Whole-Transcriptome Sequencing of Ovary Reveals the ceRNA Regulation Network in Egg Production of Gaoyou Duck
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. RNA Isolation, Library Construction, and Sequencing
2.3. Transcriptome Data Analysis
2.4. Hematoxylin–Eosin (HE) Staining and Analysis of Ovarian Follicles
2.5. Analysis through Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. Data Analysis
3. Results
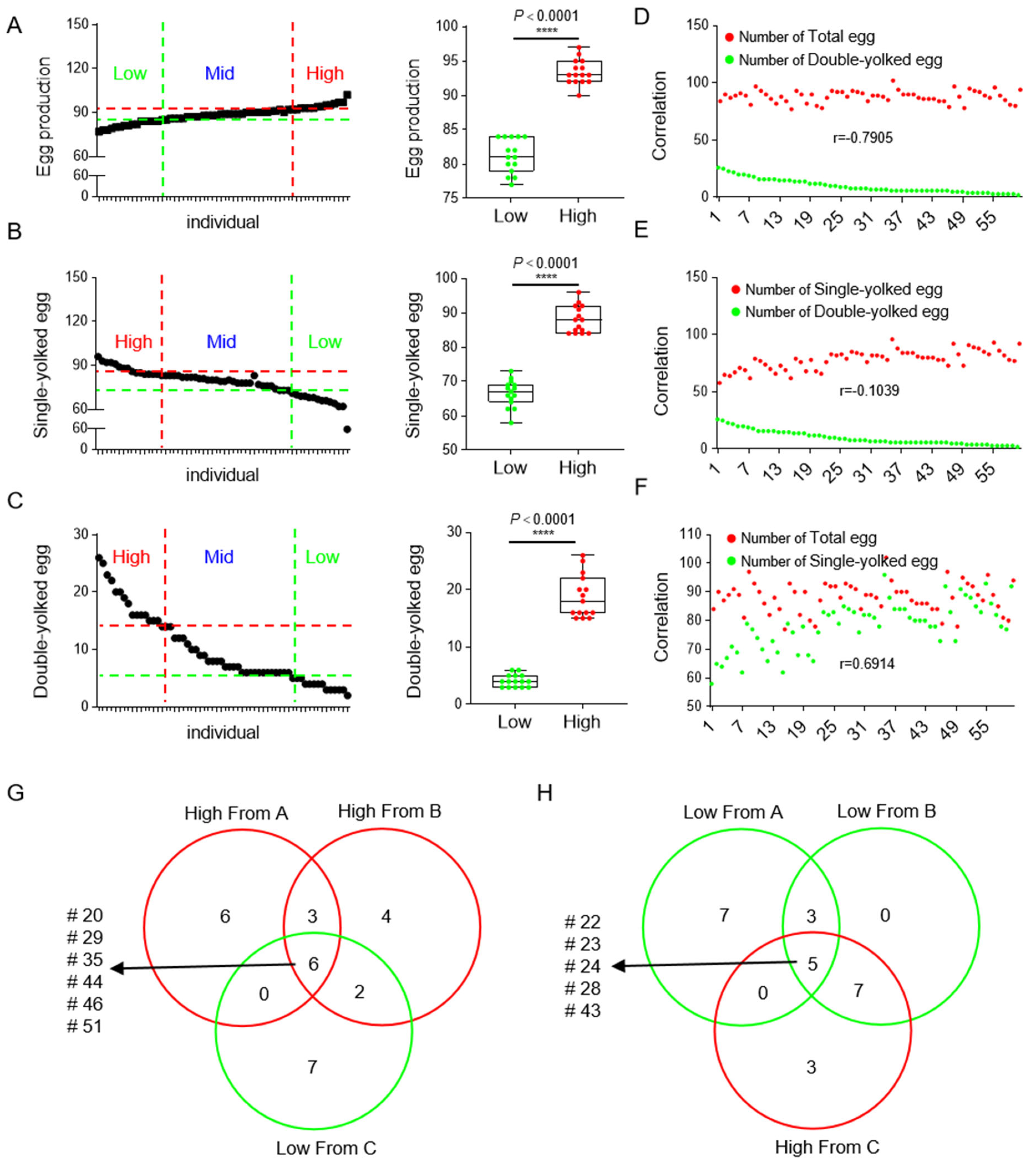
3.1. The Identification of Individuals with High- and Low-Egg Production
3.2. The Strategy of Sample Acquisition Ensures the Availability of RNA-Seq
3.3. Whole-Transcriptome Library Construction and Clean Data Statistics
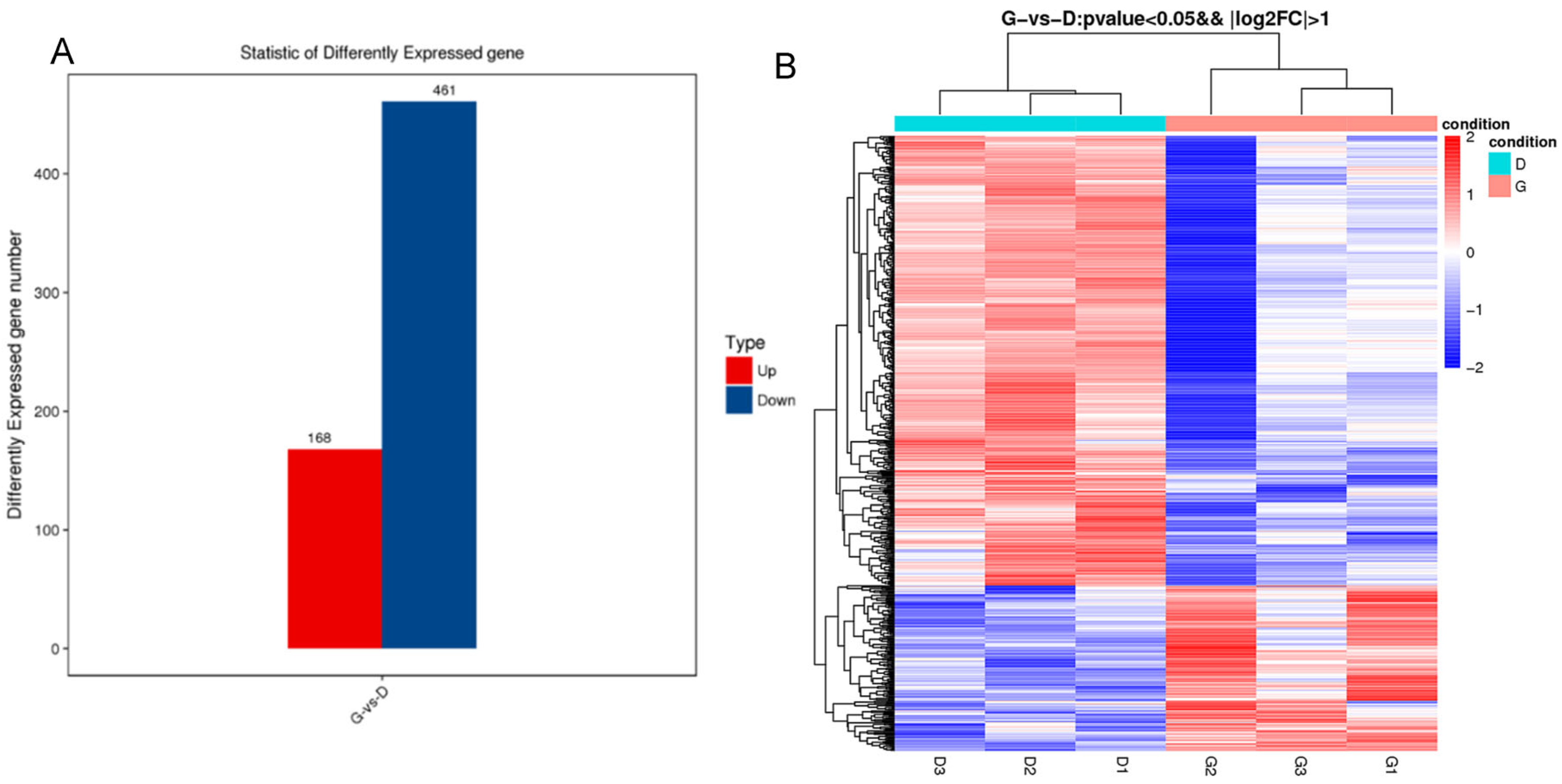
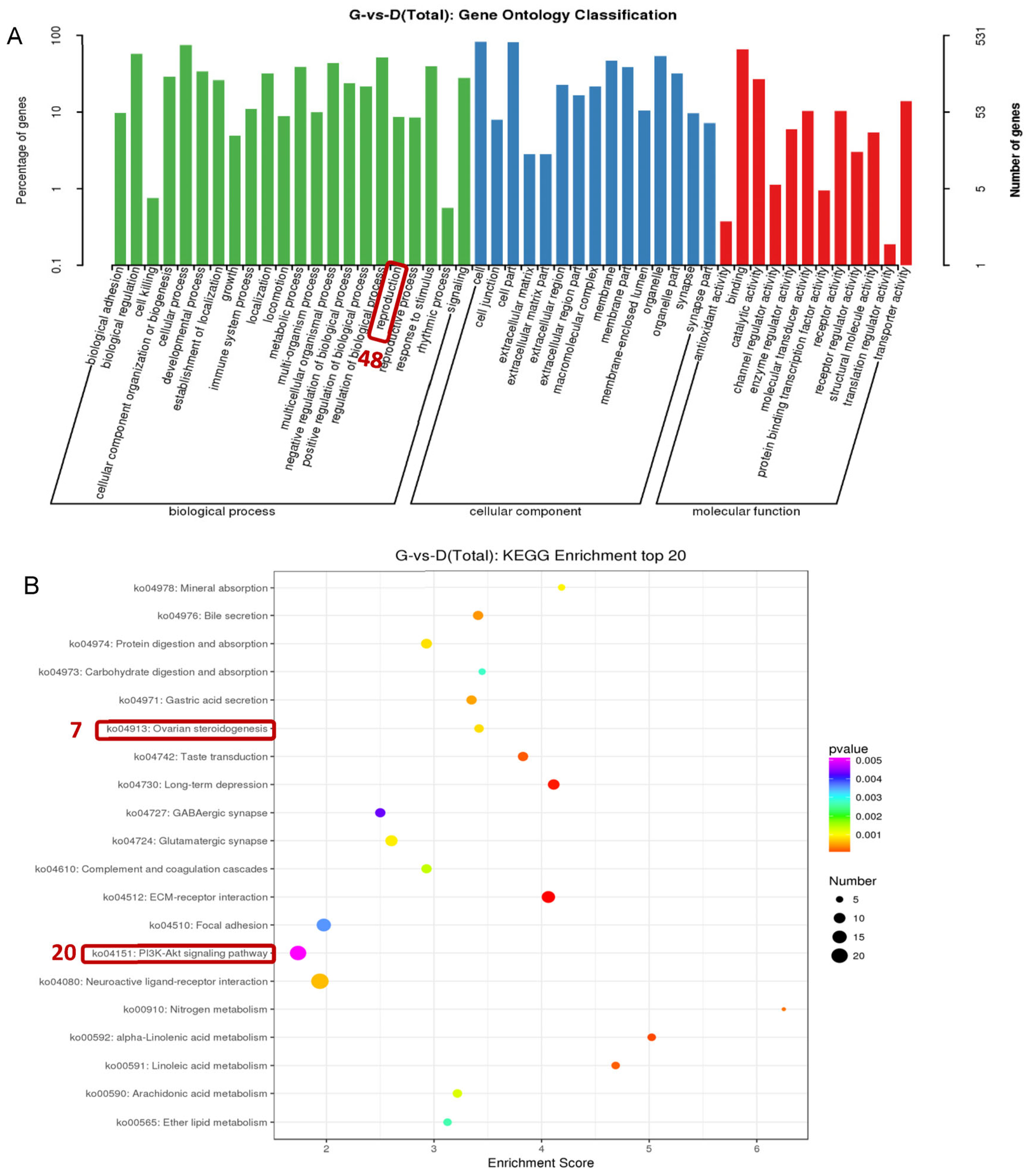
3.4. Analysis of Differentially Expressed Genes among Individuals with Different Egg Production
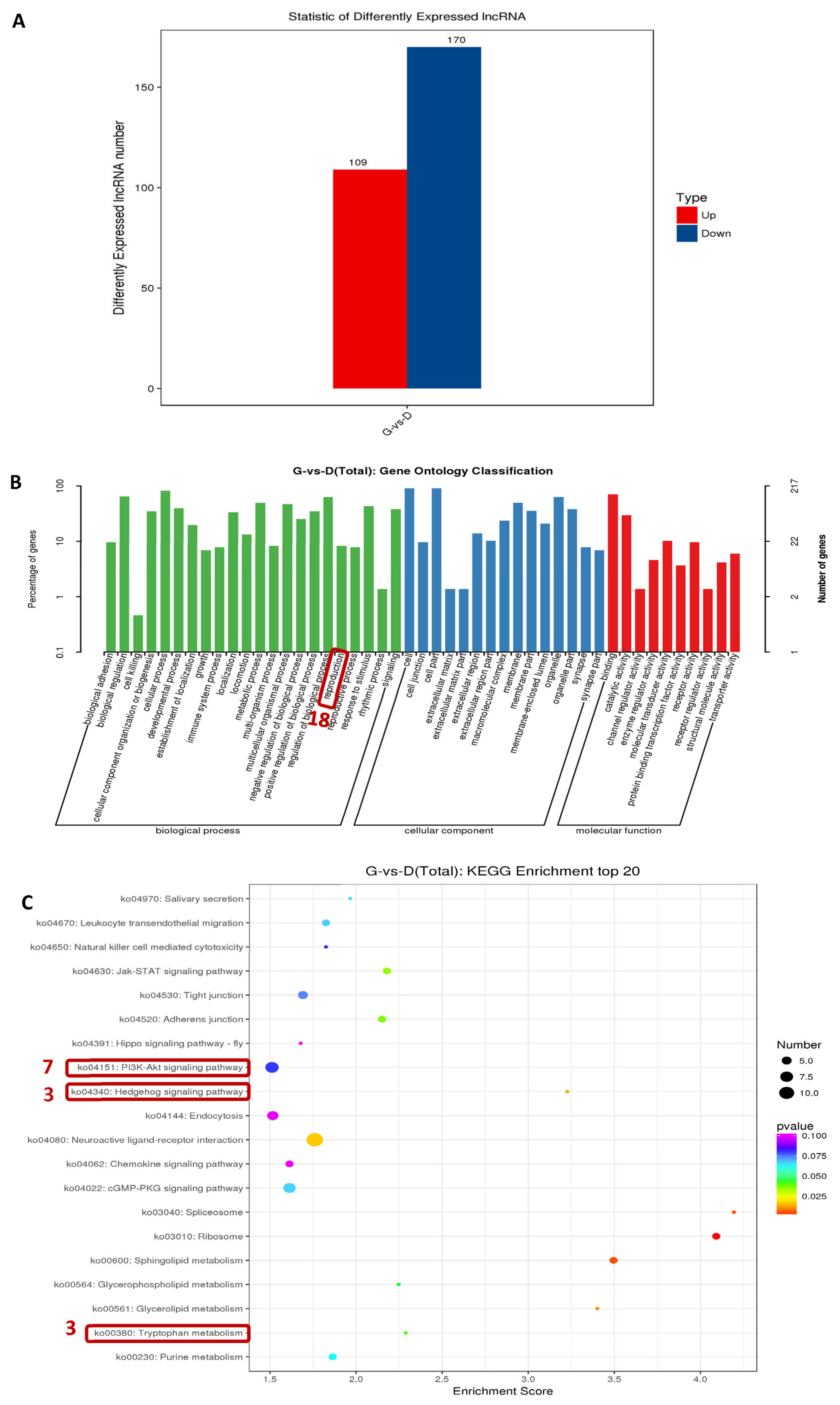
3.5. Analysis of lncRNA’s Differential Expression among Individuals with Different Egg Production
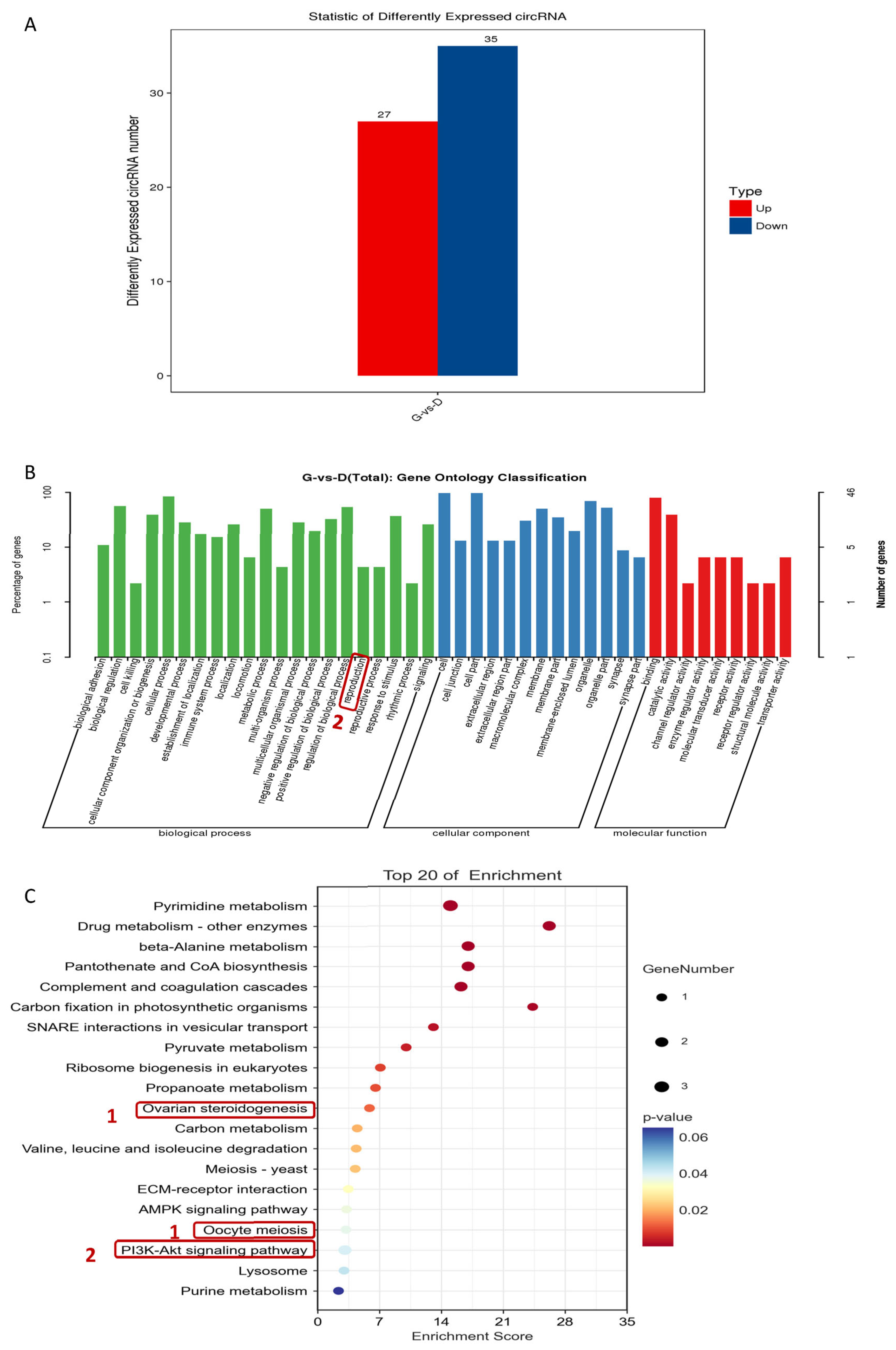
3.6. Analysis of circRNA Differential Expression among Individuals with Different Egg Production Characteristics
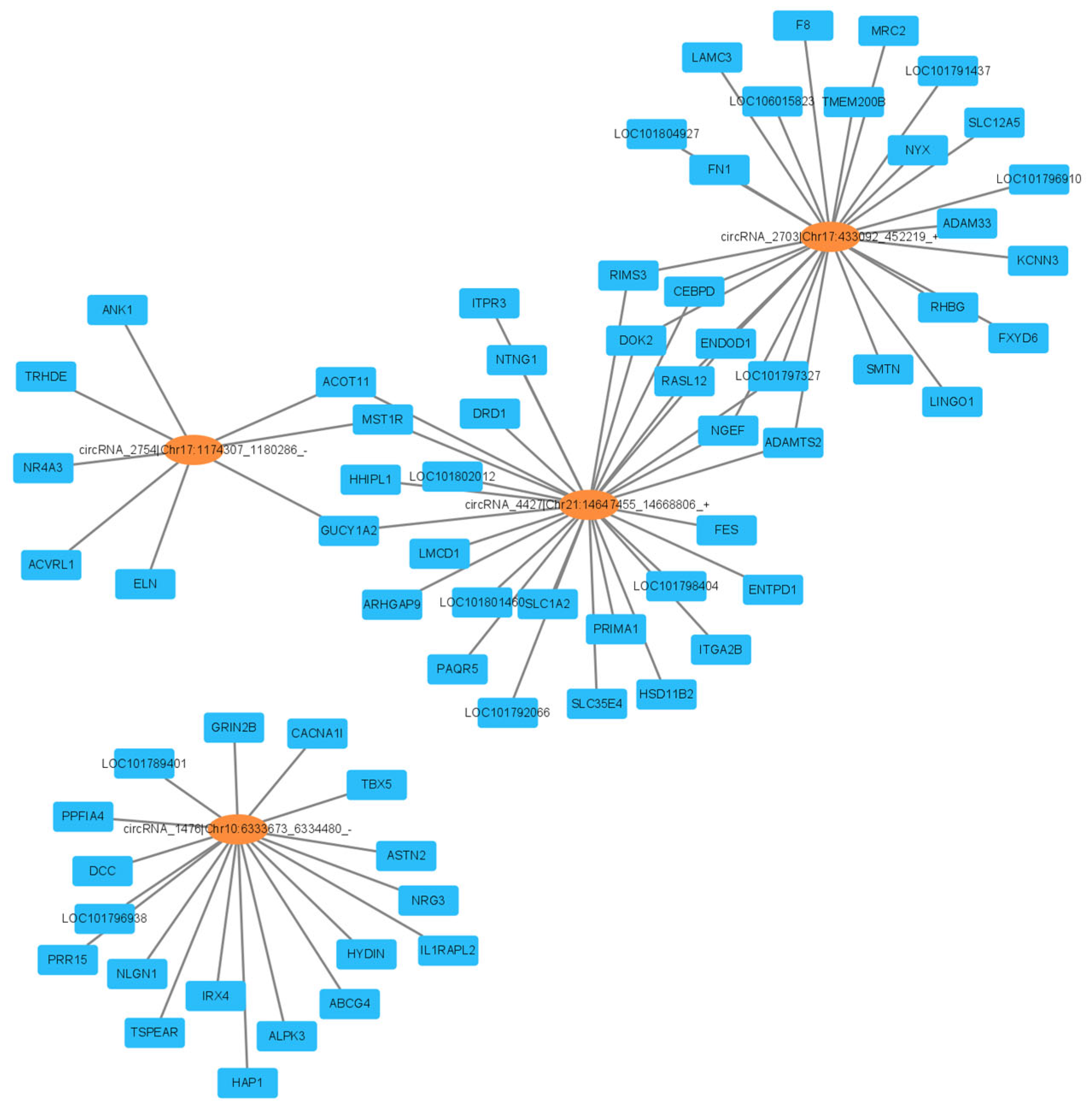
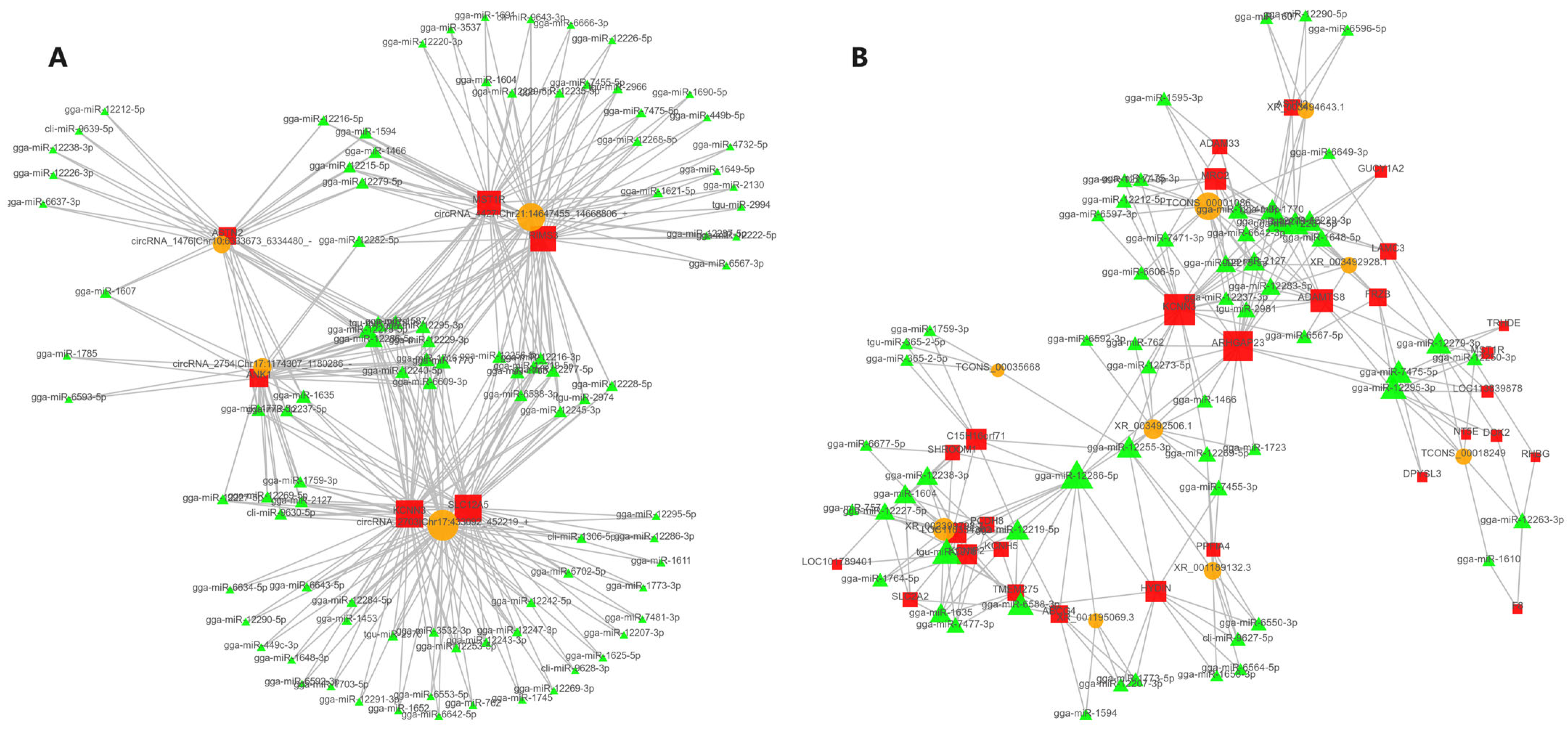
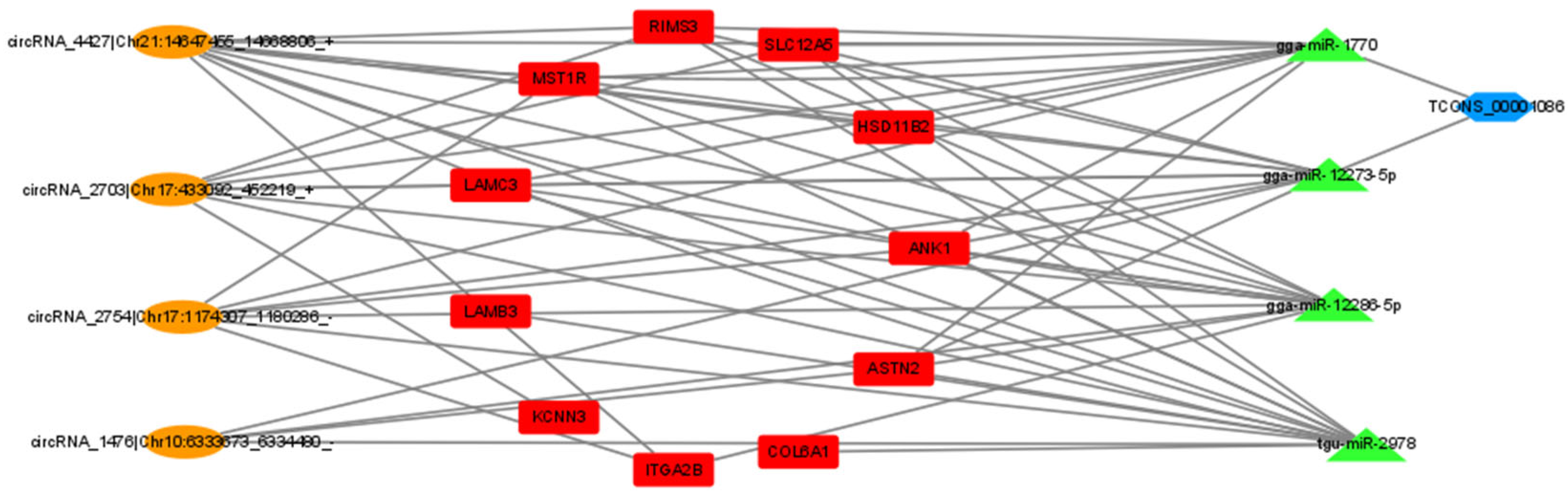
3.7. Construction of ceRNA Regulatory Network for Laying Traits in Gaoyou Ducks
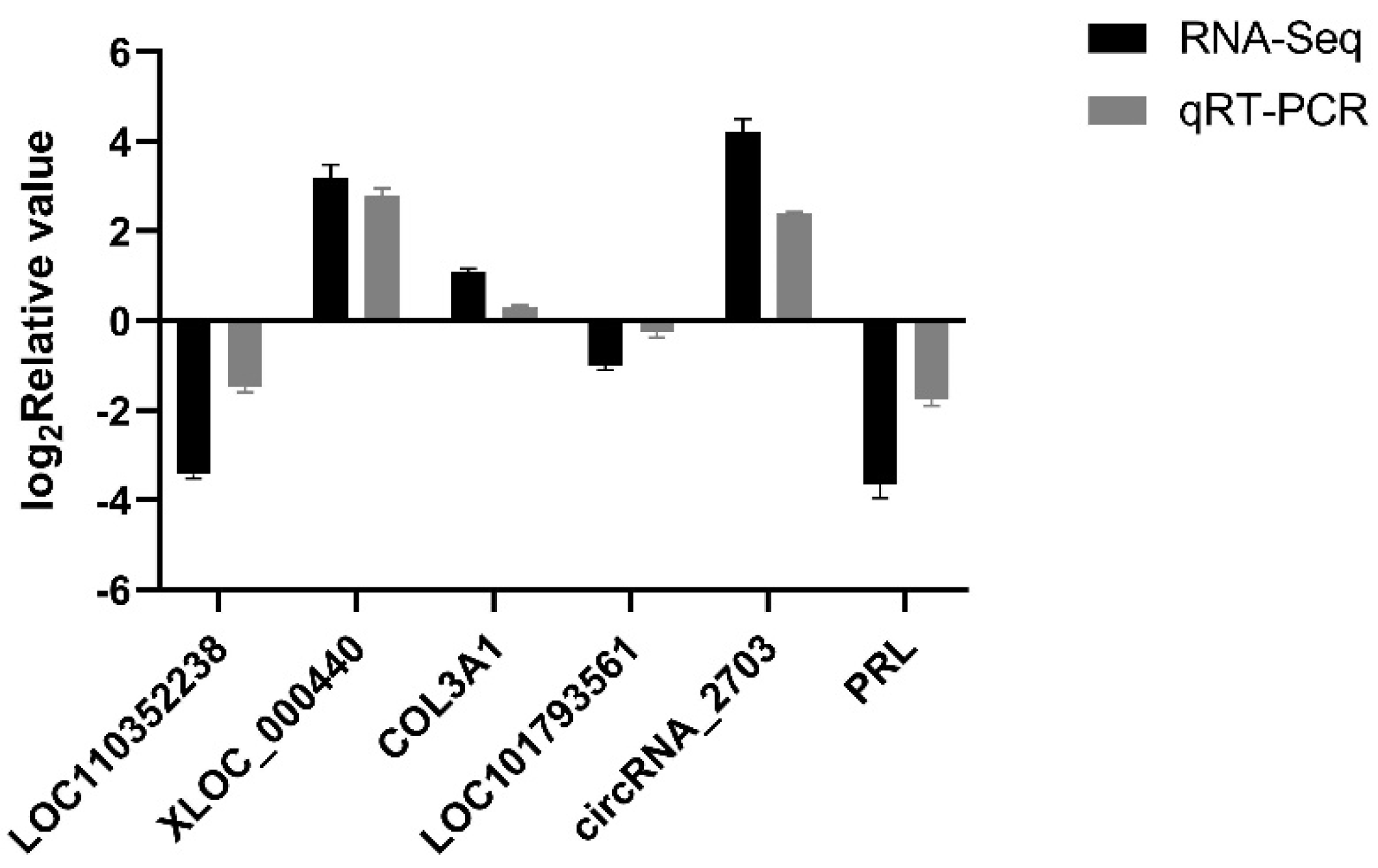
3.8. Validation of RNA-Seq Results Using qRT-PCR for Laying Traits in Gaoyou Ducks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Xie, J.; Sun, G.; Ji, R.; Li, X.; Zhang, X.; Wang, J. Identification of differentially expressed genes and signaling pathways in Gaoyou duck ovary at different physiological stages. Front. Vet. Sci. 2023, 10, 1190998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.J.; Li, H.F.; Chen, K.W.; Zhao, Y.G.; Chang, H.; Xue, M.K.; Zhang, S.F. Analysis of Fitness Predominance for Gaoyou Duck’s Double-Yolk Egg. J. Anim. Vet. Adv. 2011, 10, 367–371. [Google Scholar] [CrossRef]
- Hlokoe, V.R.; Tyasi, T.L.; Gunya, B. Chicken ovarian follicles morphology and growth differentiation factor 9 gene expression in chicken ovarian follicles: Review. Heliyon 2022, 8, e8742. [Google Scholar] [CrossRef] [PubMed]
- Apperson, K.D.; Bird, K.E.; Cherian, G.; Lohr, C.V. Histology of the Ovary of the Laying Hen (Gallus domesticus). Vet. Sci. 2017, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 2015, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.A.; Arganaraz, M.E.; Barrera, A.D.; Rodler, D.; Mutto, A.A.; Sinowatz, F. Biology and biotechnology of follicle development. Sci. World J. 2012, 2012, 938138. [Google Scholar] [CrossRef] [PubMed]
- Onagbesan, O.; Bruggeman, V.; Decuypere, E. Intra-ovarian growth factors regulating ovarian function in avian species: A review. Anim. Reprod. Sci. 2009, 111, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Salamon, A.; Kent, J.P. Yolk size and ovulation order determine fertility within double-yolked duck (Anas platyrhynchos domesticus) eggs. Reprod. Fertil. Dev. 2016, 28, 440–445. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Dai, Z.; Yang, P.; Chen, R.; Guo, B.; Lei, M.; Shi, Z. A Possible Mechanism for Double-Yolked Eggs in the Early Stage of Egg-Laying in Zhedong White Goose-Function of IGF1 and LHR Signaling. Animals 2022, 12, 2964. [Google Scholar] [CrossRef]
- Deeming, D.C. Double-yolked pheasant eggs provide an insight into the control of albumen secretion in bird eggs. Br. Poult. Sci. 2011, 52, 40–47. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, C.; Yang, Z.; Deng, J.; Yang, X. Grade follicles transcriptional profiling analysis in different laying stages in chicken. BMC Genom. 2022, 23, 492. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, X.; Zhao, J.; Ma, C.; Yan, C.; Liswaniso, S.; Xu, R.; Qin, N. Transcriptome comparative analysis of ovarian follicles reveals the key genes and signaling pathways implicated in hen egg production. BMC Genom. 2021, 22, 899. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Chen, B.; Zhu, Q.; Xu, Z.; Ning, C.; Yin, H.; Wang, Y.; Zhao, X.; Fan, X.; Yang, M.; et al. Transcriptome analysis reveals differentially expressed genes associated with high rates of egg production in chicken hypothalamic-pituitary-ovarian axis. Sci. Rep. 2020, 10, 5976. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. Hisat: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Orre, L.M.; Zhou, T.Y.; Mermelekas, G.; Johansson, H.J.; Malyutina, A.; Anders, S.; Lehtio, J. DEqMS: A Method for Accurate Variance Estimation in Differential Protein Expression Analysis. Mol. Cell Proteom. 2020, 19, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Roberts, A.; Pachter, L. Streaming fragment assignment for real-time analysis of sequencing experiments. Nat. Methods. 2013, 10, 71–73. [Google Scholar] [CrossRef]
- Zou, K.; Asiamah, C.A.; Lu, L.; Liu, Y.; Pan, Y.; Chen, T.; Zhao, Z.; Su, Y. Ovarian transcriptomic analysis and follicular development of Leizhou black duck. Poult. Sci. 2020, 99, 6173–6187. [Google Scholar] [CrossRef]
- Ravisankar, S.; Hanna, C.B.; Brooks, K.E.; Murphy, M.J.; Redmayne, N.; Ryu, J.; Kinchen, J.M.; Chavez, S.L.; Hennebold, J.D. Metabolomics analysis of follicular fluid coupled with oocyte aspiration reveals importance of glucocorticoids in primate periovulatory follicle competency. Sci. Rep. 2021, 11, 6506. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Liu, Y.; Yu, Y.C.; Huang, Y.M.; Liang, S.D.; Shi, Z.D. Prolactin plays a stimulatory role in ovarian follicular development and egg laying in chicken hens. Domest. Anim. Endocrinol. 2011, 41, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.R.; Rajkovic, G.; Daldello, E.M.; Luong, X.G.; Chen, J.; Conti, M. The RNA-binding protein DAZL functions as repressor and activator of mRNA translation during oocyte maturation. Nat. Commun. 2020, 11, 1399. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, X.; Shi, Y.; Wang, Z. The Signaling Pathways Involved in Ovarian Follicle Development. Front. Physiol. 2021, 12, 730196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Beachy, P.A. Cellular and molecular mechanisms of Hedgehog signalling. Nat. Rev. Mol. Cell Biol. 2023, 24, 668–687. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.M.; El-Senousey, H.K.; Ruan, D.; Wang, S.; Xia, W.; Zheng, C. Tryptophan in poultry nutrition: Impacts and mechanisms of action. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- John, B.; Sander, C.; Marks, D.S. Prediction of human microRNA targets. Methods Mol. Biol. 2006, 342, 101–113. [Google Scholar]
- Chand, A.L.; Ponnampalam, A.P.; Harris, S.E.; Winship, I.M.; Shelling, A.N. Mutational analysis of BMP15 and GDF9 as candidate genes for premature ovarian failure. Fertil. Steril. 2006, 86, 1009–1012. [Google Scholar] [CrossRef]
- Dong, J.; Albertini, D.F.; Nishimori, K.; Kumar, T.R.; Lu, N.; Matzuk, M.M. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996, 383, 531–535. [Google Scholar] [CrossRef]
- Andersen, C.Y.; Ezcurra, D. Human steroidogenesis: Implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod. Biol. Endocrinol. 2014, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Zhu, Z.; Xu, D.; Liu, W.; Lyu, L.; Li, P. Proteomic characterization of bovine granulosa cells in dominant and subordinate follicles. Hereditas 2019, 156, 21. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Song, Y.; Li, T.; Zhang, S.; Huang, L.; Zhang, S.; Cao, J.; Liu, X.; Zhang, J. Comparative Transcriptome Profiling of Ovary Tissue between Black Muscovy Duck and White Muscovy Duck with High- and Low-Egg Production. Genes 2020, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Lei, M.; Li, C.; Guo, Z.; Ren, Y.; Zhang, X.; Zheng, J.; Zhang, C.; Yang, C.; Mei, X.; et al. Whole transcriptome sequencing reveals that non-coding RNAs are related to embryo morphogenesis and development in rabbits. Genomics 2020, 112, 2203–2212. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Shen, Z.; Yang, Q.; Luo, L.; Li, T.X.; Ke, Z.J.; Li, T.; Meng, X.Z.; Xiang, H.; Li, C.F.; Zhou, Z.Y.; et al. Non-coding RNAs identification and regulatory networks in pathogen-host interaction in the microsporidia congenital infection. BMC Genom. 2023, 24, 420. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhu, R.; Sun, G.; Wang, J.; Zuo, Q.; Zhu, S. Whole-Transcriptome Sequencing of Ovary Reveals the ceRNA Regulation Network in Egg Production of Gaoyou Duck. Genes 2024, 15, 9. https://doi.org/10.3390/genes15010009
Zhang L, Zhu R, Sun G, Wang J, Zuo Q, Zhu S. Whole-Transcriptome Sequencing of Ovary Reveals the ceRNA Regulation Network in Egg Production of Gaoyou Duck. Genes. 2024; 15(1):9. https://doi.org/10.3390/genes15010009
Chicago/Turabian StyleZhang, Lei, Rui Zhu, Guobo Sun, Jian Wang, Qisheng Zuo, and Shanyuan Zhu. 2024. "Whole-Transcriptome Sequencing of Ovary Reveals the ceRNA Regulation Network in Egg Production of Gaoyou Duck" Genes 15, no. 1: 9. https://doi.org/10.3390/genes15010009



