203. [Google Scholar] [CrossRef]
- Dougherty, C.J.; Smith, G.W.; Dorey, C.K.; Prentice, H.M.; Webster, K.A.; Blanks, J.C. Robust hypoxia-selective regulation of a retinal pigment epithelium-specific adeno-associated virus vector. Mol. Vis. 2008, 14, 471–480. [Google Scholar] [PubMed]
- Webster, K.A. Therapeutic angiogenesis for coronary artery disease: Clinical trials of proteins, plasmids, adenovirus, and stem cells. Future Cardiol. 2005, 1, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Silver, E.; Argiro, A.; Hong, K.; Adler, E. Gene therapy vector-related myocarditis. Int. J. Cardiol. 2023, 131617. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., 3rd; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef]
| Author | Phase | Treatment | Treatment/CTRL | Major Findings |
|---|---|---|---|---|
| Lazarous et al. [13] | I | IA rFGF-2 | 13/6 | Safe. Increased calf blood flow at 6 months in treatments. |
| Rajagopalan et al. [14] (RAVE) | II | IM Ad2- VEGF121 | 15/3 | Safe. N/C ABI, PFWT, QoL. |
| Lederman et al. [15] (TRAFFIC) | II | IA rFGF-2 | 116/58 | Safe. Improved PFWT at 90 days; early improved ABI. |
| Makinen et al. [16] | II | IA Ad2- and pVEGF165 | 35/19 | Ad antibodies. Improved vascularity both treatments; N/C ABI or Rutherford class vs control. |
| Grossman et al. [17] (DELTA) | II | IM pDel-1 + poloxamer 188 | 52/53 | N/C PFWT ABI, claudication compared with control poloxamer 188 alone. |
| Tateishi-Yuyama et al. [18] (TACT) | II | IM BMMNC or PBMNC | 25 unilateral 22 bilateral | Safe. Improved ABI, TcO2, PWT, increased collateral vessels in BM-MNC vs. PBMNC. |
| Author | Phase | Treatment | Treatment/CNTR | Major Findings |
|---|---|---|---|---|
| Belch et al. [20] (TAMARIS) | III | IM pFGF1 | 259/256 | N/C amputation or death |
| Van Huyan et al. [21] | II | IA + IM BMMNC | 12/15 | Improved PFWT and ABI |
| Powell et al. [22] (HGF STAT) | II | IM pHGF | 56/23 | Increased TcPO2; N/C TBI, ABI, wound healing |
| Nikol et al. [23] (TALISMAN) | II | IM pFGF1 | 59/66 | Improved rest pain, QoL, amputation; N/C wound healing |
| Shigematsu et al. [24] | III | IM pHGF | 27/13 | Improved rest pain, ulcer size, QoL; N/C ABI or amputation |
| Creager et al. [25] | II | IM AdHIF1α | 213/76 | N/C PFWT, QoL, ABI |
| Walter et al. [26] (PROVASA) | II | IA BMMNC | 19/21 | Improved ulcer healing, rest pain, N/C ABI, amputation, death |
| Teraa et al. [27] (JUVENTAS) (2015) | II | IA EPC | 81/79 | N/C amputation, death, ABI, ulcer size, QoL, rest pain, TcPO2 |
| Author | Title | Major Findings |
|---|---|---|
| Rigato et al. [30] (2017) | Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. | Autologous cell therapy may reduce the risk of major amputation, improve the probability of wound healing, and amputation-free survival, ameliorate pain and functional capacity. Results of the primary analysis were confirmed and strengthened by secondary analysis. No change in all-cause mortality. |
| Xie et al. [31] (2018) | Autologous Stem Cell Therapy in Critical Limb Ischemia: A Meta-Analysis of Randomized Controlled Trials. | Cell therapy significantly increased the probability of ulcer healing, angiogenesis, and reduced amputation rates. ABI and PFWT were significantly improved. Higher quality and larger RCTs are required to support clinical application. |
| Gao et al. [32] (2019) | Autologous stem cell therapy for peripheral arterial disease: a systematic review and meta-analysis of randomized controlled trials. | Improved healing rate of ulcers, ABI, TcO2, and PFWT; reduction in amputation rate and rest pain scores, no significant improvement in major limb salvage. High risk of bias and low-quality evidence of outcomes. Larger, placebo controlled, RCT are needed. |
| Pu et al. [33] (2022) | A meta-analysis of randomized controlled trials on therapeutic efficacy and safety of autologous cell therapy for atherosclerosis obliterans. | No-option CLI patients show significantly improved total amputation, major amputation, ABI, TcO2, and rest pain score compared with standard care. No effect on all-cause death or ulcer size. |
| Beltrán-Camacho et al. [34] (2021) | Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia | Cell therapy may represent an alternative for no-option CLI. Variability between trials is high, reflecting a lack of consensus on cell dose, cell types or sources, administration routes, parameters to define outcome efficacy, or cohorts themselves. Further investigation is required to better understand mechanism. Much work is needed to translate to clinical practice. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webster, K.A. Translational Relevance of Advanced Age and Atherosclerosis in Preclinical Trials of Biotherapies for Peripheral Artery Disease. Genes 2024, 15, 135. https://doi.org/10.3390/genes15010135
Webster KA. Translational Relevance of Advanced Age and Atherosclerosis in Preclinical Trials of Biotherapies for Peripheral Artery Disease. Genes. 2024; 15(1):135. https://doi.org/10.3390/genes15010135
Chicago/Turabian StyleWebster, Keith A. 2024. "Translational Relevance of Advanced Age and Atherosclerosis in Preclinical Trials of Biotherapies for Peripheral Artery Disease" Genes 15, no. 1: 135. https://doi.org/10.3390/genes15010135
APA StyleWebster, K. A. (2024). Translational Relevance of Advanced Age and Atherosclerosis in Preclinical Trials of Biotherapies for Peripheral Artery Disease. Genes, 15(1), 135. https://doi.org/10.3390/genes15010135







