Transcriptome and Metabolome Analysis of BmFAMeT6 Overexpression in Bombyx mori
Abstract
:1. Introduction
2. Material and Methods
2.1. Insects and Environment
2.2. Experiment Design and Material Collection
2.3. Total RNA Isolation, Preparation, and Sequencing of Digital Gene Expression (DGE) Library
2.4. Metabolite Detection and Differential Metabolite Identification
2.5. Statistical Analysis of Data
3. Results
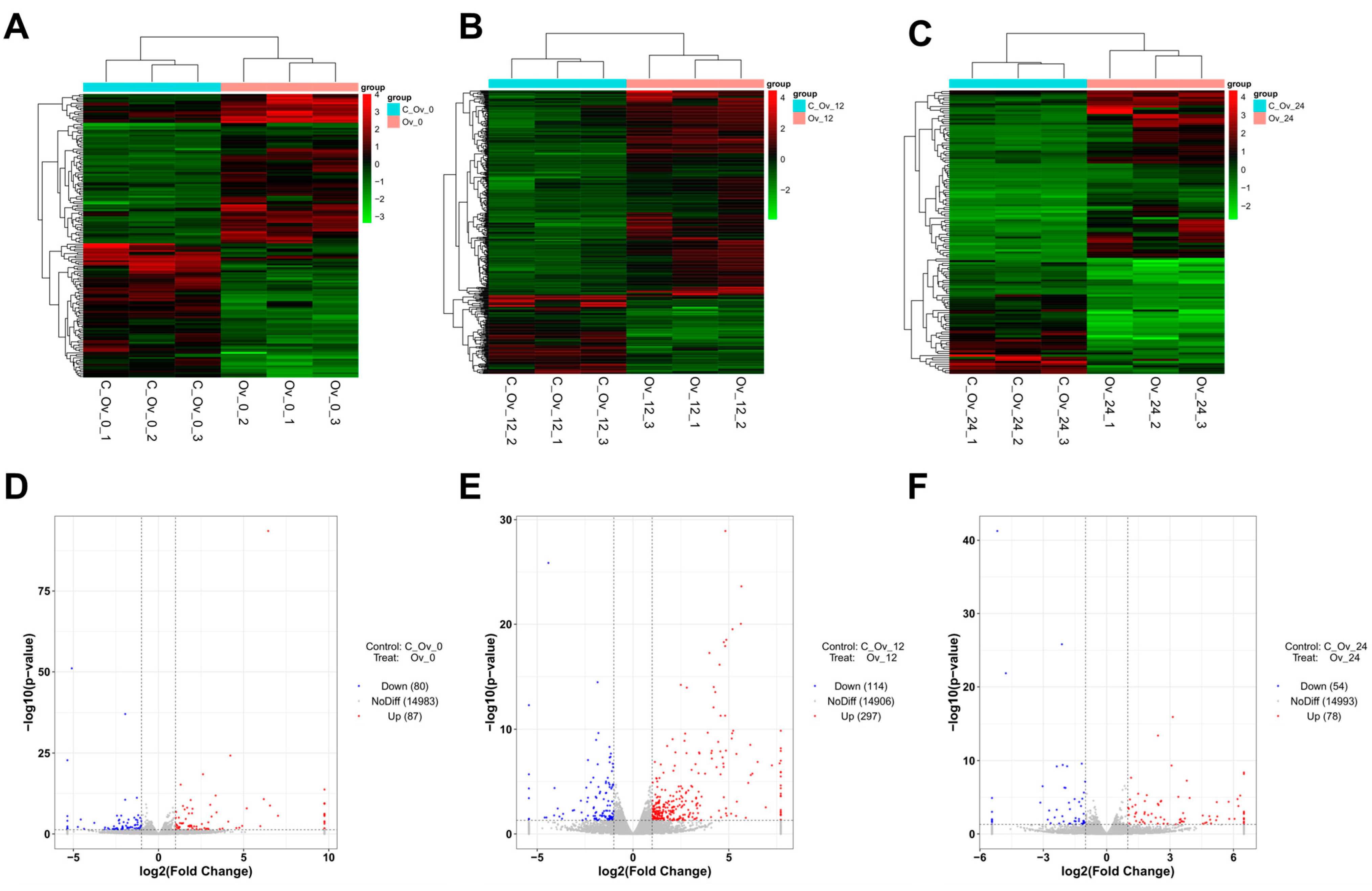
3.1. Overexpression of BmFAMeT6 Affected Gene Differential Expression in Silkworm Larvae
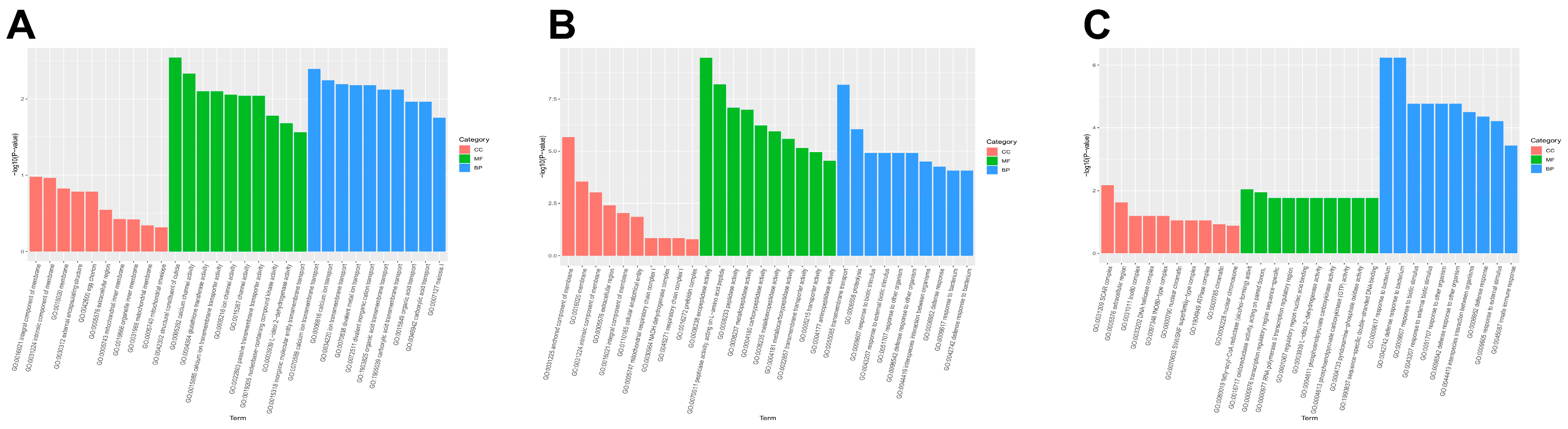
3.2. The Overexpression of BmFAMeT6 Mainly Affects the Molecular Functions and Biological Processes of Silkworm Larvae
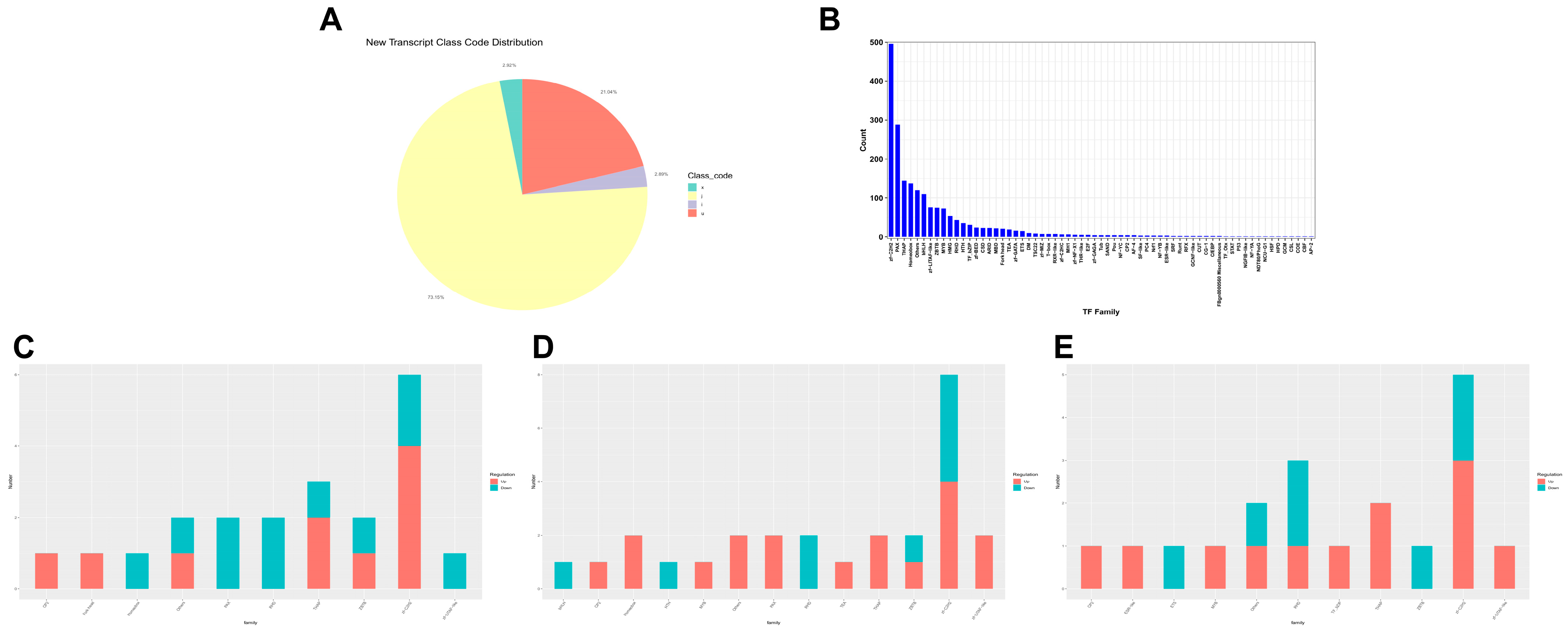
3.3. BmFAMeT6 May Modulate Larval Metabolic Pathways through Zinc Finger Transcription Factors
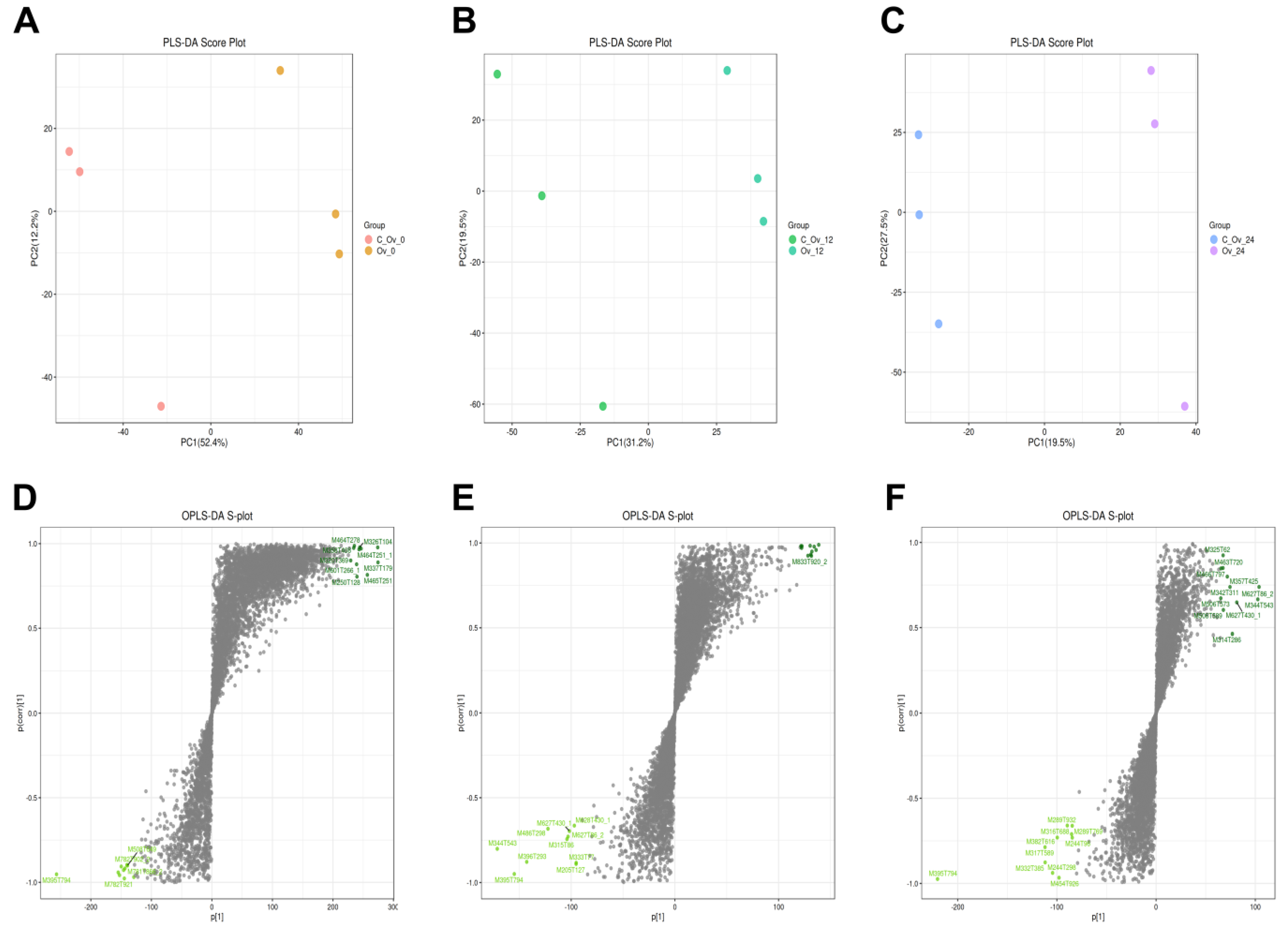
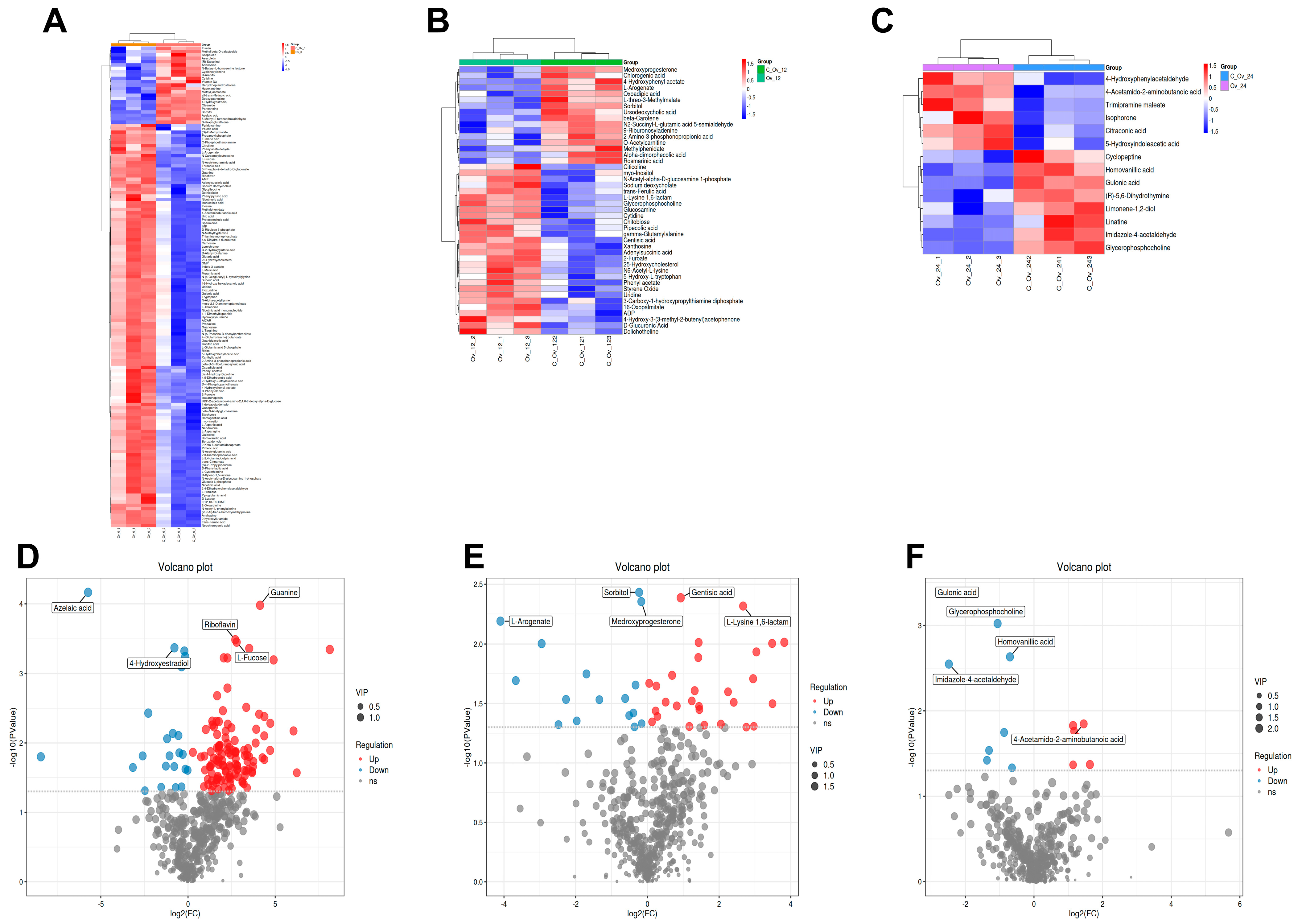
3.4. Overexpression of BmFAMeT6 Led to Metabolic Differences in the Silkworm Larvae
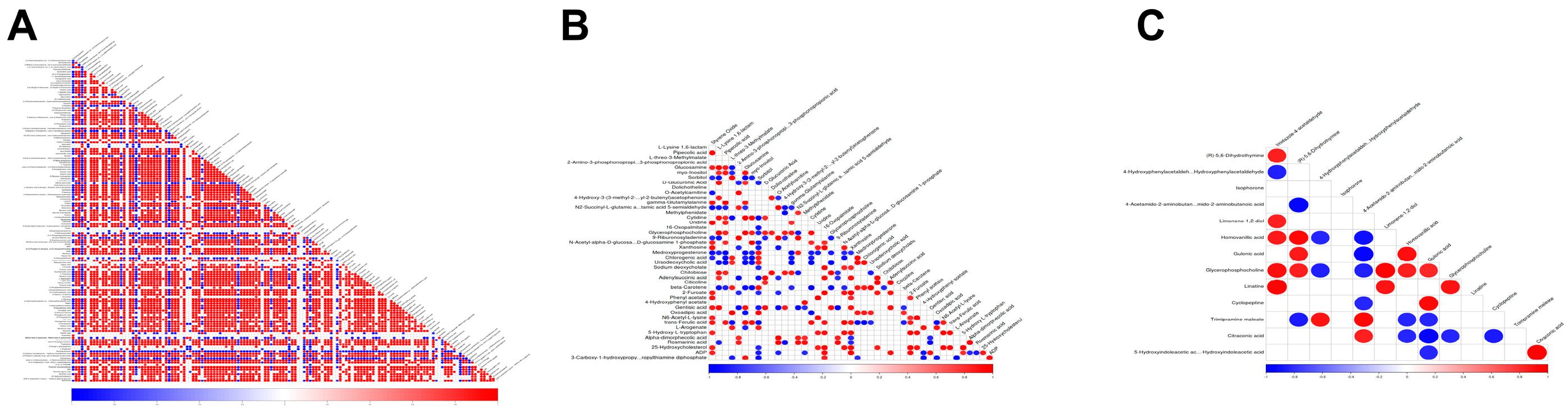
3.5. The Differential Metabolites Caused by BmFAMeT6
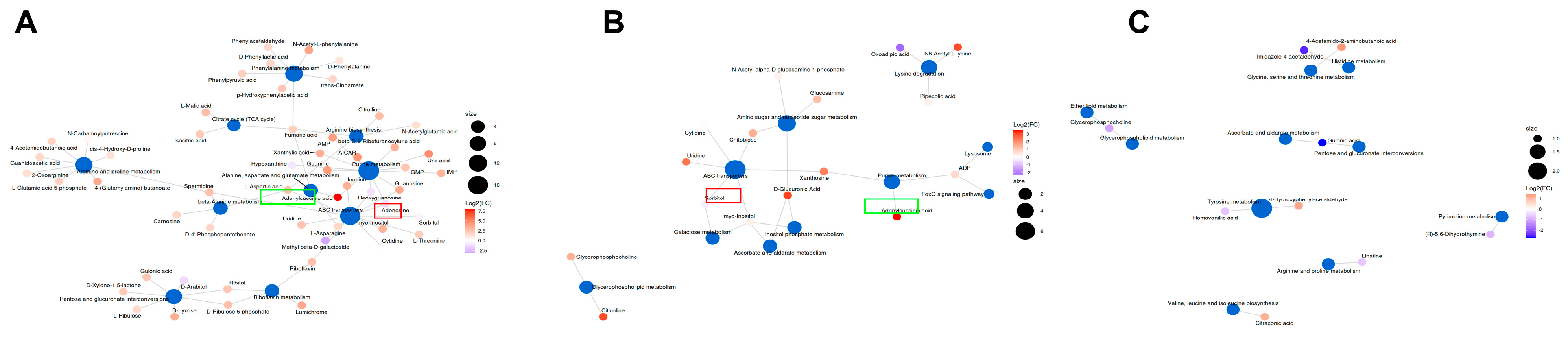
3.6. The Main Metabolism Pathway and the Same Differential Metabolite by Overexpression of BmFAMeT6
4. Discussion
4.1. The Regulatory Role of BmFAMeT6 Is Carried Out in the Context of Growth and Development
4.2. Differential Gene Enrichment in the Metabolic Pathway Indicates That BmFAMeT6 May Be Involved in the Balance between Growth and Detoxification Metabolism
4.3. The Zinc Finger C2H2 (zf-C2H2) Transcription Factor May Have Made an Important Contribution to the Regulation of BmFAMeT6
4.4. The BmFAMeT6 May Influence the Metabolism of Silkworm Larvae by Participating in the ABC Transporters, Purine Metabolism Pathway, and Tyrosine Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.S.; Takaki, K. Transcriptional regulation of cuticular genes during insect metamorphosis. Front. Biosci. 2020, 25, 106–117. [Google Scholar]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Furuta, K.; Ichikawa, A.; Murata, M.; Kuwano, E.; Shinoda, T.; Shiotsuki, T. Determination by LC-MS of juvenile hormone titers in hemolymph of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2013, 77, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Kotaki, T.; Shinada, T.; Kaihara, K.; Ohfune, Y.; Numata, H. Structure determination of a new juvenile hormone from a heteropteran insect. Org. Lett. 2009, 11, 5234–5237. [Google Scholar] [CrossRef]
- Sutherland, T.D.; Unnithan, G.C.; Andersen, J.F.; Evans, P.H.; Murataliev, M.B.; Szabo, L.Z.; Mash, E.A.; Bowers, W.S.; Feyereisen, R. A cytochrome P450 terpenoid hydroxylase linked to the suppression of insect juvenile hormone synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 12884–12889. [Google Scholar] [CrossRef] [PubMed]
- Helvig, C.; Koener, J.F.; Unnithan, G.C.; Feyereisen, R. CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc. Natl. Acad. Sci. USA 2004, 101, 4024–4029. [Google Scholar] [CrossRef]
- Meng, M.; Cheng, D.J.; Peng, J.; Tang, L.X.; Wang, Y.H.; Qian, W.L.; Xia, Q.Y. Identification and Expression Profiling of Farnesoic Acid O-methyltrans-ferase Gene in Silkworm, Bombyx mori. Seric. Sci. 2013, 39, 680–688. [Google Scholar]
- Zhang, L.; Li, X.Z.; Li, T.; Xiong, R.; Yan, D.S.; Chen, P. Farnesoic acid methyltransferase 6 (BmFAMeT6) interrelates with moltinism of dominant trimolter in silkworm, Bombyx mori. Biologia 2021, 76, 2231–2240. [Google Scholar] [CrossRef]
- Yu, Y.; Li, T.; Guo, M.; Xiong, R.; Yan, D.; Chen, P. Possible Regulation of Larval Juvenile Hormone Titers in Bombyx mori by BmFAMeT6. Insects 2023, 14, 644. [Google Scholar] [CrossRef]
- Xia, Q.; Zhou, Z.; Lu, C.; Cheng, D.; Dai, F.; Li, B.; Zhao, P.; Zha, X.; Cheng, T.; Chai, C.; et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar]
- Deng, T.; Li, X.; Yao, B. Metabonomic analysis of seminal plasma in necrozoospermia patients based on liquid chromatography-mass spectrometry. Transl. Androl. Urol. 2023, 12, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.M.; Zhang, J.; Tang, B.G.; Yu, F.F.; Lu, Y.S.; Hou, G.; Chen, J.Y.; Du, Z.X. Transcriptome and metabolome analyses of the immune response to light stress in the hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). Animal 2022, 16, 100448. [Google Scholar] [CrossRef] [PubMed]
- Al-Naama, N.; Mackeh, R.; Kino, T. C2H2-Type Zinc Finger Proteins in Brain Development, Neurodevelopmental, and Other Neuropsychiatric Disorders: Systematic Literature-Based Analysis. Front. Neurol. 2020, 11, 32. [Google Scholar] [CrossRef]
- Noll, M. Evolution and role of Pax genes. Curr. Opin. Genet Dev. 1993, 3, 595–605. [Google Scholar] [CrossRef]
- Pierpont, J.W.; Erickson, R.P. Facts on PAX. Am. J. Hum. Genet. 1993, 52, 451–454. [Google Scholar] [PubMed]
- Sabogal, A.; Lyubimov, A.Y.; Corn, J.E.; Berger, J.M.; Rio, D.C. THAP proteins target specific DNA sites through bipartite recognition of adjacent major and minor grooves. Nat. Struct Mol. Biol. 2010, 17, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, H.; Hao, F.; Li, N.; Liu, X.; Wang, G.; Wang, Y.; Tang, H. Developmental Changes for the Hemolymph Metabolome of Silkworm (Bombyx mori L.). J. Proteome Res. 2015, 14, 2331–2347. [Google Scholar] [CrossRef]
- Li, Y.X.; Kang, X.L.; Li, Y.L.; Wang, X.P.; Yan, Q.; Wang, J.X.; Zhao, X.F. Receptor tyrosine kinases CAD96CA and FGFR1 function as the cell membrane receptors of insect juvenile hormone. eLife 2024, 13, RP97189. [Google Scholar]
- Zhang, Z.J.; Liu, X.J.; Yu, Y.; Yang, F.Y.; Li, K. The receptor tyrosine kinase torso regulates ecdysone homeostasis to control developmental timing in Bombyx mori. Insect Sci. 2021, 28, 1582–1590. [Google Scholar] [CrossRef]
- Perrella, N.N.; Fuzita, F.J.; Moreti, R.; Verhaert, P.D.E.M.; Lopes, A.R. First characterization of fucosidases in spiders. Arch Insect Biochem. Physiol. 2018, 98, e21462. [Google Scholar] [CrossRef]
- Soya, S.; Şahar, U.; Karaçalı, S. Monosaccharide profiling of silkworm (Bombyx mori L.) nervous system during development and aging. Invert Neurosci. 2016, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Meng, X.; Zhang, N.; Ge, H.; Wei, J.; Qian, K.; Zheng, Y.; Park, Y.; Reddy Palli, S.; Wang, J. The pleiotropic AMPK-CncC signaling pathway regulates the trade-off between detoxification and reproduction. Proc. Natl. Acad. Sci. USA 2023, 120, e2214038120. [Google Scholar] [CrossRef]
- Amero, S.A.; Elgin, S.C.; Beyer, A.L. A unique zinc finger protein is associated preferentially with active ecdysone-responsive loci in Drosophila. Genes Dev. 1991, 5, 188–200. [Google Scholar] [CrossRef]
- Beermann, A.; Aranda, M.; Schröder, R. The Sp8 zinc-finger transcription factor is involved in allometric growth of the limbs in the beetle Tribolium castaneum. Development 2004, 131, 733–742. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Z.; Hu, B.; Yu, Y.; Tan, A. A CCCH zinc finger gene regulates doublesex alternative splicing and male development in Bombyx mori. Insect Sci. 2021, 28, 1253–1261. [Google Scholar] [CrossRef]
- Zhu, Z.D.; Hu, Q.H.; Tong, C.M.; Yang, H.G.; Zheng, S.C.; Feng, Q.L.; Deng, H.M. Transcriptomic analysis reveals the regulation network of BmKrüppel homolog 1 in the oocyte development of Bombyx mori. Insect Sci. 2021, 28, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.A.; He, Q.; Wen, D.; Zyaan, O.; Wang, J.; Xu, J.; Baumann, A.A.; Joseph, J.; Wilson, T.G.; Li, S.; et al. Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 2011, 41, 938–945. [Google Scholar] [CrossRef]
- He, Q.; Wen, D.; Jia, Q.; Cui, C.; Wang, J.; Palli, S.R.; Li, S. Heat shock protein 83 (Hsp83) facilitates methoprene-tolerant (Met) nuclear import to modulate juvenile hormone signaling. J. Biol. Chem. 2014, 289, 27874–27885. [Google Scholar] [CrossRef]
- Trowitzsch, S.; Tampé, R. ABC Transporters in Dynamic Macromolecular Assemblies. J. Mol. Biol. 2018, 430, 4481–4495. [Google Scholar] [CrossRef]
- Fujii, T.; Kakino, K.; Tanaka, M.; Lee, J.M.; Kusakabe, T.; Banno, Y. A defect in purine nucleotide metabolism in the silkworm, Bombyx mori, causes a translucent larval integument and male infertility. Insect Biochem. Mol. Biol. 2020, 126, 103458. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Tanaka, Y.; Iwata, K.; Rubio, R.O.; Yaginuma, T.; Yamashita, O.; Shiomi, K. ERK/MAPK regulates ecdysteroid and sorbitol metabolism for embryonic diapause termination in the silkworm, Bombyx mori. J. Insect Physiol. 2006, 52, 569–575. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Li, T.; Chen, P. Transcriptome and Metabolome Analysis of BmFAMeT6 Overexpression in Bombyx mori. Genes 2024, 15, 1261. https://doi.org/10.3390/genes15101261
Yu Y, Li T, Chen P. Transcriptome and Metabolome Analysis of BmFAMeT6 Overexpression in Bombyx mori. Genes. 2024; 15(10):1261. https://doi.org/10.3390/genes15101261
Chicago/Turabian StyleYu, Yang, Tian Li, and Ping Chen. 2024. "Transcriptome and Metabolome Analysis of BmFAMeT6 Overexpression in Bombyx mori" Genes 15, no. 10: 1261. https://doi.org/10.3390/genes15101261





