Abstract
Background: Adult pancreatoblastoma (PBL) is a rare pancreatic malignancy, with recent evidence suggesting a possible link to familial adenomatous polyposis (FAP). This study aims to review the latest evidence and explore a possible association between adult PBL and FAP. Methods: Two independent literature reviews were conducted: (1) on PBL and FAP, and (2) on PBL in the adult population not diagnosed with FAP. Results: Out of 26 articles on PBL and FAP screened, 5 were selected for systematic review, including 1 additional case. We identified eight FAP-related PBL cases, with a median age of 40 (IQR: 34–50). Of these, seven (87%) occurred in adults. We found 65 cases of adult PBL not FAP-related; thus, 7 out of 65 cases (10.7%) of adult PBL reported in the literature are associated with a clinical diagnosis of FAP or were carriers of APC germline pathogenic variants (GPVs). Conclusion: Data suggest a non-random association between adult PBL and FAP. Further research is essential to optimise surveillance protocols and develop more effective treatment strategies.
1. Introduction
Familial adenomatous polyposis (FAP) is a rare hereditary syndrome characterised by the early onset of hundreds to thousands of colonic adenomas, with a lifetime risk of colorectal cancer of approximately 100%. This condition results from a germline mutation in the adenomatous polyposis coli (APC) gene, a tumour suppressor gene located on chromosome 5. Following a dominant inheritance pattern with almost complete penetrance, FAP is also associated with duodenal adenomas, desmoids and other neoplastic (e.g., thyroid and duodenal carcinoma, hepatoblastoma, medulloblastoma) and non-neoplastic features (e.g., congenital hypertrophy of the retinal pigment epithelium and supernumerary teeth) [1,2]. While the gastrointestinal manifestations of FAP have been extensively investigated, recent evidence suggests a possible association between FAP and a specific type of pancreatic malignancy known as pancreatoblastoma (PBL) [3,4]. PBL is a rare and aggressive form of pancreatic cancer, primarily affecting children and young adults. It accounts for approximately 25% of pancreatic neoplasms in children, while it remains an extremely rare entity in the adult population [5,6]. PBL exhibits aggressive behaviour, frequently associated with local invasion, recurrence and distal metastases. It typically appears as a circumscribed mass with cystic solid components, which poses a challenge in determining the presence of local infiltration [7,8,9,10]. Radical surgery is considered the best treatment option, and resection of metastases has also been contemplated [11,12,13]. Recent genetic research has revealed key molecular alterations in PBL, including the loss of heterozygosity of chromosome 11p, which is associated with Beckwith–Wiedemann syndrome in pediatric patients [14]. Furthermore, alterations in the Wnt/β-catenin pathway are widespread, with CTNNB1 or APC gene mutations being frequent [4,15]. To date, only about 70 cases of adult PBL have been reported in the literature, and due to its rarity and the limited number of reports, the pathogenesis of adult PBL remains largely unknown [3]. Currently, emerging evidence suggests a possible association between FAP and adult PBL. Several case reports and small studies have documented PBL in individuals with FAP, suggesting a possible genetic connection [3,4]. However, the exact mechanism by which the APC gene may lead to PBL still remains unclear. The aim of this study is to present the case of a patient diagnosed with FAP who subsequently developed adult PBL. In addition, we conducted a systematic review of the recent literature in search of a possible association between adult PBL and FAP to postulate a rare but non-random association between these two conditions.
2. Case Report
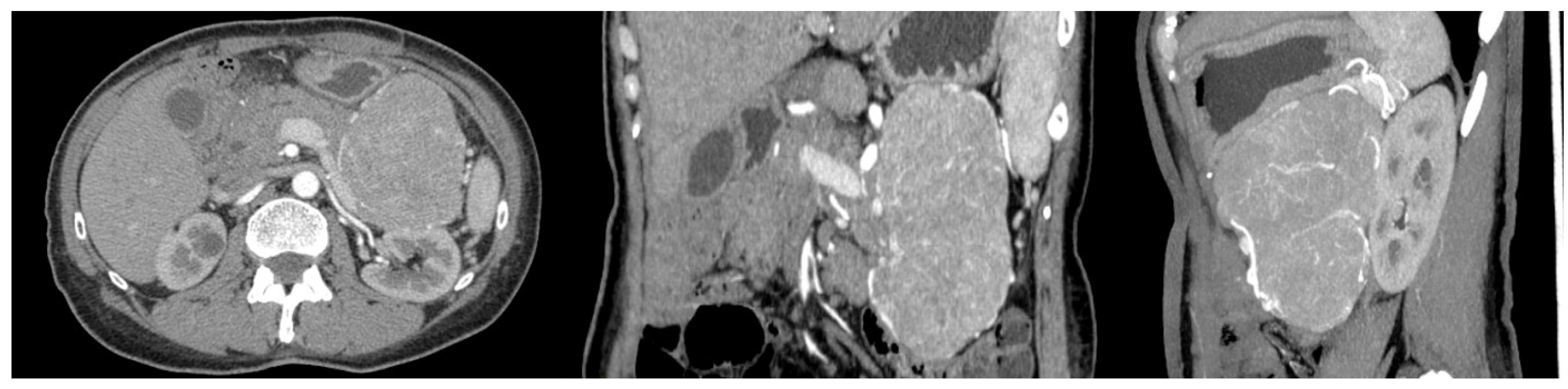
A 56-year-old woman with FAP was admitted to the University Hospital of Padua, complaining of dyspepsia and upper abdominal pain. She was a carrier of a deleterious transversion C to A at nucleotide 2805 of the APC gene, causing a premature stop codon and resulting in a truncated protein (Y935X). Her medical history revealed that she underwent a total colectomy with low ileorectal anastomosis at the age of 21 due to extensive colonic polyposis. Subsequent regular surveillance revealed no extracolonic manifestations except for gastric fundic gland polyps and duodenal adenomas. Some 35 years after colectomy, clinical examination suggested a mass in the left hypochondrium. This suspicion was confirmed by a CT scan, which showed a well-defined retroperitoneal mass measuring 19 × 8.5 cm. The mass originated from the tail of the pancreas with no evidence of nodal or distant metastases (Figure 1). The patient underwent a radical distal splenopancreatectomy. Histologically, the neoplasm showed acinar differentiation with squamous nests and strong, diffuse cytoplasmic and nuclear β-catenin staining (Figure 2). Systemic therapy was not recommended, and the patient remains alive with no evidence of local recurrence or distant metastases exceeding 100 months of follow-up.
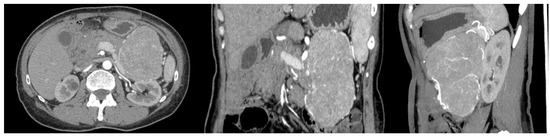
Figure 1.
CT scan of the case report.
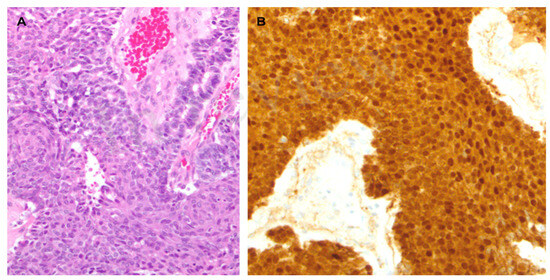
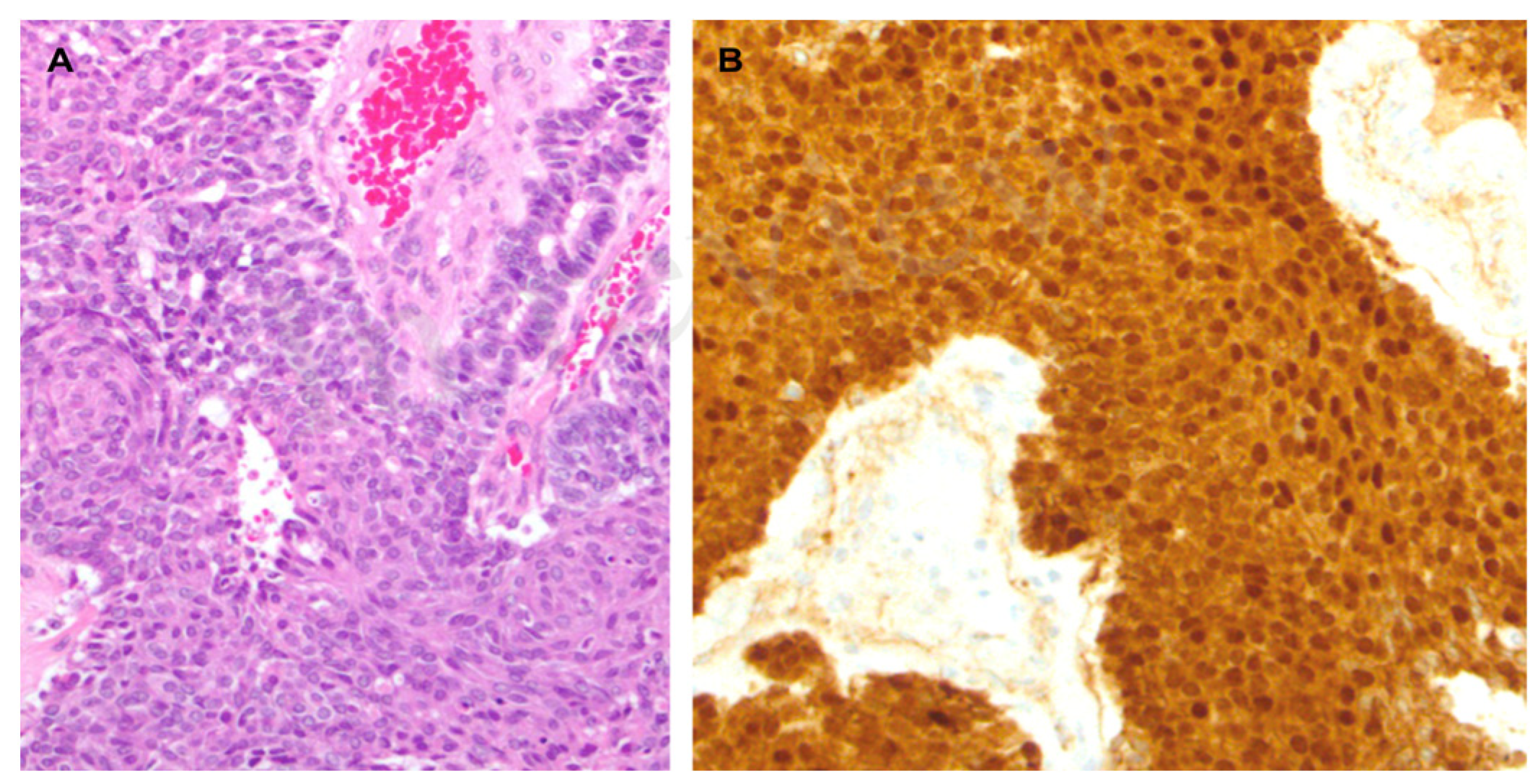
Figure 2.
(A). Microscopic examination revealed a hypercellular neoplasm with a solid and acinar growth pattern admixed with pale/roundish areas with a whorled aspect, representing the so-called squamoid nestes (hematoxylin-eosin). (B). The neoplasm showed strong and diffuse cytoplasmic and nuclear β-catenin staining.
3. Materials and Methods
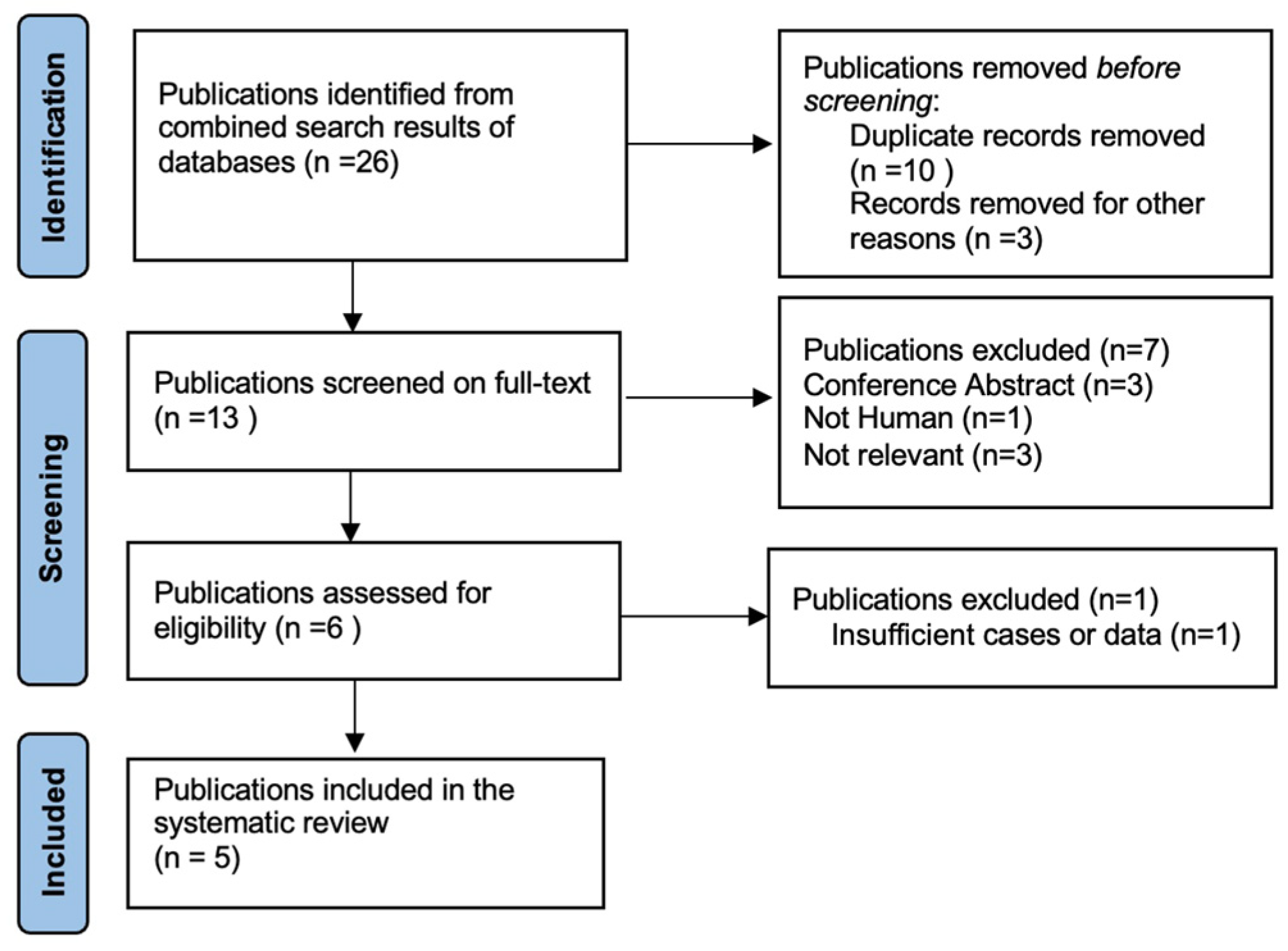
Two independent literature searches were designed to identify: (1) studies that reported a histological diagnosis of PBL and a clinical and/or molecular diagnosis of FAP, and (2) studies that reported a histological diagnosis of adult PBL (i.e., age ≥ 18 years) without clinical and/or molecular diagnosis of FAP. A comprehensive search of the Pubmed, Ovid and Scopus archives was performed using the following medical subject heading (MeSH) search terms: (1) pancreatoblastoma, familial adenomatous polyposis, and (2) pancreatoblastoma, adult (Supplementary Tables S1 and S2). Articles from 1946 to 31 September 2023 were screened. The reference lists of all articles found were also searched to identify additional relevant studies. Articles not written in English were excluded. We included articles that met our eligibility criteria, represented by PBL in FAP patients and adult PBL in those not diagnosed with FAP. Two reviewers (S.N and R.Q.B.) independently performed literature searches and matched their results according to the established inclusion criteria. Data on authors, year of publication, patient numbers, demographics, tumour characteristics, oncological outcomes and treatment modalities were analysed and recorded separately by the reviewers. A dedicated database of selected papers was created, and disagreements were resolved by two additional blinded reviewers (A.R. and E.D.L.U.). The systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) (Figure 3, Supplementery Figure S1) guidelines. This study was approved by the AOUPD Ethics Committee (protocol number 007401, 24 November 2022). Data were reported in accordance with the tenets of the Declaration of Helsinki, and the patient gave informed consent for the study.
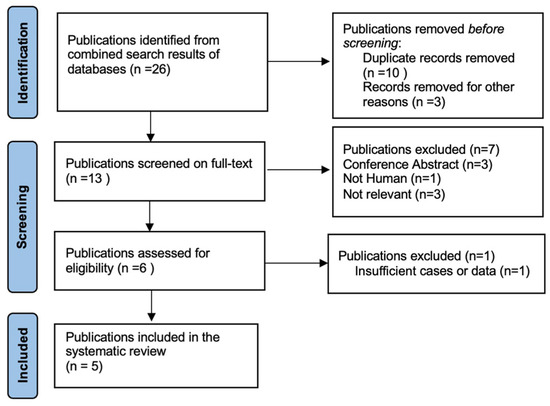
Figure 3.
Selection of studies for review “PBL and FAP”.
4. Results
4.1. Study Inclusion and Characteristics of Included Studies
- PBL and FAP
A total of 26 articles were initially screened, and 13 met the criteria for full-text review. After removing unavailable and not-relevant articles, five studies were selected for the systematic review (Figure 3). Of these five included studies, three were retrospective multicentre studies, one was a case report, and one was a letter to the editor.
- PBL in the Adult Population
A total of 409 studies were initially screened, and 180 were considered for full-text review (excluding those focused on: review of pancreatic tumours with acinar differentiation, n = 15; pseudo-papillary tumours of the pancreas, n = 11; miscellanea, n = 19). After removing unavailable and not-relevant articles, 44 studies were selected for the systematic review (Supplementary Figure S1).
4.2. Synthesis of Clinicopathological Data
- PBL and FAP
Information on demographics, tumour characteristics, treatment, and outcomes of FAP-related PB are summarised in Table 1. The five studies and our case report included a total of eight patients with PBL and FAP colonic phenotype or a germline APC deleterious variant: seven were adults and one was an infant. The median age at diagnosis of FAP-related PBLs was 40 (IQR: 34–50) years, with a female/male ratio of 3/1(6/2 cases). PBLs had no preferred location in the gland (head, isthmus or tail). The median tumour size was five (IQR: 3.4–7.5) cm. At a median follow-up of 32 (IQR: 13–100) months, four PBL patients were alive and three had died of the disease (follow-up data were not available for one patient). The age at diagnosis of FAP and of the PBL was reported for only six cases: in four of them, the pancreatic tumour was detected more than 10 years after the diagnosis of FAP. In one case, with an APC variant C.5503A>G (p.R1835G), PBL was diagnosed without any signs of polyposis coli. Information on the APC mutations found was only available in four cases, so a genotype/phenotype correlation was not possible. Data on systemic treatments administered to PBL were too limited to be reported in detail.

Table 1.
Study participants’ characteristics “PBL and FAP”.
- PBL in the Adult Population
Systematic review of the literature found 65 cases of adult PB not diagnosed with FAP. The median age at diagnosis of PBL was 37 (IQR: 27–61) years, with a female/male ratio of 1/1.5 (26/39 cases). The median tumour size was 5.8 (IQR: 4.3–9) cm (Supplementary Table S3).
5. Discussion
In this review, we investigated the characteristics of a rare tumour in the context of a rare syndrome. We found eight cases (including our additional case) of FAP-related PBL. Of these, seven (87%) occurred in the adult population. In total, 65 cases of PBL in the adult population not diagnosed with FAP have been described in the literature. Taken together, these findings indicate that more than 7 out of 65 cases (10.7%) of adult PBL may be associated with a clinical diagnosis of FAP or were carriers of an APC GPVs, suggesting a non-random association between adult PBL and FAP. It is crucial to identify rare clinical conditions associated with inherited syndromes, as this awareness significantly enhances the possibility of recognising the disease within the population and optimising surveillance for carriers. Numerous examples exist where clinical conditions initially considered extremely rare in the context of an inherited disease eventually become integral components of the syndrome. Muir, E.G., and Torre, D. independently made the initial reports of sebaceous neoplasms and keratoacanthomas associated with visceral malignancies in 1967 [19,20]. In 1991, Cohen, P.R., et al. collected 120 cases of sebaceous neoplasms associated with abdominal cancers, formalising the definition of Muir–Torre syndrome 25 [21]. However, it was only recently that Muir–Torre syndrome was officially associated with one of the clinical conditions related to a deficiency in the germline mismatch repair (MMR) system, specifically Lynch syndrome [22]. Similar considerations could be made for hepatoblastoma (HB) and FAP. The first association between FAP and HB was reported in 1983 [23]. Subsequently, more than 50 cases were documented in 2001 and by 2022 over 100 patients were identified [24]. Surveillance for HB in infants from FAP families is now recommended 28 [25].
PBL is considered to be a rare paediatric cancer. However, if we consider only FAP patients, there is only one report of PBL in children with FAP, as mentioned in a paper focusing on medulloblastoma and APC mutation carriers [18]. Although PBL is much less common in adulthood, eight cases of adult PBL in FAP have been reported. Taken together, these data suggest a non-random association between FAP and PBL, particularly in the adult population.
Excluding the one paediatric case, the median age of PBL diagnosis in our series of FAP patients was 40 years, which is similar to that reported for all patients with adult PBL not selected for FAP (median 37 years; IQR: 27–61; p = 0.6). Recently, a review by Omiyale et al. reported a 27-month OS of 58% for adults PBL [3]. Among these patients, 42% died due to the disease, 4% died due to unrelated causes, 16% survived with the disease, and the remaining 38% demonstrated no evidence of the disease (NED). In our study, even considering the small cases enrolled, four (50%) of FAP-related PBLs remained alive and with NED after a median follow-up of 46 (IQR:18.5–90) months. A possible explanation for the good outcome observed in our series is that the female gender may lead to a better cancer outcome, as is already the case for several cancers [26]. In support of this, the gender distribution showed a higher proportion of females (3:1 female to male ratio) in FAP-related PBL compared to adult PBL not selected for FAP (1/1.5 female to male ratio). On the other hand, since the largest FAP-related PBLs (6, 7.5 and 19 cm) were alive after a long follow-up period (60, 143, 100 months, respectively), the presence of a germline APC deficiency may indicate less aggressive behaviour of the tumour in this population. These data are in line with previous reports by Machado and Suemitsu et al., who reported better survival outcomes in those PBLs with a somatic mutation on the APC gene compared to those that were wild-type for the mutation [27]. Typically, FAP is diagnosed at an average age of 20 years. Therefore, based on our case series, the diagnosis of PBL would be expected to occur later, at around 40 years of age on average. However, there are cases where the diagnosis of PBL precedes that of polyposis. For instance, Ikenoue et al. reported a case of PBL in a patient with an attenuated form of familial adenomatous polyposis (AFAP) before the identification of the syndrome itself [28]. Furthermore, Yamaguchi et al. suggest that PBL could be diagnosed prior to the syndrome in individuals carrying a deleterious APC variant, even without a personal or familial history of polyposis [17]. Several previous studies have found an association between FAP and pancreatic cancer. Giardiello et al. analysed FAP families in the Johns Hopkins registry and found a relative risk of pancreatic adenocarcinoma in these patients of 4.46 (95% CI: 1.2–11.4) [29]. Karstensen et al. recently confirmed previous findings, reporting a risk of approximately 6.45 (95% CI, 2.02–20.64) for pancreatic cancer among FAP patients [30]. Despite the fact that current guidelines do not include FAP as an inherited condition requiring pancreatic surveillance, these results suggest that the spectrum of pancreatic malignancies may be more relevant in FAP patients than previously assumed, thus warranting regular monitoring for individuals with FAP syndrome. Complex surveillance programs have already been established internationally for those at the highest risk of pancreatic cancer (i.e., CDKN2A GPVs carriers, BRCA1 and 2 GPVs carriers). Periodic magnetic resonance imaging and endoscopic ultrasounds could reduce the risk of advanced cancer in these populations [31]. Given the low incidence of pancreatic carcinoma in FAP patients, no recommendation could be made. However, the likelihood of pancreatic tumours suggests the need for a prompt evaluation of any signs or symptoms that could be related to pancreatic disease. Moreover, as somatic molecular profiling is now increasingly used in oncology, the results of our review suggest that if a somatic APC pathogenic variant is detected on PBL, germline investigation would be indicated.
Several limitations of this study should be discussed.
This is the first comprehensive review regarding the association of FAP and PBL. As a result of the rarity of the disease, there are limited published series available. Furthermore, most published studies are made up of small sample sizes and case reports. Additionally, there is a lack of homogeneity in the definition of polyposis coli across many studies, and the majority of them do not include molecular classification of the polyposis. However, even without the possibility of statistically proving a non-random association between PBL and FAP, our review provides good data to support the hypothesis of a reasonable association between FAP and adult PBL. Therefore, FAP care providers may be encouraged to report on PBL cases, and PBL care providers may be encouraged to screen for polyposis coli and germline APC mutation. It is possible that PBL in FAP patients is more common than previously thought, thus necessitating the development of new surveillance strategies to manage cancer risk.
6. Conclusions
PBL in the context of FAP is a rare and complex condition that poses challenges for both surveillance and treatment. While the genetic link between APC mutation and PBL development is becoming clearer, much still needs to be understood about this unique conceivable association. Further research is essential to improve our knowledge of adult PBL, optimise surveillance protocols and develop more effective treatment strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes15010044/s1, Figure S1: PRISMA flowchart of study selection “PBL and adult”; Table S1: Detailed Search Strategy “PBL and FAP”; Table S2: Detailed Search Strategy “PBL and adult”; Table S3: Study characteristics “PBL and adult”.
Author Contributions
Conceptualization, A.R. and E.D.L.U.; methodology, A.R., E.D.L.U., S.N., R.Q.B. and E.d.; formal analysis, S.N. and R.Q.B.; data curation, S.N.; writing—original draft preparation, A.R. and S.N.; writing—review and editing, S.N., R.A., G.C., I.M., R.I., F.B., M.F., M.V., S.P. and M.A.; supervision, E.D.L.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the AOUPD Ethics Committee (protocol number 007401, 24 November 2022).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bodmer, W.F.; Bailey, C.J.; Bodmer, J.; Bussey, H.J.R.; Ellis, A.; Gorman, P.; Lucibello, F.C.; Murday, V.A.; Rider, S.H.; Scambler, P.; et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature 1987, 328, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Galiatsatos, P.; Foulkes, W.D. Familial adenomatous polyposis. Am. J. Gastroenterol. 2006, 101, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Omiyale, A.O. Adult pancreatoblastoma: Current concepts in pathology. World J. Gastroenterol. 2021, 27, 4172–4181. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.C.; Wu, T.T.; Klimstra, D.S.; Finn, L.S.; Lee, J.-H.; Yeo, C.J.; Cameron, J.L.; Hruban, R.H. Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: Frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am. J. Pathol. 2001, 159, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.M.; Travis, M.D.; Conran, R.M. Pancreatic tumors in children: Radiologic-pathologic correlation. RadioGraphics 2006, 26, 1211–1238. [Google Scholar] [CrossRef]
- Shorter, N.A.; Glick, R.D.; Klimstra, D.S.; Brennan, M.F.; LaQuaglia, M.P. Malignant pancreatic tumors in childhood and adolescence: The Memorial Sloan-Kettering experience, 1967 to 222 present. J. Pediatr. Surg. 2002, 37, 887–892. [Google Scholar] [CrossRef]
- Tsvetkova, V.; Magro, G.; Broggi, G.; Luchini, C.; Cappello, F.; Caporalini, C.; Santoro, L. New insights in gastrointestinal “pediatric” neoplasms in adult patients: Pancreatoblastoma, hepatoblastoma and embryonal sarcoma of the liver. A practical approach by GIPPI-GIPAD Groups. Pathologica 2022, 114, 64–78. [Google Scholar] [CrossRef]
- Gruppioni, F.; Casadei, R.; Fusco, F.; Calculli, L.; Marrano, D.; Gavelli, G. Adult pancreatoblastoma. A case report. Radiol. Med. 2002, 103, 119–122. [Google Scholar]
- Montemarano, H.; Lonergan, G.J.; Bulas, D.I.; Selby, D.M. Pancreatoblastoma: Imaging findings in 10 patients and review of the literature. Radiology 2000, 214, 476–482. [Google Scholar] [CrossRef]
- Savastano, S.; D’Amore, E.S.G.; Zuccarotto, D.; Banzato, O.; Beghetto, M.; Famengo, B. Pancreatoblastoma in an adult patient. A case report. JOP 2009, 10, 192–195. [Google Scholar]
- Salman, B.; Brat, G.; Yoon, Y.-S.; Hruban, R.H.; Singhi, A.D.; Fishman, E.K.; Herman, J.M.; Wolfgang, C.L. The Diagnosis and surgical treatment of pancreatoblastoma in adults: A case series and review of the literature. J. Gastrointest. Surg. 2013, 17, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Charlton-Ouw, K.M.; Kaiser, C.L.; Tong, G.X.; Allendorf, J.D.; Chabot, J.A. Revisiting metastatic adult pancreatoblastoma. A case and review of the literature. JOP 2008, 9, 733–738. [Google Scholar] [PubMed]
- Benoist, S.; Penna, C.; Julié, C.; Malafosse, R.; Rougier, P.; Nordlinger, B. Prolonged survival after resection of pancreatoblastoma and synchronous liver metastases in an adult. World J. Gastroenterol. 2001, 48, 1340–1342. [Google Scholar]
- Drut, R.; Jones, M.C. Congenital pancreatoblastoma in Beckwith-Wiedemann syndrome: An emerging association. Pediatr. Pathol. 1988, 8, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.D.; Bhattarai, S.; Graham, R.P.; Pehlivanoglu, B.; Sigel, C.S.; Shi, J.; Saqi, A.; Shirazi, M.; Xue, Y.; Basturk, O.; et al. Pancreatoblastoma: Cytologic and histologic analysis of 12 adult cases reveals helpful criteria in their diagnosis and distinction from common mimics. Cancer Cytopathol. 2019, 127, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Moussata, D.; Senouci, L.; Berger, F.; Scoazec, J.Y.; Pinson, S.; Walter, T.; Lombard-Bohas, C.; Saurin, J.C. Familial Adenomatous Polyposis and Pancreatic Cancer. Pancreas 2015, 44, 512–513. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Fujii, T.; Izumi, Y.; Fukumura, Y.; Han, M.; Yamaguchi, H.; Akita, T.; Yamashita, C.; Kato, S.; Sekiya, T.; et al. Identification and characterization of a novel adenomatous polyposis coli mutation in adult pancreatoblastoma. Oncotarget 2018, 6, 10818–10827. [Google Scholar] [CrossRef][Green Version]
- Massimino, M.; Signoroni, S.; Boschetti, L.; Chiapparini, L.; Erbetta, A.; Biassoni, V.; Schiavello, E.; Ferrari, A.; Spreafico, F.; Terenziani, M.; et al. Medulloblastoma and familial adenomatous polyposis: Good prognosis and good quality of life in the long-term? Pediatr. Blood Cancer 2021, 68, e28912. [Google Scholar] [CrossRef]
- Muir, E.G.; Bell, A.J.Y.; Barlow, K.A. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br. J. Surg. 1967, 54, 191–195. [Google Scholar] [CrossRef]
- Torre, D. Society transactions: New York Dermatological Society, Oct 24, 1967 (multiple sebaceous tumors). Arch. Dermatol. 1968, 98, 549–551. [Google Scholar]
- Cohen, P.R.; Kohn, S.R.; Kurzrock, R. Association of sebaceous gland tumors and internal malignancy: The Muir-Torre syndrome. Am. J. Med. 1991, 90, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; de Leon, M.P. Muir-Torre syndrome. Lancet Oncol. 2005, 6, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Kingston, J.E.; Herbert, A.; Draper, G.J.; Mann, J.R. Association between hepatoblastoma and polyposis coli. Arch. Dis. Child. 1983, 58, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-K.; Abdalla, E.K.; Law, C.H.L.; Kohlmann, W.; Rashid, A.; Vauthey, J.-N. Biallelic inactivation of the APC gene is associated with hepatocellular carcinoma in familial adenomatous polyposis coli. Cancer 2001, 92, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh-Lotrario, A.D.; López-Terrada, D.; Li, P.; Feusner, J.H. Hepatoblastoma in patients with molecularly proven familial adenomatous polyposis: Clinical characteristics and rationale for surveillance screening. Pediatr. Blood Cancer 2018, 65, e27103. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Suemitsu, Y.; Ono, Y.; Mizukami, Y.; Ye, J.; Yamakawa, K.; Takamoto, T.; Nakano-Narusawa, Y.; Mukai, Y.; Takamatsu, M.; Nakazawa, A.; et al. A Case of Adult Pancreatoblastoma with Novel APC Mutation and Genetic Heterogeneity. Front. Oncol. 2021, 11, 725290. [Google Scholar] [CrossRef] [PubMed]
- Ikenoue, T.; Yamaguchi, K.; Komura, M.; Imoto, S.; Yamaguchi, R.; Shimizu, E.; Kasuya, S.; Shibuya, T.; Hatakeyama, S.; Miyano, S.; et al. Attenuated familial adenomatous polyposis with desmoids caused by an APC mutation. Hum. Genome Var. 2015, 2, 15011. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Offerhaus, G.J.; Lee, D.H.; Krush, A.J.; Tersmette, A.C.; Booker, S.V.; Kelley, N.C.; Hamilton, S.R. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 1993, 34, 1394–1396. [Google Scholar] [CrossRef]
- Karstensen, J.G.; Bülow, S.; Højen, H.; Jelsig, A.M.; Jespersen, N.; Andersen, K.K.; Wewer, M.D.; Burisch, J.; Pommergaard, H.C. Cancer in Patients with Familial Adenomatous Polyposis: A Nationwide Danish Cohort Study with Matched Controls. Gastroenterology 2023, 165, 573–581.e3. [Google Scholar] [CrossRef]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).