Next-Generation Sequencing to Determine Changes in the Intestinal Microbiome of Juvenile Sturgeon Hybrid (Acipenser gueldenstaedtii♀ × Acipenser baerii♂) Resulting from Sodium Butyrate, Β-Glucan and Vitamin Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Origin of the Experimental Fish
2.2. Experimental Feed
2.3. Feeding Experiment
2.4. Periodic Measurement of Fish and Sampling
2.5. DNA Isolation, NGS Sequencing and Bioinformatic Analysis
2.6. Analysis of Cortisol Concentration and Lysozyme Activity in the Blood Plasma
2.7. Statistical Analysis
3. Results
3.1. Breeding Indicators
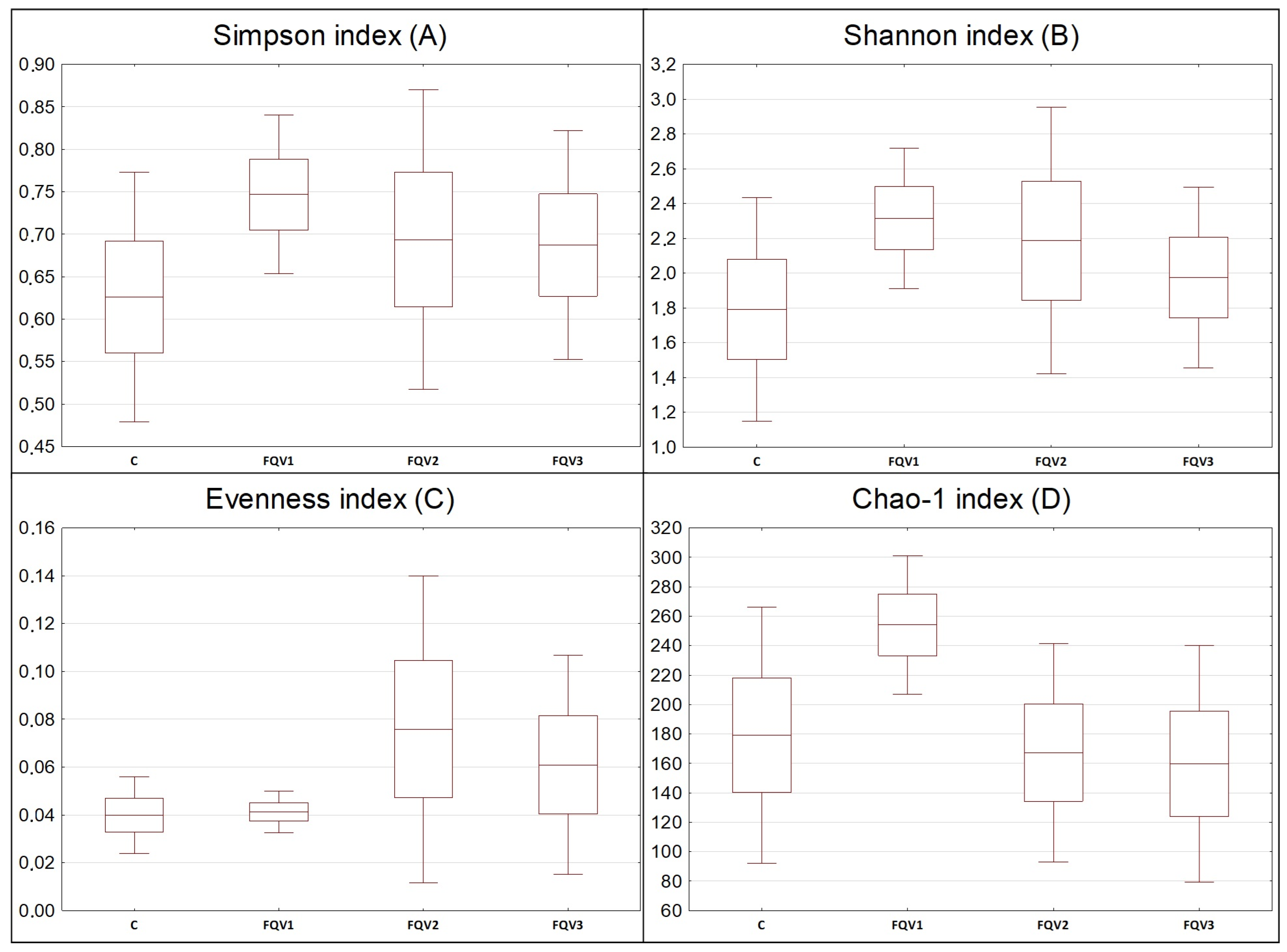
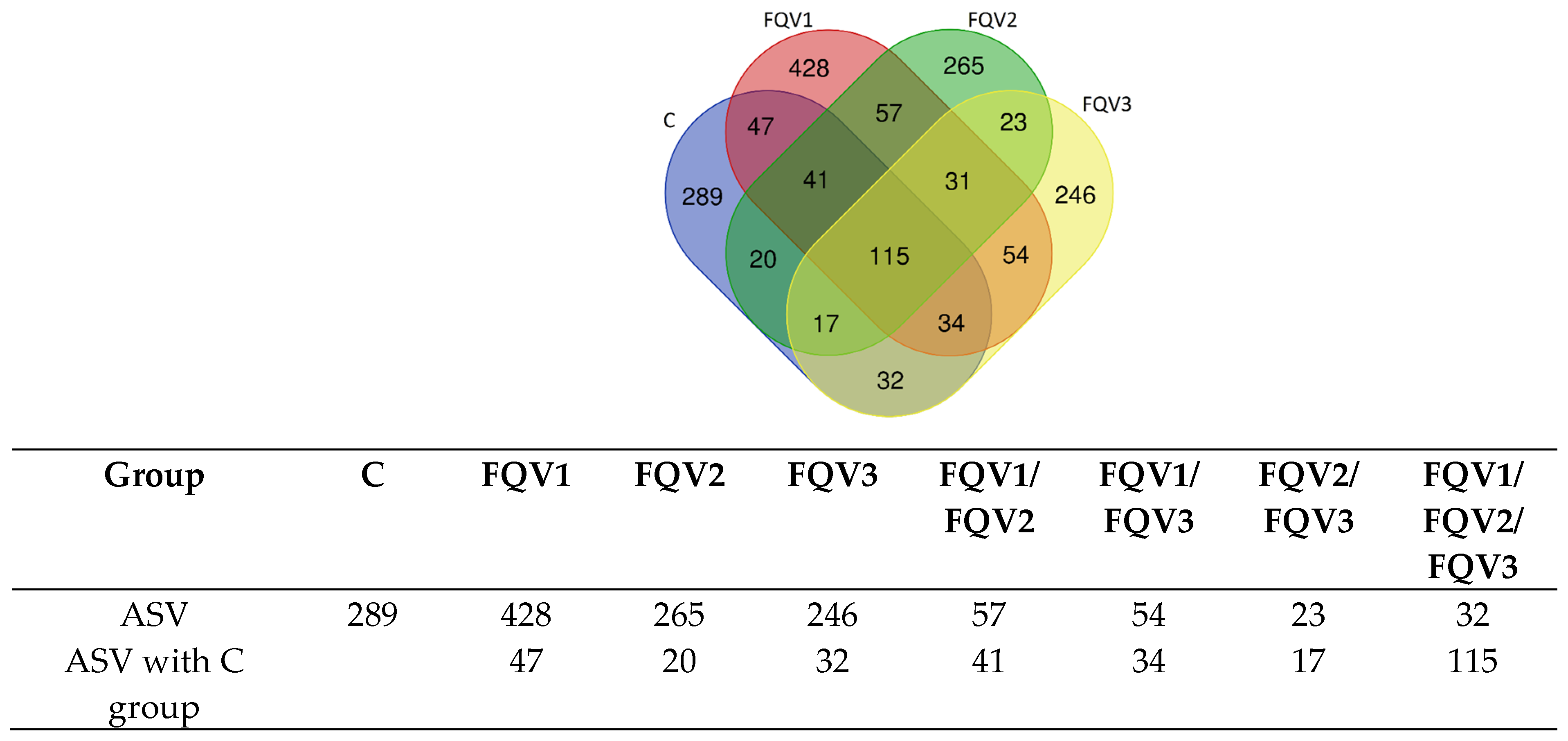
3.2. Sequencing Data and Diversity of Gut Microbiome
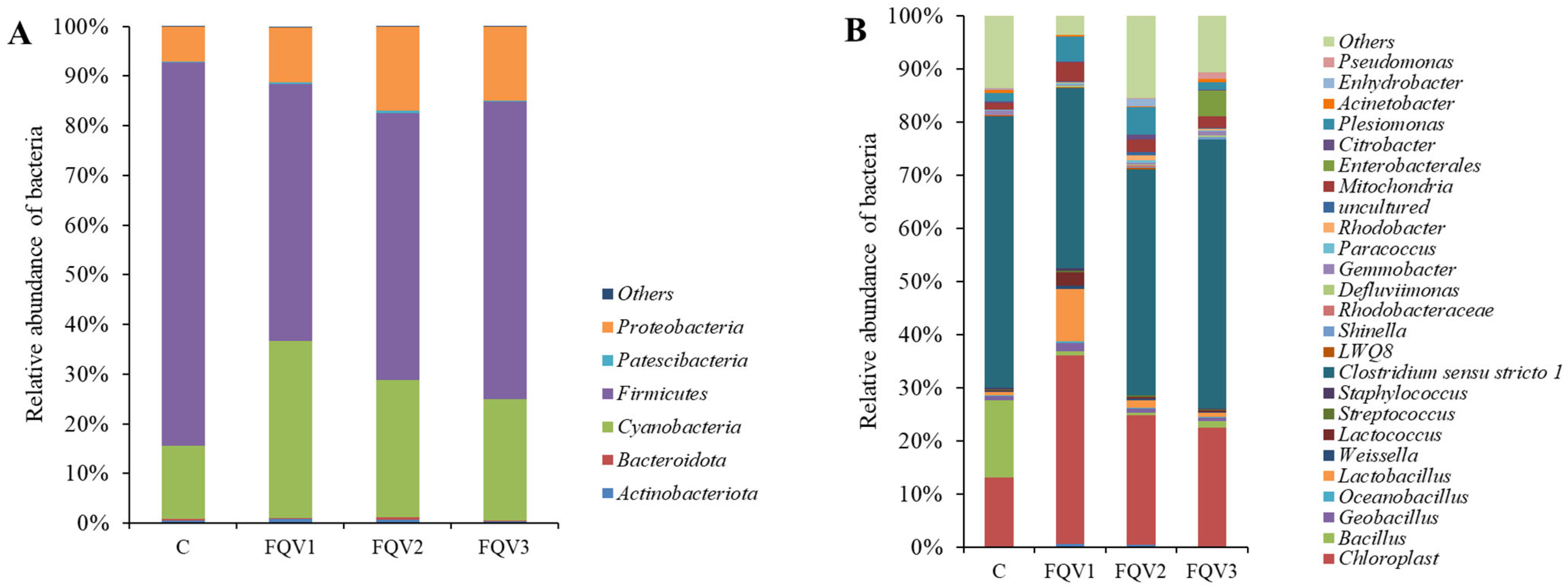
3.3. Profile of Gut Microbiome
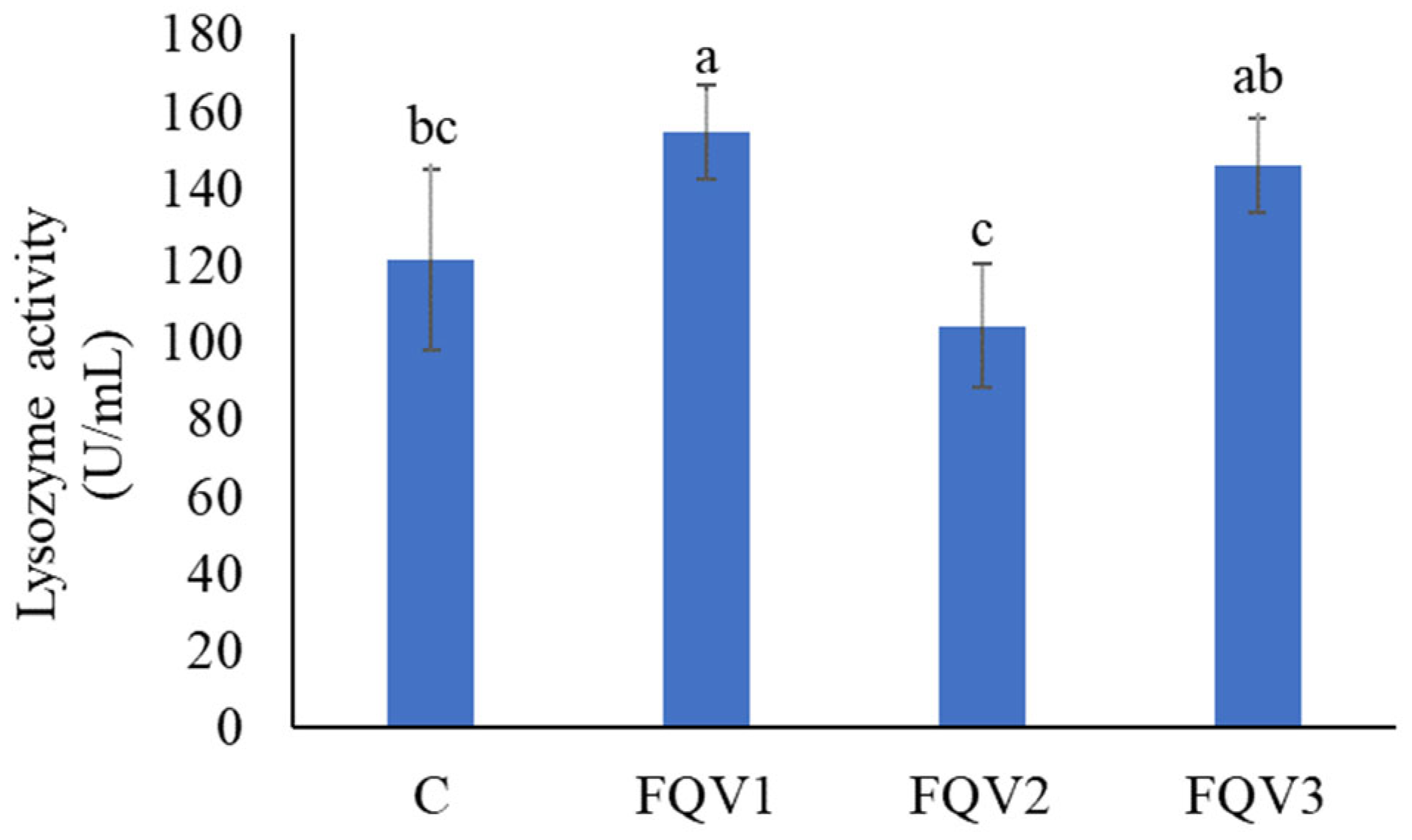
3.4. Cortisol Concentration and Lysozyme Activity in Blood Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stakenas, S.; Pilinkovskij, A. Migration patterns and survival of stocked Atlantic sturgeon (Acipenser oxyrinchus Mitchill, 1815) in nemunas basin, Baltic Sea. J. Appl. Ichthyol. 2019, 35, 128–137. [Google Scholar] [CrossRef]
- EUMOFA. Sturgeon Meat and Other by-Products of Caviar. Production, Trade, and Consumption In and Outside the EU; Maritime Affairs and Fisheries: Brussel, Belgium, 2023. [Google Scholar]
- Havelka, M.; Arai, K. Hybridization and Polyploidization in Sturgeon. In Sex Control in Aquaculture, 1st ed.; Wang, H.P., Piferrer, F., Chen, S.L., Shen, Z.G., Eds.; Wiley: Oxford, UK, 2018; Volume 1, pp. 669–687. [Google Scholar] [CrossRef]
- Shivaramu, S.; Vuong, D.T.; Havelka, M.; Šachlová, H.; Lebeda, I.; Kašpar, V.; Flajšhans, M. Influence of interspecific hybridization on fitness-related traits in Siberian sturgeon and Russian sturgeon. Czech J. Anim. Sci. 2019, 64, 78–88. [Google Scholar] [CrossRef]
- Lee, S.; Zhao, H.; Li, Y.; Binkowski, F.; Deng, D.-F.; Shepherd, B.; Hung, S.; Bai, S. Evaluation of formulated feed for juvenile lake sturgeon (Acipenser fulvescens) based on growth performance and nutrient retention. N. Am. J. Aquac. 2018, 80, 223–236. [Google Scholar] [CrossRef]
- Yamamoto, F.Y.; Castillo, S.; de Cruz, C.R.; Chen, K.; Hume, M.E.; Gatlin, D.M. Synergistic effects of the β-1,3 glucan paramylon and vitamin C on immunological responses of hybrid striped bass (Morone chrysops × M. saxatilis) were pronounced in vitro but more moderate in vivo. Aquaculture 2020, 526, 735394. [Google Scholar] [CrossRef]
- Hussain, S.M.; Jamil, M.; Ali, S.; Shahzad, M.M.; Hussain, M.; Ahmad, N.; Riaz, D. Gene expression, growth performance and body composition of Labeo rohita fingerlings fed on polyphenols supplement. Sains Malays. 2023, 52, 3357–3370. [Google Scholar] [CrossRef]
- Mathiessen, H.; Jaafar, R.M.; Al-Jubury, A.; von Gersdorff Jørgensen, L.; Kania, P.W.; Buchmann, K. Comparative In Vitro and In Vivo Effects of Feed Additives on Rainbow Trout Response to Ichthyophthirius multifiliis. N. Am. J. Aquac. 2021, 83, 67–77. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Alagawany, M.; Faggio, C.; Nowosad, J.; Kucharczyk, D. Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish Shellfish Immunol. 2022, 122, 115–130. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.-Z.; Caipang, C.M. Short-chain fatty acids as feed supplements for sustainable aquaculture: An updated view. Aquac. Res. 2017, 48, 1380–1391. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Metwally, A.E.-S.; El-Sharawy, M.E.; Atta, A.M.; Elbialy, Z.I.; Abdel-Latif, H.M.R.; Paray, B.A. The role of β-glucan in the growth, intestinal morphometry, and immune-related gene and heat shock protein expressions of Nile tilapia (Oreochromis niloticus) under different stocking densities. Aquaculture 2020, 523, 735205. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Shahin, M.G. Synbiotic Effects of Aspergillus oryzae and β-Glucan on Growth and Oxidative and Immune Responses of Nile Tilapia, Oreochromis niloticus. Probiotics Antimicrob. Proteins 2020, 12, 172–183. [Google Scholar] [CrossRef]
- Lopes, L.M.F.; Marinho de Mello, M.M.; Urbinati, E.C. β-Glucan reduces cortisol plasma levels, enhances innate immune system after A. hydrophila inoculation, and has lipolytic effects on the pacu (Piaractus mesopotamicus). Aquaculture 2022, 546, 737411. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Angulo, M.; Sanchez, V.; Guluarte, C.; Angulo, C. β-D-glucan from marine yeast Debaryomyces hansenii BCS004 enhanced intestinal health and glucan-expressed receptor genes in Pacific red snapper Lutjanus peru. Microb. Pathog. 2020, 143, 104141. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, L.; Xie, Q.; Tan, P. Effects of dietary sodium butyrate on growth, diet conversion, body chemical compositions and distal intestinal health in yellow drum (Nibea albiflora, Richardson). Aquac. Res. 2020, 51, 69–79. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, L.; Sun, R.; Zhou, H.; Xu, W.; Zhang, W.; Mai, K. Effects of dietary citric acid on growth performance, mineral status and intestinal digestive enzyme activities of large yellow croaker Larimichthys crocea (Richardson, 1846) fed high plant protein diets. Aquaculture 2016, 453, 147–153. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.; Wassef, E.; El-Bermawy, N.; Abdel-Meguid, N.; Saleh, N.; Barakat, K.; Shaltout, O. Advantageous effects of dietary butyrate on growth, immunity response, intestinal microbiota and histomorphology of European Seabass (Dicentrarchus labrax) fry. Egypt. J. Aquat. Biol. Fish. 2018, 22, 93–110. [Google Scholar] [CrossRef]
- Rimoldi, S.; Gliozheni, E.; Ascione, C.; Gini, E.; Terova, G. Effect of a specific composition of short-and medium-chain fatty acid 1-monoglycerides on growth performances and gut microbiota of gilthead sea bream (Sparus aurata). PeerJ 2018, 31, 5355. [Google Scholar] [CrossRef]
- Johny, T.K.; Puthusseri, R.M.; Bhat, S.G. A primer on metagenomics and next-generation sequencing in fish gut microbiome research. Aquac. Res. 2021, 52, 4574–4600. [Google Scholar] [CrossRef]
- Gallo, B.D.; Farrell, J.M.; Leydet, B. Use of next generation sequencing to compare simple habitat and species level differences in the gut microbiota of an invasive and native freshwater fish species. PeerJ 2020, 8, 10237. [Google Scholar] [CrossRef]
- Rimoldi, S.; Terova, G.; Ascione, C.; Giannico, R.; Brambilla, F. Next generation sequencing for gut microbiome characterization in rainbow trout (Oncorhynchus mykiss) fed animal by-product meals as an alternative to fishmeal protein sources. PLoS ONE 2018, 13, 0193652. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; Boutin, S.; Hoseinifar, S.H.; Derome, N. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 2014, 5, 207. [Google Scholar] [CrossRef]
- Diwan, A.D.; Harke, S.N.; Gopalkrishna; Panche, A.N. Aquaculture industry prospective from gut microbiome of fish and shellfish: An overview. J. Anim. Physiol. Anim. Nutr. 2022, 106, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Tarnecki, A.M.; Burgos, F.A.; Ray, C.L.; Arias, C.R. Fish intestinal microbiome: Diversity and symbiosis unraveled by metagenomics. J. Appl. Microbiol. 2017, 123, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Fopp-Bayat, D.; Ciemniewski, T.; Cejko, B.I. Embryonic Development and Survival of Siberian Sturgeon × Russian Sturgeon (Acipenser baerii × Acipenser gueldenstaedtii) Hybrids Cultured in a RAS System. Animals 2023, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, J.; Jasiński, S.; Arciuch-Rutkowska, M.; Abdel-Latif, H.M.R.; Wróbel, M.; Mikiewicz, M.; Zielonka, Ł.; Kotsyumbas, I.Y.; Muzyka, V.P.; Brezvyn, O.M.; et al. Effects of Bee Pollen on Growth Performance, Intestinal Microbiota and Histomorphometry in African Catfish. Animals 2023, 13, 132. [Google Scholar] [CrossRef]
- Arciuch-Rutkowska, M.; Nowosad, J.; Jasiński, S.; Łuczyński, M.; Kucharczyk, D. Effects of the diet enrichment with β-glucan, sodium salt of butyric acid and vitamins on growth parameters and the profile of the gut microbiome of juvenile African catfish (Clarias gariepinus). Anim. Feed Sci. Technol. 2024, 310, 115941. [Google Scholar] [CrossRef]
- Sikora, M.; Nowosad, J.; Biegaj, M.; Kucharczyk, D.; Dębowski, M. The possibility of application of agglomerate elastomers (EPP) as media for biological bed in aquaculture. Aquac. Res. 2018, 49, 2988–2994. [Google Scholar] [CrossRef]
- Suratip, N.; Charoenwattanasak, S.; Klahan, R.; Herault, M.; Yuangsoi, B. An investigation into the effects of using protein hydrolysate in low fish meal diets on growth performance, feed utilization and health status of snakehead fish (Channa striata) fingerling. Aquac. Rep. 2023, 30, 101623. [Google Scholar] [CrossRef]
- El-Asely, A.M.; Abbass, A.A.; Austin, B. Honey bee pollen improves growth, immunity and protection of Nile tilapia (Oreochromis niloticus) against infection with Aeromonas hydrophila. Fish Shellfish Immunol. 2014, 40, 500–506. [Google Scholar] [CrossRef]
- Riaz, D.; Hussain, S.M.; Hussain, M.; Muhammad Zubair-ul-Hassan Arsalan, M.Z.-H.; Naeem, E. Probiotics Supplementation for Improving Growth Performance, Nutrient Digestibility and Hematology of Catla catla Fingerlings Fed Sunflower Meal-Based Diet. Pak. J. Zool. 2024, 56, 1369–1377. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Dulski, T.; Kozłowski, K.; Ciesielski, S. Habitat and seasonality shape the structure of tench (Tinca tinca L.) gut microbiome. Sci. Rep. 2020, 10, 4460. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Sasada, R.; Weinstein, M.; Prem, A.; Jin, M.; Bhasin, J. FIGARO: An efficient and objective tool for optimizing microbiome rRNA gene trimming parameters. J. Biomol. Tech. 2020, 31, S2. [Google Scholar]
- Novriadi, R.; Fadhilah, R.; Wahyudi, A.E.; Trullàs, C. Effects of hydrolysable tannins on the growth performance, total haemocyte counts and lysozyme activity of pacific white leg shrimp Litopenaeus vannamei. Aquac. Rep. 2021, 21, 100796. [Google Scholar] [CrossRef]
- Luan, Y.; Li, M.; Zhou, W.; Yao, Y.; Yang, Y.; Zhang, Z.; Ringø, E.; Olsen, R.E.; Clarke, J.L.; Xie, S.; et al. The Fish Microbiota: Research Progress and Potential Applications. Engineering 2023, 29, 137–146. [Google Scholar] [CrossRef]
- Wah-Suárez, M.I.; Vázquez, M.A.M.; Bosques-Padilla, F.J. Inflammatory bowel disease: The role of commensal microbiome in immune regulation. Gastroenterol. Hepatol. (Engl. Ed.) 2022, 45, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, X.; Liu, D.; Wei, Y.; Li, P.; Hou, H.; Yao, J.; Chen, A.; Liang, Y.; Zhou, Z.; et al. A microbiome abundant environment remodels the intestinal microbiota and improves resistance to obesity induced by chlorpyrifos in mice. Environ. Pollut. 2022, 315, 120415. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; de Wouters, T.; Walter, J.; Clavel, T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022, 15, 1095–1113. [Google Scholar] [CrossRef]
- Arciuch-Rutkowska, M.; Nowosad, J.; Gil, Ł.; Czarnik, U.; Kucharczyk, D. Synergistic Effect of Dietary Supplementation with Sodium Butyrate, β-Glucan and Vitamins on Growth Performance, Cortisol Level, Intestinal Microbiome and Expression of Immune-Related Genes in Juvenile African Catfish (Clarias gariepinus). Int. J. Mol. Sci. 2024, 25, 4619. [Google Scholar] [CrossRef]
- Singh, R.P.; Bhardwaj, A. b-glucans: A potential source for maintaining gut microbiota and the immune system. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, D.; Rasmussen, J.A.; Carøe, C.; Sveier, H.; Nordøy, K.; Gilbert, M.T.P.; Limborg, M.T. Salmon gut microbiota correlates with disease infection status: Potential for monitoring health in farmed animals. Anim. Microbiome 2021, 3, 30. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Hu, C.; Niu, X.; Chen, S.; Wen, J.; Bao, M.; Mohyuddin, S.G.; Yong, Y.; Liu, X.; Wu, L.; Yu, Z.; et al. A Comprehensive Analysis of the Colonic Flora Diversity, Short Chain Fatty Acid Metabolism, Transcripts, and Biochemical Indexes in Heat-Stressed Pigs. Front. Immunol. 2021, 12, 717723. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, X.-Q.; Jiang, W.-D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Sodium butyrate improved intestinal immune function associated with NF-jB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 66, 548–563. [Google Scholar] [CrossRef]
- Carballo, C.; Pinto, P.I.S.; Mateus, A.P.; Berbel, C.; Guerreiro, C.C.; Martinez-Blanch, J.F.; Codoñer, F.M.; Mantecon, L.; Power, D.M.; Manchado, M. Yeast β-glucans and microalgal extracts modulate the immune response and gut microbiome in Senegalese sole (Solea senegalensis). Fish Shellfish Immunol. 2019, 92, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.; Sharifinia, M.; Ghaedi, G. β-glucan as a promising food additive and immunostimulant in aquaculture industry. Ann. Anim. Sci. 2022, 22, 817–827. [Google Scholar] [CrossRef]
- Ai, Q.; Mai, K.; Zhang, L.; Tan, B.; Zhang, W.; Xu, W.; Li, H. Effects of dietary β-1, 3 glucan on innate immune response of large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol. 2007, 22, 394–402. [Google Scholar] [CrossRef]
- Misra, C.K.; Das, B.K.; Mukherjee, S.C.; Pattnaik, P. Effect of Multiple Injections of β-Glucan on Non-Specific Immune Response and Disease Resistance in Labeo rohita Fingerlings. Fish Shellfish Immunol. 2006, 20, 305–319. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Moss, A.S.; Dossou, S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Marinho de Mello, M.M.; de Fátima Pereira de Faria, C.; Zanuzzo, F.S.; Urbinati, E.C. β-glucan modulates cortisol levels in stressed pacu (Piaractus mesopotamicus) inoculated with heat-killed Aeromonas hydrophila. Fish Shellfish Immunol. 2019, 93, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Abdel-Razik, N.I.; Gewaily, M.S.; Sewilam, H.; Paray, B.A.; Soliman, A.A.; Abdelhiee, E.Y.; Aboubakr, M.; Doan, H.V.; El-Sabagh, M.; et al. β-Glucan improved the immunity, hepato-renal, and histopathology disorders induced by chlorpyrifos in Nile tilapia. Aquac. Rep. 2020, 18, 100549. [Google Scholar] [CrossRef]
- Voloski, A.P.; de Figueiredo Soveral, L.; Dazzi, C.C.; Sutili, F.; Frandoloso, R.; Kreutz, L.C. β-Glucan improves wound healing in silver catfish (Rhamdia quelen). Fish Shellfish Immunol. 2019, 93, 575–579. [Google Scholar] [CrossRef]
- El-Sharkawy, E.A.; El-Razek, I.M.A.; Amer, A.A.; Soliman, A.A.; Shukry, M.; Gewaily, M.S.; Téllez-Isaías, G.; Kari, Z.A.; Dawood, M.A.O. Effects of sodium butyrate on the growth performance, digestive enzyme activity, intestinal health, and immune responses of Thinlip Grey Mullet (Liza ramada) juveniles. Aquac. Rep. 2023, 30, 101530. [Google Scholar] [CrossRef]
- Chen, W.; Gao, S.; Chang, K.; Zhao, X.; Niu, B. Dietary sodium butyrate supplementation improves fish growth, intestinal microbiota composition, and liver health in largemouth bass (Micropterus salmoides) fed high-fat diets. Aquaculture 2023, 564, 739040. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Q.; Guo, X.; Pan, X.; Li, X. Effects of dietary sodium butyrate on growth performance, antioxidant capacity, intestinal histomorphology and immune response in juvenile Pengze crucian carp (Carassius auratus Pengze). Aquac. Rep. 2021, 21, 100828. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Effect of dietary sodium butyrate on growth performance, enzyme activities and intestinal proliferation-related gene expression of juvenile golden pompano Trachinotus ovatus. Aquac. Nutr. 2019, 25, 1261–1271. [Google Scholar] [CrossRef]
- Luz, J.; Ramos, A.P.; Melo, J.F.; Braga, L.G. Use of sodium butyrate in the feeding of Arapaima gigas (Schinz, 1822) juvenile. Aquaculture 2019, 510, 248–255. [Google Scholar] [CrossRef]
- Aramli, M.S.; Kamangar, B.; Nazari, R.M. Effects of dietaryβ-glucan on the growth and innate immune response of juvenile Persian sturgeon, Acipenser persicus. Fish Shellfish Immunol. 2015, 47, 606–610. [Google Scholar] [CrossRef]
- Do Huu, H.; Sang, M.H.; Thanh Thuy, N.T. Dietary β-glucan improved growth performance, Vibrio counts, haematological parameters and stress resistance of pompano fish, Trachinotus ovatus Linnaeus, 1758. Fish Shellfish Immunol. 2016, 54, 402–410. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Ghaedi, G.; Sharifinia, M. Effects of diets containing β-glucan on survival, growth performance, haematological, immunity and biochemical parameters of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquac. Res. 2021, 53, 1842–1850. [Google Scholar] [CrossRef]
- Dou, X.; Huang, H.; Li, Y.; Deng, J.; Tan, B. Effects of dietary β-glucan on growth rate, antioxidant status, immune response, and resistance against Aeromonas hydrophila in genetic improvement of farmed tilapia (GIFT, Oreochromis niloticus). Aquac. Rep. 2023, 29, 101480. [Google Scholar] [CrossRef]
- Sealey, W.M.; Barrows, F.T.; Hang, A.; Johansen, K.A.; Overturf, K.; LaPatra, S.E.; Hardy, R.W. Evaluation of the ability of barley genotypes containing different amounts of β-glucan to alter growth and disease resistance of rainbow trout Oncorhynchus mykiss. Anim. Feed Sci. Technol. 2008, 141, 115–128. [Google Scholar] [CrossRef]
- Jami, M.J.; Kenari, A.A.; Paknejad, H.; Mohseni, M. Effects of dietary b-glucan, mannan oligosaccharide, Lactobacillus plantarum and their combinations on growth performance, immunity and immune related gene expression of Caspian trout, Salmo trutta caspius (Kessler, 1877). Fish Shellfish Immunol. 2019, 91, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.M.; Shah, T.M.; Reddy, B.; Deshpande, S.; Rank, D.N.; Joshi, C.G. Taxonomic and gene-centric metagenomics of the fecal microbiome of low and high feed conversion ratio (FCR) broilers. J. Appl. Genet. 2014, 55, 145–154. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Tiezzi, F.; Howard, J.; Huang, Y.J.; Gray, K.A.; Schillebeeckx, C.; McNulty, N.P.; Maltecca, C. Gut microbiome composition differences among breeds impact feed efficiency in swine. Microbiome 2020, 8, 110. [Google Scholar] [CrossRef]
- Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Y.; Zhang, J.; Gatlin, D.M.; Ringø, E.; Zhou, Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr. 2014, 112, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. Benefits of Dietary Butyric Acid, Sodium Butyrate, and Their Protected Forms in Aquafeeds: A Review. Rev. Fish. Sci. Aquac. 2020, 28, 421–448. [Google Scholar] [CrossRef]
- Ng, W.-K.; Koh, C.-B. The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev. Aquac. 2017, 9, 342–368. [Google Scholar] [CrossRef]
- Ahsan, U.; Cengiz, Ö.; Raza, I.; Kuter, E.; Chacher, M.F.A.; Iqbal, Z.; Umar, S.; Çakir, S. Sodium butyrate in chicken nutrition: The dynamics of performance, gut microbiota, gut morphology, and immunity. World’s Poult. Sci. J. 2016, 72, 265–275. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Gooneratne, R.; Lai, R.; Zeng, C.; Zhan, F.; Wang, W. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Sci. Rep. 2016, 6, 24340. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 6, 101–110. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Shan, C.; Li, M.; Liu, Z.; Xu, R.; Qiao, F.; Du, Z.-Y.; Zhang, M.-L. Pediococcus pentosaceus enhances host resistance against pathogen by increasing IL-1β production: Understanding probiotic effectiveness and administration duration. Front. Immunol. 2021, 12, 766401. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Cheng, C.-H.; Gua, W.-R.; Guu, Y.-K.; Cheng, W. Dietary administration of the probiotic, Saccharomyces cerevisiae P13, enhanced the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol. 2010, 29, 1053–1059. [Google Scholar] [CrossRef]
- Fu, W.; Duan, P.; Wang, Q.; Song, J.; Wang, Y.; Zhang, Z.; Wang, P.; Jiang, H.; Zhang, X.; Song, G.; et al. Effect of Pseudomonas stutzeri F2 on rearing water quality and growth, innate immunity, visceral morphology and gut microbiota structure of juvenile spotted seabass (Lateolabrax maculatus). Aquac. Rep. 2023, 30, 101536. [Google Scholar] [CrossRef]
- Say, P.; Nimitkul, S.; Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Effects of the combination of chitosan and Acinetobacter KU011TH on the growth and health performances and disease resistance of juvenile hybrid catfish (Clarias gariepinus × C. macrocephalus). Fish Shellfish Immunol. 2023, 142, 109177. [Google Scholar] [CrossRef]


| Active Ingredients * | Groups | |||
|---|---|---|---|---|
| C | FQV1 | FQV2 | FQV3 | |
| sodium butyrate (mg/kg) | Not included | 50 | 150 | 50 |
| β-glucan (mg/kg) | Not included | 20 | 20 | 60 |
| vitamin C (mg/kg) | Not included | 30 | 30 | 30 |
| vitamin E (mg/kg) | Not included | 10 | 10 | 10 |
| vitamin K (mg/kg) | Not included | 0.4 | 0.4 | 0.4 |
| vitamin A (IU/kg) | 7500 | 8700 | 8700 | 8700 |
| vitamin D3 (IU/kg) | 1125 | 1925 | 1925 | 1925 |
| Breeding Indicators | Groups | |||
|---|---|---|---|---|
| C | FQV1 | FQV2 | FQV3 | |
| IW (g) | 8.56 ± 1.50 | 8.56 ± 1.50 | 8.56 ± 1.50 | 8.56 ± 1.50 |
| IL (cm) | 12.50 ± 0.68 | 12.50 ± 0.68 | 12.50 ± 0.68 | 12.50 ± 0.68 |
| FW (g) | 49.63 ± 7.53 b | 51.68 ± 5.23 ab | 54.14 ± 11.16 a | 47.20 ± 8.27 b |
| FL (cm) | 22.37 ± 1.15 ab | 22.55 ± 0.95 ab | 22.91 ± 1.35 a | 22.05 ± 1.04 b |
| WG (g) | 40.62 ± 5.64 b | 44.04 ± 4.67 ab | 47.24 ± 10.54 a | 39.66 ± 8.68 b |
| LG (cm) | 9.89 ± 0.95 ab | 10.21 ± 0.88 ab | 10.57 ± 1.31 a | 9.76 ± 0.98 b |
| SGR (%/day) | 4.56 ± 0.52 ab | 4.71 ± 0.34 ab | 4.82 ± 0.67 a | 4.38 ± 0.57 b |
| K (K/fish) | 0.44 ± 0.03 | 0.45 ± 0.03 | 0.45 ± 0.02 | 0.43 ± 0.05 |
| SR (%) | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 97.2 ± 4.8 |
| Group | Counts | Observations |
|---|---|---|
| C | 94,505.20 ± 17,161.98 | 179.40 ± 87.45 |
| FQV1 | 105,357.20 ± 12,016.35 | 254.80 ± 46.91 |
| FQV2 | 107,145.60 ± 37,173.80 | 168.40 ± 73.19 |
| FQV3 | 94,608.00 ± 27,899.12 | 170.60 ± 66.45 |
| Total number of counts taxonomically classified | 2,008,080.00 | |
| Mean number of counts per sample | 100,404.00 ± 23,546.31 | |
| Total number of ASV | 1785.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arciuch-Rutkowska, M.; Nowosad, J.; Łuczyński, M.K.; Hussain, S.M.; Kucharczyk, D. Next-Generation Sequencing to Determine Changes in the Intestinal Microbiome of Juvenile Sturgeon Hybrid (Acipenser gueldenstaedtii♀ × Acipenser baerii♂) Resulting from Sodium Butyrate, Β-Glucan and Vitamin Supplementation. Genes 2024, 15, 1276. https://doi.org/10.3390/genes15101276
Arciuch-Rutkowska M, Nowosad J, Łuczyński MK, Hussain SM, Kucharczyk D. Next-Generation Sequencing to Determine Changes in the Intestinal Microbiome of Juvenile Sturgeon Hybrid (Acipenser gueldenstaedtii♀ × Acipenser baerii♂) Resulting from Sodium Butyrate, Β-Glucan and Vitamin Supplementation. Genes. 2024; 15(10):1276. https://doi.org/10.3390/genes15101276
Chicago/Turabian StyleArciuch-Rutkowska, Martyna, Joanna Nowosad, Michał Krzysztof Łuczyński, Syed Makhdoom Hussain, and Dariusz Kucharczyk. 2024. "Next-Generation Sequencing to Determine Changes in the Intestinal Microbiome of Juvenile Sturgeon Hybrid (Acipenser gueldenstaedtii♀ × Acipenser baerii♂) Resulting from Sodium Butyrate, Β-Glucan and Vitamin Supplementation" Genes 15, no. 10: 1276. https://doi.org/10.3390/genes15101276






