Unveiling the Roles of LncRNA MOIRAs in Rice Blast Disease Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Pathogen
2.2. Pathogen Inoculation
2.3. Generation of Transgenic Plants
2.4. RNA Isolation and Quantitative qRT-PCR
2.5. Statistical Analysis
3. Results
3.1. Identification of Three LncRNAs Responsive to M. oryzae Infection
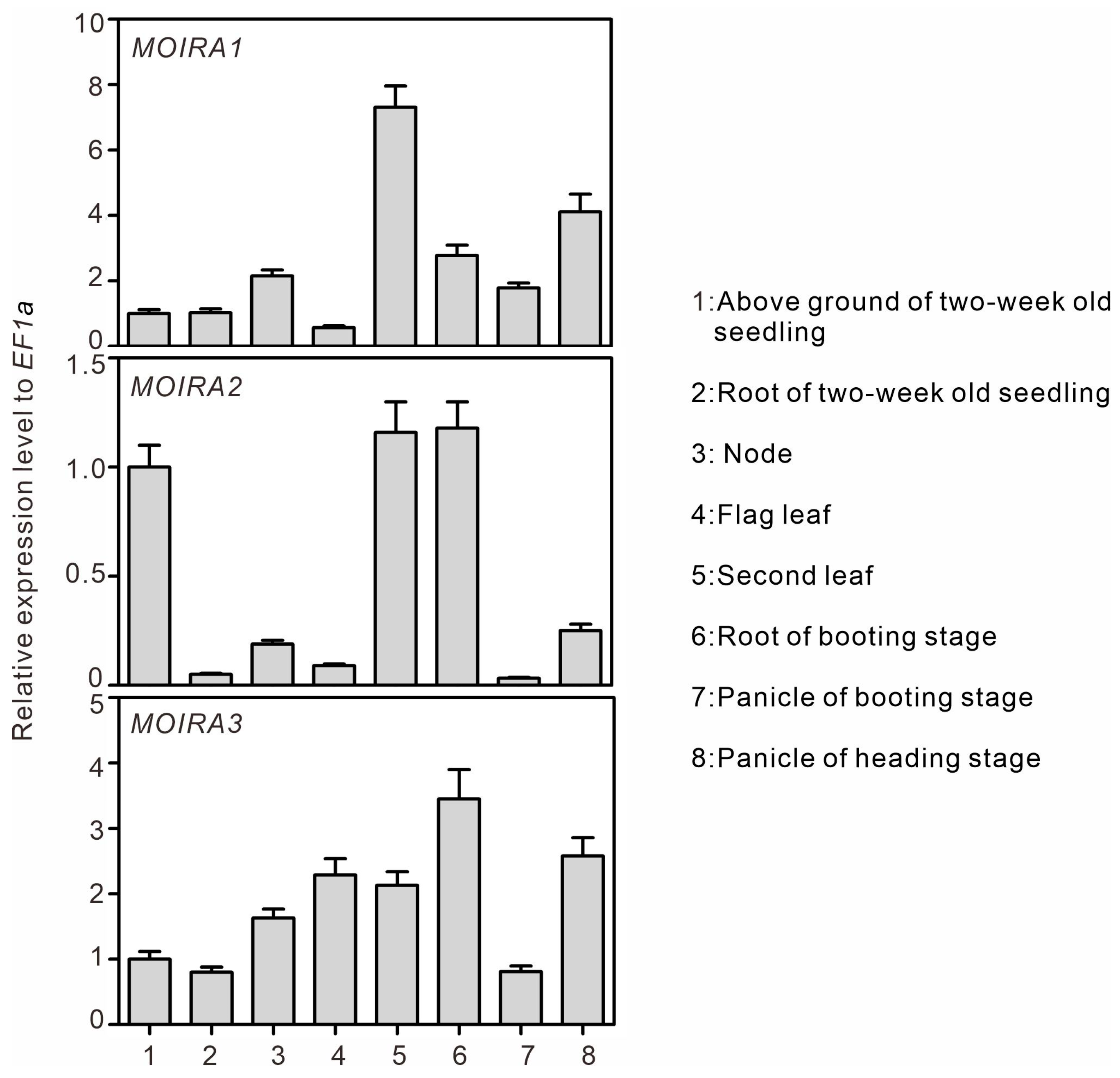
3.2. Tissue-Specific Expressions of MOIRAs
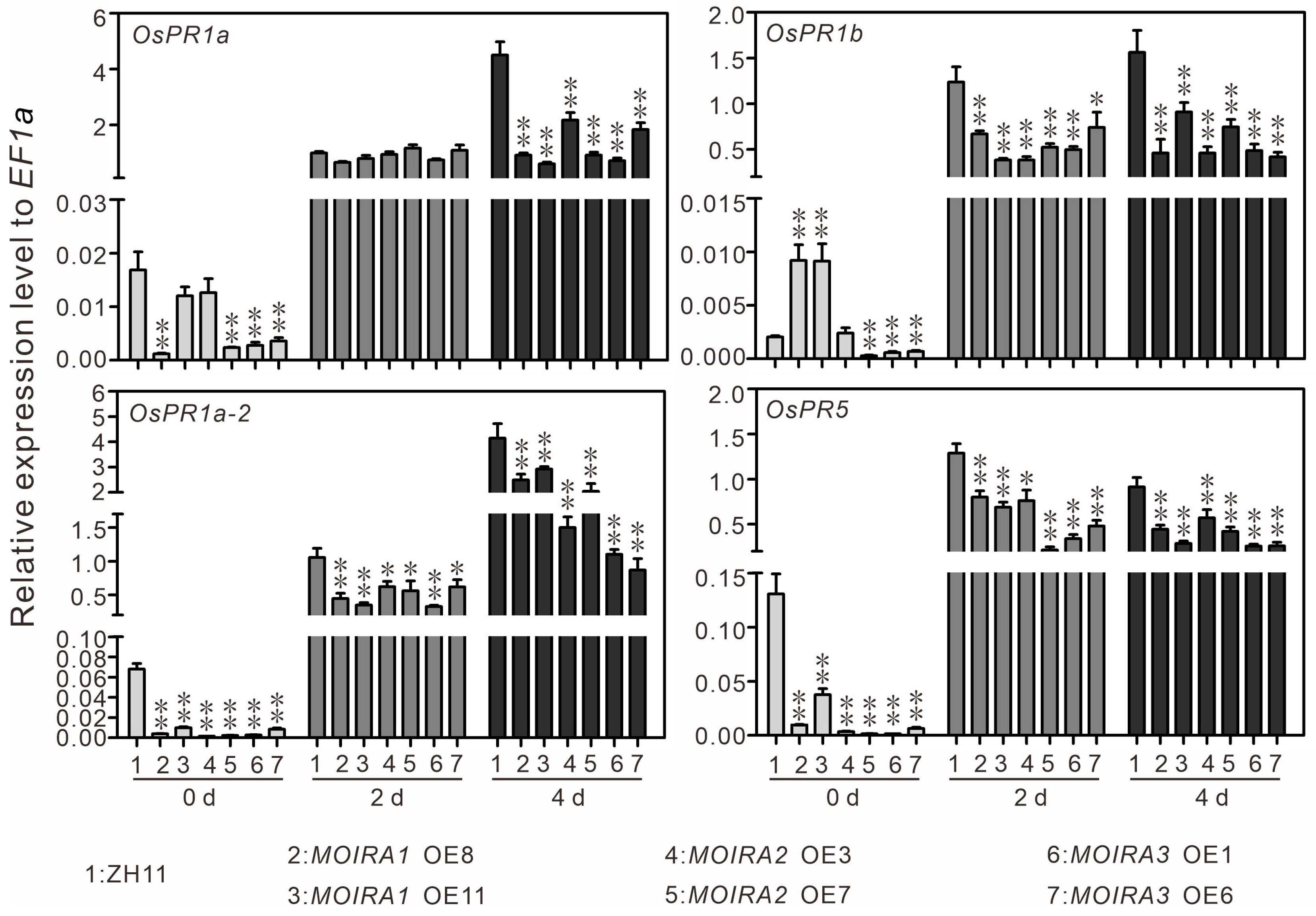
3.3. Overexpression of MOIRAs Enhances Rice Susceptibility to Blast Fungus
3.4. Association of MOIRAs with Yield Traits in Rice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burdon, J.J.; Thrall, P.H. Coevolution of plants and their pathogens in natural habitats. Science 2009, 324, 755–756. [Google Scholar] [CrossRef]
- Xue, J.; Lu, Z.; Liu, W.; Wang, S.; Lu, D.; Wang, X.; He, X. The genetic arms race between plant and Xanthomonas: Lessons learned from TALE biology. Sci. China Life Sci. 2021, 64, 51–65. [Google Scholar] [CrossRef]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Rice blast: A disease with implications for global food security. Agronomy 2019, 9, 451. [Google Scholar] [CrossRef]
- Fernandez, J. The Phantom Menace: Latest findings on effector biology in the rice blast fungus. aBIOTECH 2023, 4, 140–154. [Google Scholar] [CrossRef]
- Nejat, N.; Mantri, N. Emerging roles of long non-coding RNAs in plant response to biotic and abiotic stresses. Crit. Rev. Biotechnol. 2018, 38, 93–105. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Hu, W.; Ji, W. The emerging role of long non-coding RNAs in plant defense against fungal stress. Int. J. Mol. Sci. 2020, 21, 2659. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, T.; Shen, D.; Wang, J.; Ling, X.; Hu, Z.; Chen, T.; Hu, J.; Huang, J.; Yu, W. Tomato yellow leaf curl virus intergenic siRNAs target a host long noncoding RNA to modulate disease symptoms. PLoS Pathog. 2019, 15, e1007534. [Google Scholar] [CrossRef]
- Song, L.; Fang, Y.; Chen, L.; Wang, J.; Chen, X. Role of non-coding RNAs in plant immunity. Plant Commun. 2021, 2, 100180. [Google Scholar] [CrossRef]
- Feng, Y.-Z.; Zhu, Q.-F.; Xue, J.; Chen, P.; Yu, Y. Shining in the dark: The big world of small peptides in plants. aBIOTECH 2023, 4, 238–256. [Google Scholar] [CrossRef]
- He, H.; Zhou, Y.-F.; Yang, Y.-W.; Zhang, Z.; Lei, M.-Q.; Feng, Y.-Z.; Zhang, Y.-C.; Chen, Y.-Q.; Lian, J.-P.; Yu, Y. Genome-Wide Analysis Identified a Set of Conserved lncRNAs Associated with Domestication-Related Traits in Rice. Int. J. Mol. Sci. 2021, 22, 4742. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Chen, X.; Chen, Y. Plant noncoding RNAs: Hidden players in development and stress responses. Annu. Rev. Cell Dev. Biol. 2019, 35, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, S.; Qi, H.; Cai, H.; Xu, M. Research progress on plant long non-coding RNA. Plants 2020, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, A.T.; Blevins, T.; Swiezewski, S. Long noncoding RNAs in plants. Annu. Rev. Plant Biol. 2021, 72, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, Y.; Li, Q.; Cao, Y.; Yang, D.; Liu, S.; Wang, X.; Mi, Y.; Liu, Y.; Ding, C. A lncRNA fine-tunes salicylic acid biosynthesis to balance plant immunity and growth. Cell Host Microbe 2022, 30, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Cui, J.; Hou, X.; Yang, G.; Xiao, Y.; Han, L.; Meng, J.; Luan, Y. Sl-lncRNA15492 interacts with Sl-miR482a and affects Solanum lycopersicum immunity against Phytophthora infestans. Plant J. 2020, 103, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Cui, J.; Shi, Y.; Yang, G.; Zhou, X.; Hou, X.; Meng, J.; Luan, Y. Tomato lncRNA23468 functions as a competing endogenous RNA to modulate NBS-LRR genes by decoying miR482b in the tomato-Phytophthora infestans interaction. Hortic. Res. 2019, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhu, J.; Qian, N.; Guo, J.; Yan, C. Bacillus subtilis SL18r Induces Tomato Resistance Against Botrytis cinerea, Involving Activation of Long Non-coding RNA, MSTRG18363, to Decoy miR1918. Front Plant Sci 2021, 11, 634819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, M.; Li, N.; Wang, H.; Qiu, P.; Pei, L.; Xu, Z.; Wang, T.; Gao, E.; Liu, J.; et al. Long noncoding RNAs involve in resistance to Verticillium dahliae, a fungal disease in cotton. Plant Biotechnol. J. 2018, 16, 1172–1185. [Google Scholar] [CrossRef]
- Wang, L.-L.; Jin, J.-J.; Li, L.-H.; Qu, S.-H. Long Non-coding RNAs Responsive to Blast Fungus Infection in Rice. Rice 2020, 13, 77. [Google Scholar] [CrossRef]
- Jain, P.; Sharma, V.; Dubey, H.; Singh, P.K.; Kapoor, R.; Kumari, M.; Singh, J.; Pawar, D.V.; Bisht, D.; Solanke, A.U.; et al. Identification of long non-coding RNA in rice lines resistant to Rice blast pathogen Maganaporthe oryzae. Bioinformation 2017, 13, 249–255. [Google Scholar] [CrossRef]
- Choi, G.; Jeon, J.; Lee, H.; Zhou, S.; Lee, Y.-H. Genome-wide profiling of long non-coding RNA of the rice blast fungus Magnaporthe oryzae during infection. BMC Genom. 2022, 23, 132. [Google Scholar] [CrossRef]
- Liang, M.; Ye, H.; Shen, Q.; Jiang, X.; Cui, G.; Gu, W.; Zhang, L.H.; Naqvi, N.I.; Deng, Y.Z. Tangeretin inhibits fungal ferroptosis to suppress rice blast. J. Integr. Plant Biol. 2021, 63, 2136–2149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, T.; Yu, T.; Zhang, S.; Mao, X.; Zhao, J.; Wang, X.; Dong, J.; Liu, B. Integrating small RNA sequencing with QTL mapping for identification of miRNAs and their target genes associated with heat tolerance at the flowering stage in rice. Front. Plant Sci. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, Y.-F.; Feng, Y.-Z.; He, H.; Lian, J.-P.; Yang, Y.-W.; Lei, M.-Q.; Zhang, Y.-C.; Chen, Y.-Q. Transcriptional landscape of pathogen-responsive lncRNAs in rice unveils the role of ALEX1 in jasmonate pathway and disease resistance. Plant Biotechnol. J. 2020, 18, 679–690. [Google Scholar] [CrossRef]
- Datta, R.; Paul, S. Long non-coding RNAs: Fine-tuning the developmental responses in plants. J. Biosci. 2019, 44, 77. [Google Scholar] [CrossRef]
- Ariel, F.; Romero-Barrios, N.; Jégu, T.; Benhamed, M.; Crespi, M. Battles and hijacks: Noncoding transcription in plants. Trends Plant Sci. 2015, 20, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Waititu, J.K.; Zhang, C.; Liu, J.; Wang, H. Plant non-coding RNAs: Origin, biogenesis, mode of action and their roles in abiotic stress. Int. J. Mol. Sci. 2020, 21, 8401. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Liu, W.; Wang, G.-L. Balancing immunity and yield in crop plants. Trends Plant Sci. 2017, 22, 1069–1079. [Google Scholar] [CrossRef]
- Chandran, V.; Wang, H.; Gao, F.; Cao, X.-L.; Chen, Y.-P.; Li, G.-B.; Zhu, Y.; Yang, X.-M.; Zhang, L.-L.; Zhao, Z.-X. miR396-OsGRF s module balances growth and rice blast disease-resistance. Front. Plant Sci. 2019, 9, 1999. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.-P.; Zhou, X.-H.; Yang, X.-M.; He, X.-R.; Feng, Q.; Zhu, Y.; Li, G.-B.; Wang, H.; Zhao, J.-H. Rice miR1432 fine-tunes the balance of yield and blast disease resistance via different modules. Rice 2021, 14, 87. [Google Scholar] [CrossRef]
- Xiao, N.; Pan, C.; Li, Y.; Wu, Y.; Cai, Y.; Lu, Y.; Wang, R.; Yu, L.; Shi, W.; Kang, H. Genomic insight into balancing high yield, good quality, and blast resistance of japonica rice. Genome Biol. 2021, 22, 283. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Feng, Y.; Xue, J.; Chen, P.; Zhang, A.; Yu, Y. Advances in Receptor-like Protein Kinases in Balancing Plant Growth and Stress Responses. Plants 2023, 12, 427. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Xue, J.; Zhang, L.; Jiang, L.; Li, C. Unveiling the Roles of LncRNA MOIRAs in Rice Blast Disease Resistance. Genes 2024, 15, 82. https://doi.org/10.3390/genes15010082
Liu Q, Xue J, Zhang L, Jiang L, Li C. Unveiling the Roles of LncRNA MOIRAs in Rice Blast Disease Resistance. Genes. 2024; 15(1):82. https://doi.org/10.3390/genes15010082
Chicago/Turabian StyleLiu, Qing, Jiao Xue, Lanlan Zhang, Liqun Jiang, and Chen Li. 2024. "Unveiling the Roles of LncRNA MOIRAs in Rice Blast Disease Resistance" Genes 15, no. 1: 82. https://doi.org/10.3390/genes15010082



