Multiomics Reveals the Key Microorganisms and Metabolites in the Resistance to Root Rot Disease of Paris polyphylla
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Isolation and Identification of the Pathogen
2.3. DNA Extraction and Sequencing
2.4. Genome Sequencing
2.5. Metabolome Measurement
2.6. Transcriptome Analysis
2.7. Statistical Analysis
3. Results
3.1. Morphology of Healthy and Diseased P. polyphylla and Isolation of Pathogens
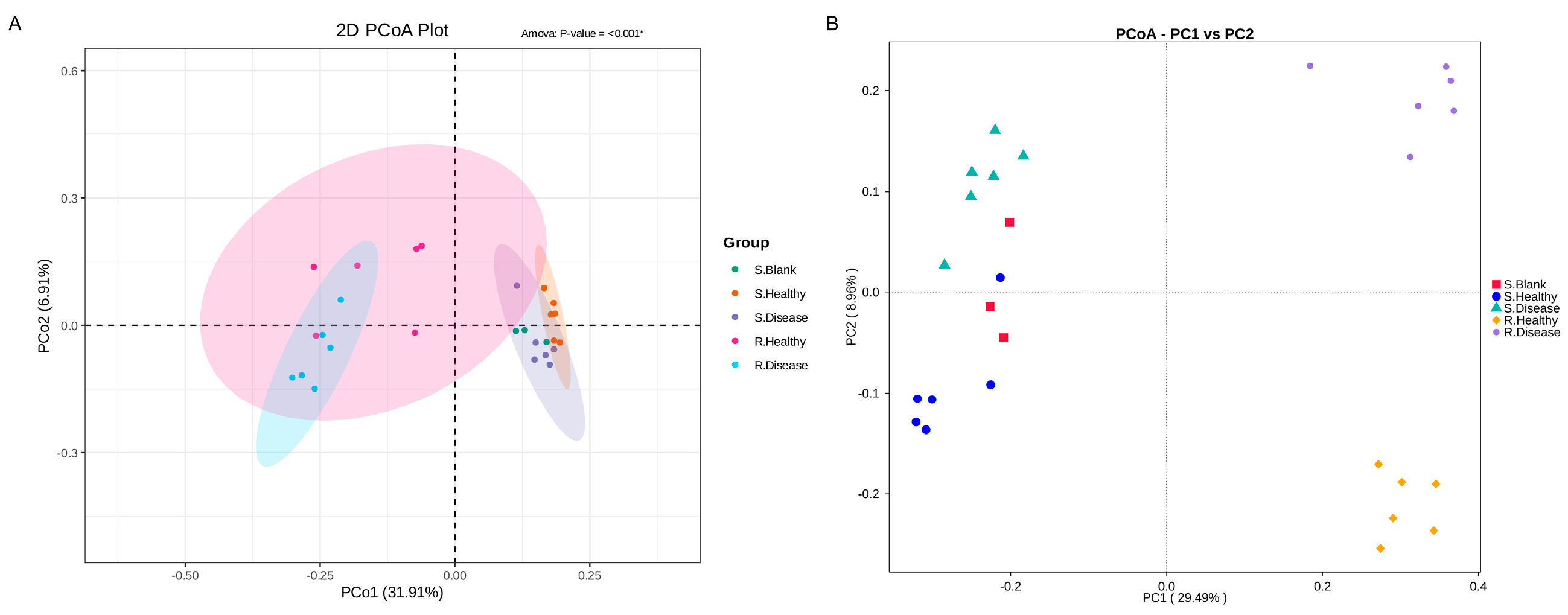
3.2. Differences in Microbial α- and β-Diversity of Healthy and Diseased P. polyphylla
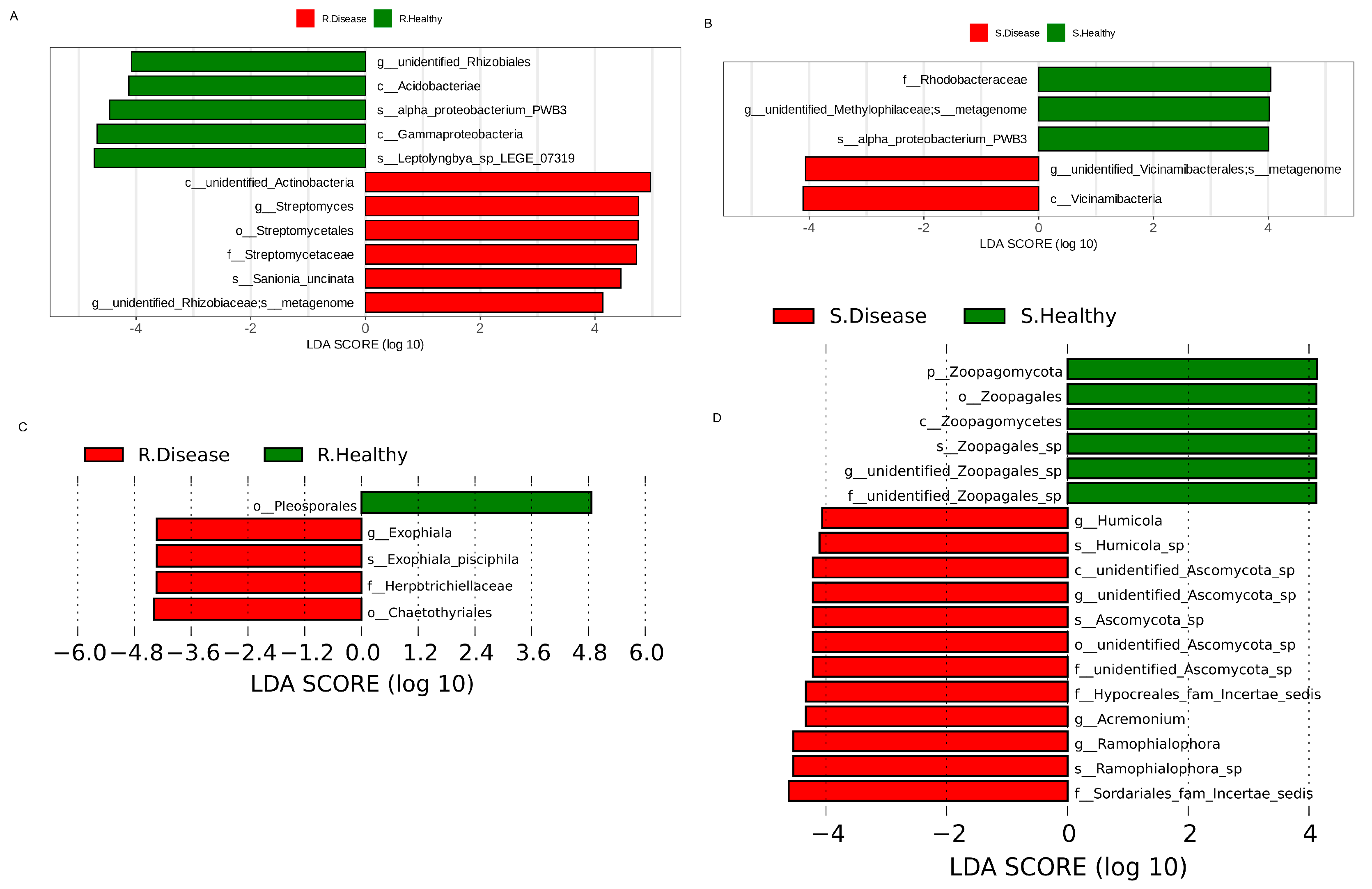
3.3. Differences in the Microbial Community of Healthy and Diseased P. polyphylla
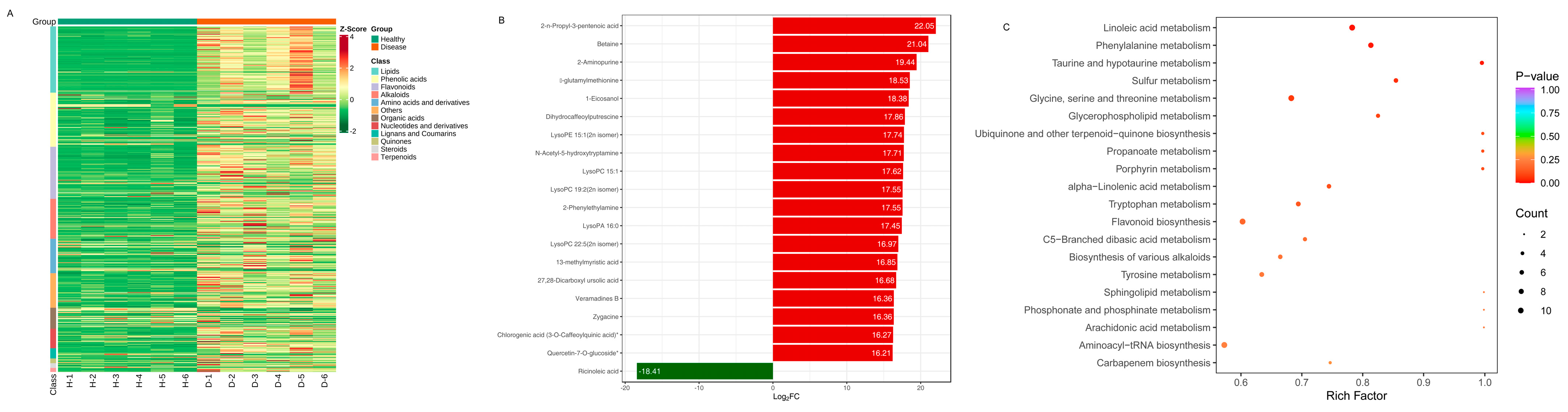
3.4. Metabolomic Changes between the Healthy and Diseased P. polyphylla
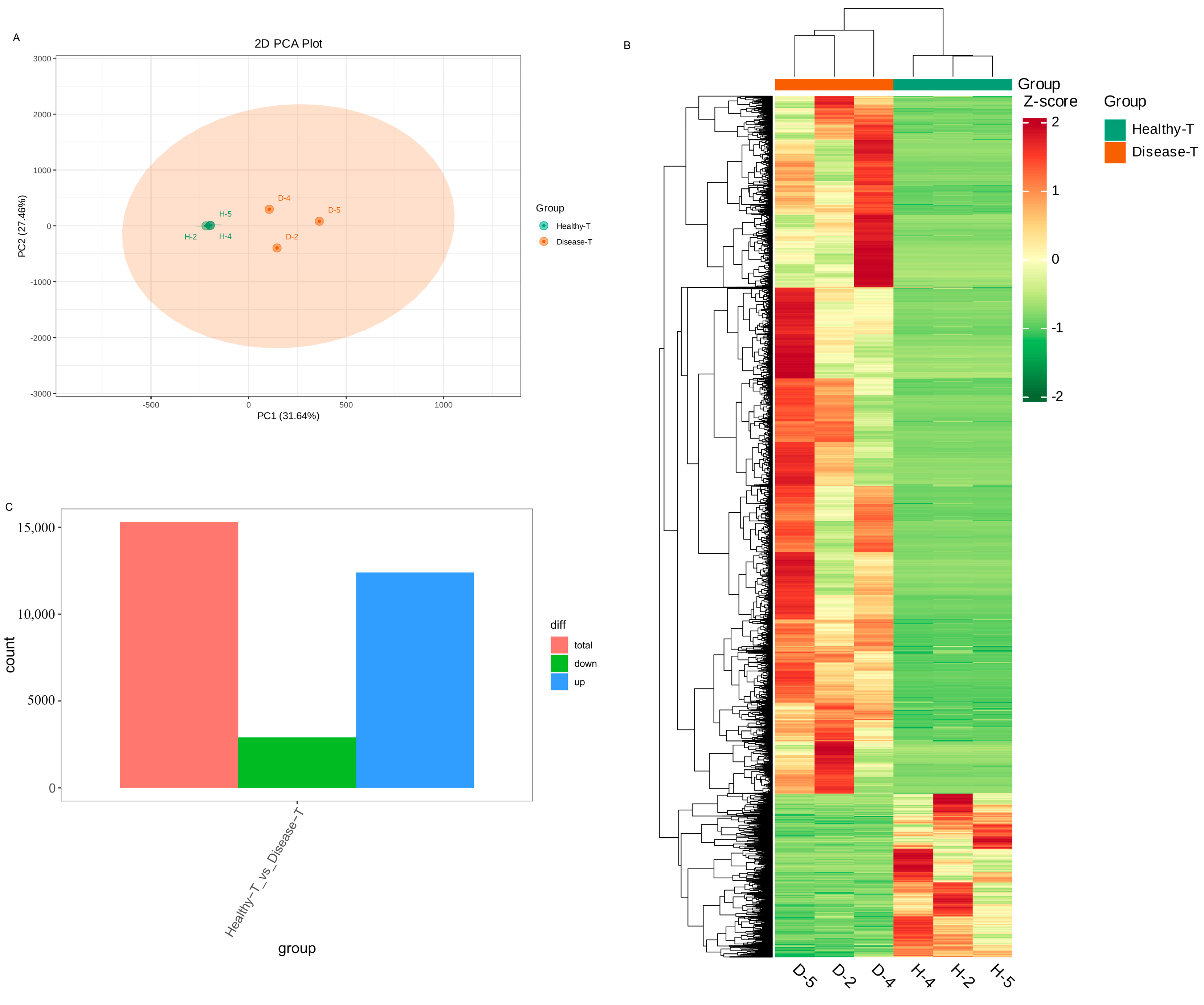
3.5. Transcriptomic Profiling of Healthy and Diseased P. polyphylla
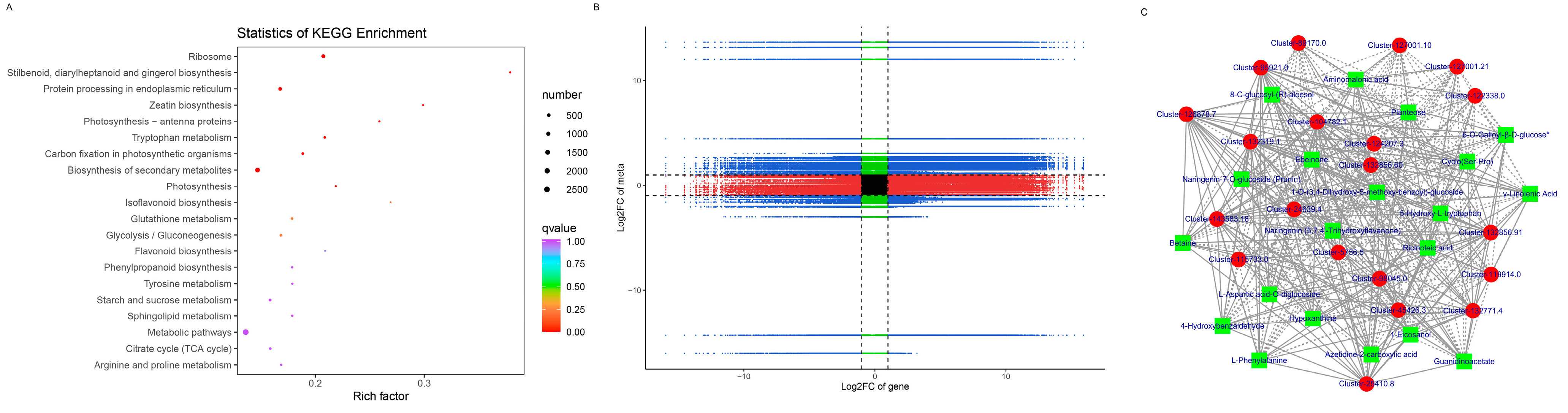
3.6. Differentially Accumulated Metabolites in Healthy and Diseased P. polyphylla
3.7. Metabolic Pathway Analysis and Changing Trends of DAMs and DEGs in P. polyphylla
3.8. Integrative Analysis of DEGs and DAMs Reveals the Differential Regulatory Network of Metabolites Biosynthesis in P. polyphylla
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Zhang, M.Q.; Du, Y.N.; Chen, S.; Zhang, Z.K.; Zhao, L.H. First Report of Tobacco Mosaic Virus Infection in Paris polyphylla var. yunnanensis in China. Plant Dis. 2023, 107, 1251. [Google Scholar] [CrossRef] [PubMed]
- Li, H. The phylogeny of the genus Paris L. Acta Bot. Yunnanica 1984, 6, 351–362. [Google Scholar]
- Ling, L.Z.; Zhang, S.D.; Zhao, F.; Yang, J.L.; Song, W.H.; Guan, S.M.; Li, X.S.; Huang, Z.J.; Cheng, L. Transcriptome-Wide Identification and Prediction of miRNAs and Their Targets in Paris polyphylla var. yunnanensis by High-Throughput Sequencing Analysis. Int. J. Mol. Sci. 2017, 18, 219. [Google Scholar]
- Chen, T.Z.; Lin, J.; Yang, J.; Tang, Y.N.; Zhang, C.M.; Zhang, T.; Wen, F.Y.; Fang, Q.M.; Zhang, H. Determination of Inorganic Elements in the Rhizome of Paris polyphylla Smith Var. chinensis (Franch.) Hara by Using Inductively Coupled Plasma Mass Spectrometry. J. Anal. Methods Chem. 2019, 2019, 4946192. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Gao, W.Y.; Duan, H.Q.; Jia, W.J.C.T.; Drugs, H. Advances in studies on chemical constituents and pharmacological activities of Rhizoma Paridis. Chin. Tradit. Herb. Drugs 2004, 35, 344–346. [Google Scholar]
- Duan, B.Z.; Wang, Y.P.; Fang, H.L.; Xiong, C.; Li, X.W.; Wang, P.; Chen, S.L. Authenticity analyses of Rhizoma Paridis using barcoding coupled with high resolution melting (Bar-HRM) analysis to control its quality for medicinal plant product. Chin. Med. 2018, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Zhang, W.; Lu, Y.Y.; Liu, Y.; Wang, X.Y.; Li, H.; Tang, H.F. Steroidal saponins and lignan glycosides from the rhizomes of Paris polyphylla var. latifolia. Biochem. Syst. Ecol. 2018, 81, 27–29. [Google Scholar] [CrossRef]
- Berlec, A. Novel techniques and findings in the study of plant microbiota: Search for plant probiotics. Plant Sci. 2012, 193, 96–102. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xu, J.; Riera, N.; Jin, T.; Li, J.Y.; Wang, N. Huanglongbing impairs the rhizosphere-to-rhizoplane enrichment process of the citrus root-associated microbiome. Microbiome 2017, 5, 97. [Google Scholar] [CrossRef]
- Liu, H.W.; Li, J.Y.; Carvalhais, L.C.; Percy, C.D.; Verma, J.P.; Schenk, P.M.; Singh, B.K. Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 2021, 229, 2873–2885. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Larousse, M.; Rancurel, C.; Syska, C.; Palero, F.; Etienne, C.; Industri, B.; Nesme, X.; Bardin, M.; Galiana, E. Tomato root microbiota and Phytophthora parasitica-associated disease. Microbiome 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Vargas, J.C.; Schlatter, D.C.; Hagerty, C.; Hulbert, S.H.; Paulitz, T.C. Rhizosphere community selection reveals bacteria associated with reduced root disease. Microbiome 2021, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.W.; Raaijmakers, J.M.; de Hollander, M.; Mendes, R.; Tsai, S.M. Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J. 2018, 12, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.L.; Wang, W.S.; Zhang, H.Y.; Liu, X.Q.; Yu, S.B.; Xiong, L.Z.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Ma, X.X.; Meng, Y.J.; Wang, P.; Tang, Z.H.; Wang, H.Z.; Xie, T. Bioinformatics-assisted, integrated omics studies on medicinal plants. Brief. Bioinform. 2020, 21, 1857–1874. [Google Scholar] [CrossRef]
- Bai, B.; Liu, W.D.; Qiu, X.Y.; Zhang, J.; Zhang, J.Y.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef]
- Huang, S.H.; Zhang, J.Y.; Tao, Z.; Lei, L.; Yu, Y.H.; Huang, L.Q. Enzymatic conversion from pyridoxal to pyridoxine caused by microorganisms within tobacco phyllosphere. Plant Physiol. Biochem. 2014, 85, 9–13. [Google Scholar] [CrossRef]
- van de Mortel, J.E.; de Vos, R.C.H.; Dekkers, E.; Pineda, A.; Guillod, L.; Bouwmeester, K.; van Loon, J.J.A.; Dicke, M.; Raaijmakers, J.M. Metabolic and Transcriptomic Changes Induced in Arabidopsis by the Rhizobacterium Pseudomonas fluorescens SS101. Plant Physiol. 2012, 160, 2173–2188. [Google Scholar] [CrossRef] [PubMed]
- Ryffel, F.; Helfrich, E.J.N.; Kiefer, P.; Peyriga, L.; Portais, J.C.; Piel, J.; Vorholt, J.A. Metabolic footprint of epiphytic bacteria on Arabidopsis thaliana leaves. Isme J. 2016, 10, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Qiu, L.; Zhang, Z.J.; Liu, K.; Xia, X.; Xiong, S.L.; Zhao, S.M.; Zhao, Z.Q.; Hu, Y.M.; Liang, Y.X. Control of Streptomyces alfalfae XY25T Over Clubroot Disease and Its Effect on Rhizosphere Microbial Community in Chinese Cabbage Field Trials. Front. Microbiol. 2021, 12, 641556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Zhu, A.P.; Tan, H.M.; Cao, L.X.; Zhang, R.D. Engineering banana endosphere microbiome to improve Fusarium wilt resistance in banana. Microbiome 2019, 7, 74. [Google Scholar] [CrossRef]
- Kusstatscher, P.; Zachow, C.; Harms, K.; Maier, J.; Eigner, H.; Berg, G.; Cernava, T. Microbiome-driven identification of microbial indicators for postharvest diseases of sugar beets. Microbiome 2019, 7, 112. [Google Scholar] [CrossRef]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.M.; Zhou, X.; Zhang, A.M.; Cai, L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef]
- van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottova, D.; Kristufek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef]
- Cao, L.; Qiu, Z.; You, J.; Tan, H.; Zhou, S. Isolation and characterization of endophytic streptomycete antagonists of Fusarium wilt pathogen from surface-sterilized banana roots. FEMS Microbiol. Lett. 2005, 247, 147–152. [Google Scholar] [CrossRef]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askari, H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Nassar, A.H.; Hardy, G.; Sivasithamparam, K. Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J. Appl. Microbiol. 2009, 106, 13–26. [Google Scholar] [CrossRef]
- Chaudhary, V.; Prasanna, R.; Nain, L.; Dubey, S.C.; Gupta, V.; Singh, R.; Jaggi, S.; Bhatnagar, A.K. Bioefficacy of novel cyanobacteria-amended formulations in suppressing damping off disease in tomato seedlings. World J. Microbiol. Biotechnol. 2012, 28, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; He, Q.F. Cyanobacteria as cell factories to produce plant secondary metabolites. Front. Bioeng. Biotechnol. 2015, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Y.; Zhao, Y.; Li, G.; Zhang, W.; Wu, Y.; Huang, L. iTRAQ and RNA-Seq analyses revealed the effects of grafting on fruit development and ripening of oriental melon (Cucumis melo L. var. makuwa). Gene 2021, 766, 145142. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]




| Group | Genus | Mean (R. Healthy/S. Healthy) | Mean (R. Disease/S. Healthy) | p-Value |
|---|---|---|---|---|
| Lysinibacillus | 8.77 × 10−05 | 3.11 × 10−05 | 0.01 | |
| unidentified_Hydrogenedentes | 7.3 × 10−05 | 8.49 × 10−06 | 0.01 | |
| unidentified_Elusimicrobiota | 9.91 × 10−05 | 3.96 × 10−05 | 0.02 | |
| Edaphobaculum | 0.0005 | 6.79 × 10−05 | 0.02 | |
| R. Healthy vs. R. Disease | Anaeromyxobacter | 0.0003 | 5.09 × 10−05 | 0.02 |
| unidentified_Alphaproteobacteria | 0.0005 | 9.06 × 10−05 | 0.03 | |
| Halomonas | 0.0002 | 6.51 × 10−05 | 0.03 | |
| unidentified_Tepidisphaerales | 9.34 × 10−05 | 3.3 × 10−05 | 0.03 | |
| unidentified_Rhizobiales | 0.0028 | 0.0004 | 0.03 | |
| Muricauda | 0.0003 | 2.55 × 10−05 | 0.03 | |
| unidentified_Rhodospirillaceae | 0.0005 | 3.40 × 10−05 | 4.41 × 10−08 | |
| Pirellula | 0.0017 | 0.0003 | 5.80 × 10−07 | |
| unidentified_Chloroflexi | 0.0022 | 0.0002 | 7.73 × 10−07 | |
| unidentified_Alphaproteobacteria | 0.0021 | 9.06 × 10−05 | 1.26 × 10−06 | |
| Ferruginibacter | 0.0021 | 0.0001 | 1.41 × 10−06 | |
| S. Healthy vs. S. Disease | Luteitalea | 0.0007 | 0.0001 | 2.41 × 10−06 |
| Pajaroellobacter | 0.0042 | 0.0004 | 3.06 × 10−06 | |
| Edaphobaculum | 0.0025 | 6.79 × 10−06 | 4.86 × 10−06 | |
| Sandaracinus | 0.0001 | 1.13 × 10−06 | 5.36 × 10−06 | |
| unidentified_Micropepsaceae | 0.0015 | 0.0002 | 7.44 × 10−06 | |
| Lysinibacillus | 0.0015 | 5.09 × 10−06 | 1.16 × 10−05 |
| Gene ID | Log2 FC | p-Value | H-2_fpkm | Regulation |
|---|---|---|---|---|
| Cluster-132319.1 | 5.839804085 | 9 × 10−86 | 2.84 | up |
| Cluster-127001.21 | −5.115231299 | 3.2 × 10−75 | 14,976.77 | down |
| Cluster-122338.0 | −5.240803144 | 2.8 × 10−56 | 24.91 | down |
| Cluster-89170.0 | −6.99819639 | 1 × 10−50 | 49.48 | down |
| Cluster-126878.7 | 5.03189586 | 1.4 × 10−41 | 13.45 | up |
| Cluster-25410.8 | 6.269167442 | 2.5 × 10−41 | 11.15 | up |
| Cluster-127001.10 | −2.899061791 | 7.3 × 10−39 | 13,951.98 | down |
| Cluster-143583.18 | 8.710087805 | 8.2 × 10−34 | 6.47 | up |
| Cluster-132771.4 | 4.376939557 | 3.3 × 10−33 | 4.21 | up |
| Cluster-24639.4 | −5.618000462 | 1.4 × 10−31 | 66.66 | down |
| Cluster-124207.3 | −2.490346547 | 8.4 × 10−31 | 139.07 | down |
| Cluster-98045.0 | 15.95590433 | 7.3 × 10−30 | 0 | up |
| Cluster-132856.60 | −12.95953203 | 8.7 × 10−29 | 84.91 | down |
| Cluster-115733.0 | −8.138256466 | 1.9 × 10−28 | 205.57 | down |
| Cluster-132856.91 | 7.690022495 | 5.8 × 10−28 | 7.3 | up |
| Cluster-95921.0 | 3.168091923 | 7.6 × 10−28 | 14.28 | up |
| Cluster-5756.5 | −6.957003683 | 1.4 × 10−27 | 443.32 | down |
| Cluster-45426.3 | −15.9143436 | 2.9 × 10−27 | 448.77 | down |
| Cluster-119914.0 | −6.225555532 | 6.8 × 10−27 | 194.03 | down |
| Cluster-104782.1 | 3.916116249 | 6.8 × 10−27 | 3.37 | up |
| Compounds | VIP | p-Value | Fold_Change | Log2 FC | Regulation |
|---|---|---|---|---|---|
| Cyclo(Ser-Pro) | 1.48 | 8.52 × 10−08 | 1.09 × 10+04 | 3.58 × 10+00 | up |
| L-Aspartic acid-O-diglucoside | 1.36 | 1.84 × 10−07 | 9.55 × 10+03 | 6.54 × 10+00 | up |
| 1-Eicosanol | 1.49 | 7.12 × 10−07 | 8.66 × 10+03 | 3.41 × 10+05 | up |
| Guanidinoacetate | 1.42 | 6.62 × 10−06 | 8.37 × 10+03 | 3.51 × 10+00 | up |
| Betaine | 1.49 | 1.23 × 10−05 | 6.34 × 10+03 | 2.16 × 10+06 | up |
| Hypoxanthine | 1.39 | 1.62 × 10−05 | 4.59 × 10+03 | 2.58 × 10+00 | up |
| Ricinoleic acid | 1.49 | 2.13 × 10−05 | 3.96 × 10+03 | 2.87 × 10−06 | down |
| 1-O-(3,4-Dihydroxy-5-methoxy-benzoyl)-glucoside | 1.41 | 2.59 × 10−05 | 3.36 × 10+03 | 3.07 × 10+00 | up |
| 5-Hydroxy-L-tryptophan | 1.26 | 2.76 × 10−05 | 3.33 × 10+03 | 2.67 × 10+00 | up |
| Naringenin (5,7,4′-Trihydroxyflavanone) | 1.38 | 3.80 × 10−05 | 3.29 × 10+03 | 4.51 × 10+00 | up |
| Naringenin-7-O-glucoside (Prunin) | 1.15 | 7.78 × 10−05 | 2.35 × 10−04 | 2.71 × 10+01 | up |
| Planteose | 1.43 | 9.54 × 10−05 | 2.32 × 10−04 | 3.03 × 10+00 | up |
| Ebeinone | 1.32 | 1.13 × 10−04 | 1.88 × 10−04 | 3.89 × 10+00 | up |
| L-Phenylalanine | 1.38 | 1.30 × 10−04 | 1.51 × 10−04 | 2.59 × 10+00 | up |
| 6-O-Galloyl-β-D-glucose * | 1.40 | 1.32 × 10−04 | 1.47 × 10−04 | 7.02 × 10+00 | up |
| 8-C-glucosyl-(R)-aloesol | 1.19 | 1.39 × 10−04 | 1.06 × 10−04 | 4.91 × 10+00 | up |
| Azetidine-2-carboxylic acid | 1.30 | 1.49 × 10−04 | 1.00 × 10−04 | 3.22 × 10+00 | up |
| γ-Linolenic Acid | 1.49 | 1.51 × 10−04 | 5.96 × 10−05 | 4.22 × 10+04 | up |
| Aminomalonic acid | 1.37 | 1.51 × 10−04 | 3.12 × 10−05 | 4.33 × 10−01 | down |
| 4-Hydroxybenzaldehyde | 1.38 | 1.61 × 10−04 | 3.19 × 10−06 | 4.50 × 10+00 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Su, H.; Zheng, G.; Meng, H.; Wang, W.; Guo, Y. Multiomics Reveals the Key Microorganisms and Metabolites in the Resistance to Root Rot Disease of Paris polyphylla. Genes 2024, 15, 21. https://doi.org/10.3390/genes15010021
Ye T, Su H, Zheng G, Meng H, Wang W, Guo Y. Multiomics Reveals the Key Microorganisms and Metabolites in the Resistance to Root Rot Disease of Paris polyphylla. Genes. 2024; 15(1):21. https://doi.org/10.3390/genes15010021
Chicago/Turabian StyleYe, Ting, Hailan Su, Guohua Zheng, Hongyan Meng, Wenhua Wang, and Ying Guo. 2024. "Multiomics Reveals the Key Microorganisms and Metabolites in the Resistance to Root Rot Disease of Paris polyphylla" Genes 15, no. 1: 21. https://doi.org/10.3390/genes15010021
APA StyleYe, T., Su, H., Zheng, G., Meng, H., Wang, W., & Guo, Y. (2024). Multiomics Reveals the Key Microorganisms and Metabolites in the Resistance to Root Rot Disease of Paris polyphylla. Genes, 15(1), 21. https://doi.org/10.3390/genes15010021





