Refinement and Enhancement of Agrobacterium-Mediated Transient Transformation for Functional Gene Examination in Mulberry (Morus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Formulation of Agrobacterium Strains Used in Infiltration
2.3. Optimization of Experimental Parameters
2.4. Green Fluorescent Protein (GFP) Microscopy and Quantitative Assays
2.5. Analysis of Anthocyanin Content
2.6. Statistical Analysis
3. Results
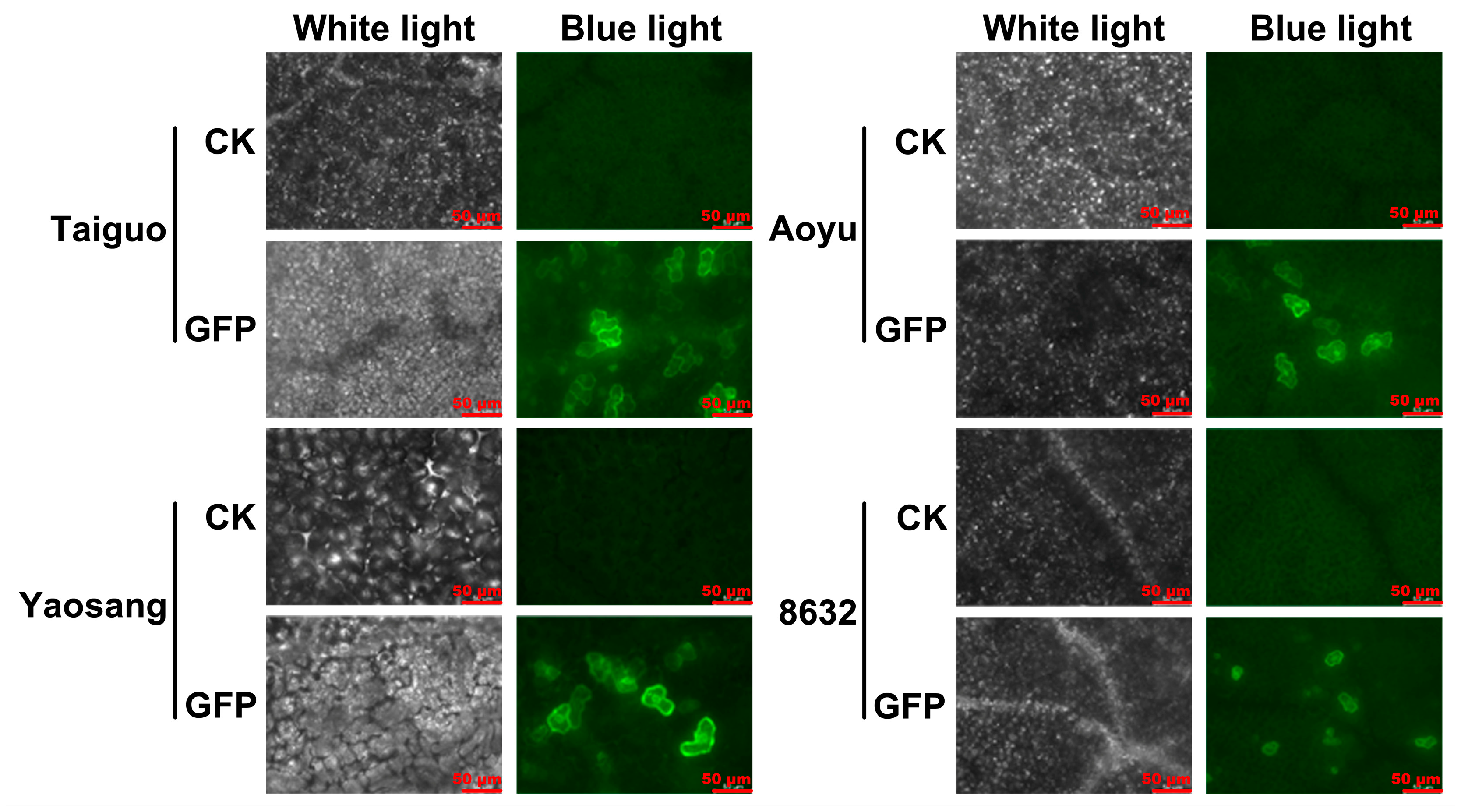
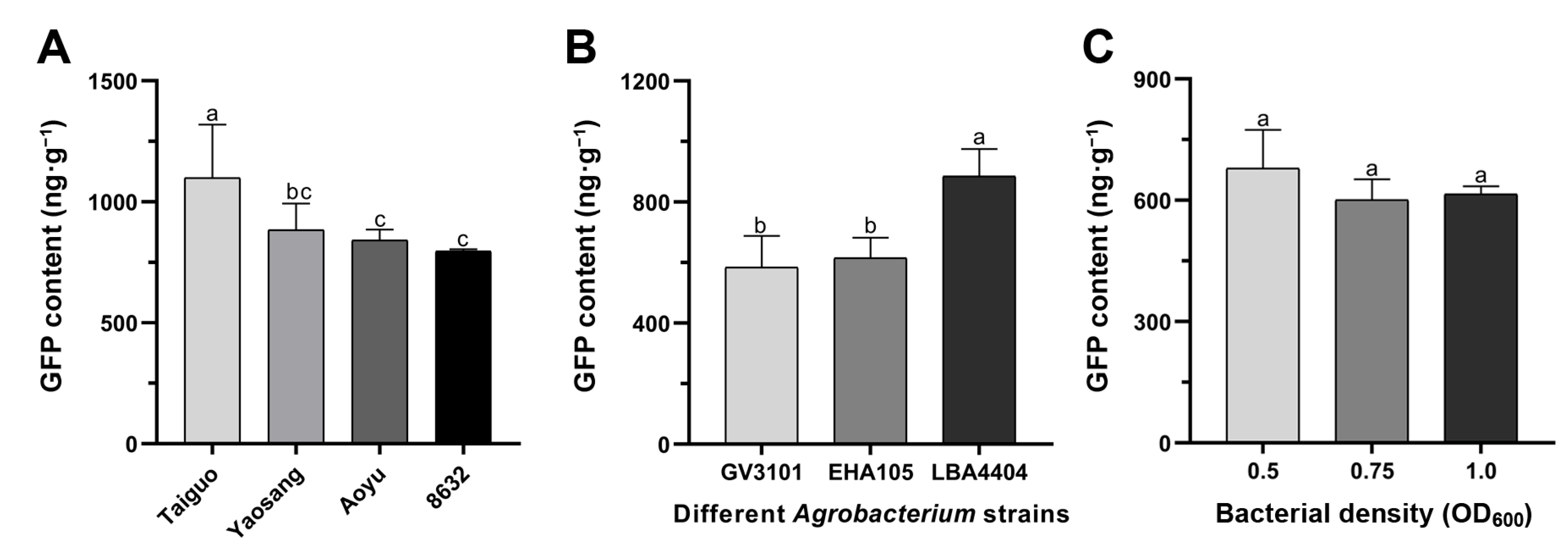
3.1. Transient GFP Expression in the Leaves of Various Mulberry Genotypes
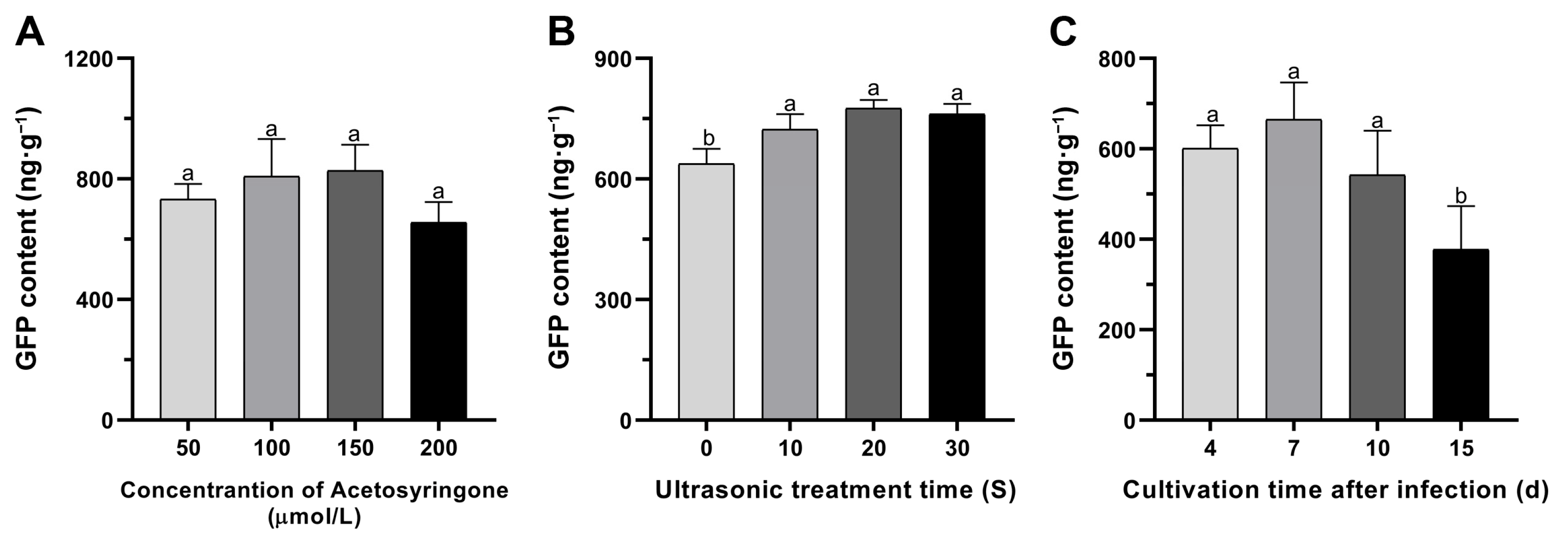
3.2. Improvement of the In Vitro Transient Transformation Systems in Mulberry Leaves
3.3. Transient Overexpression of MaANS and MaDFR in Mulberry Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vijayan, K.; Chauhan, S.; Das, N.K.; Chakraborti, S.P.; Roy, B.N. Leaf yield component combining abilities in mulberry (Morus spp.). Euphytica 1997, 98, 47–52. [Google Scholar] [CrossRef]
- Wang, D.; Dong, Z.; Zhang, Y.; Guo, K.; Guo, P.; Zhao, P.; Xia, Q. Proteomics provides insight into the interaction between mulberry and silkworm. J. Proteome Res. 2017, 16, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, K.; Doss, S.; Chakraborti, S.; Ghosh, P. Breeding for salinity resistance in mulberry (Morus spp.). Euphytica 2009, 169, 403–411. [Google Scholar] [CrossRef]
- Qin, J.; He, N.; Wang, Y.; Xiang, Z. Ecological issues of mulberry and sustainable development. J. Resour. Ecol. 2012, 3, 330–339. [Google Scholar]
- Qin, J.; He, N.; Huang, X.; Xiang, Z. Development of mulberry ecological industry and sericulture. Sci. Seric. 2010, 36, 984–989. [Google Scholar]
- Sun, X.; Liu, M.; Wang, B. The ecosystem service function analysis of Xiajin yellow river ancient mulberry trees group in Shandong province. World Agric. 2015, 11, 107–113+256. [Google Scholar]
- Yuan, B.; Zhao, L. The mulberry (Morus alba L.) fruit-a review of characteristic components and health benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Jiang, Y.; Nie, W.J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2015; Volume 1, p. 300. [Google Scholar]
- Lin, C.Y.; Lay, H.L. Characteristics of fruit growth, component analysis and antioxidant activity of mulberry (Morus spp.). Sci. Hortic. 2013, 162, 285–292. [Google Scholar] [CrossRef]
- Raman, S.T.; Ganeshan, A.K.; Chen, C.; Jin, C.; Li, S.H.; Chen, H.J.; Gui, Z.Z. In vitro and in vivo antioxidant activity of flavonoid extracted from mulberry fruit (Morus alba L.). Pharmacogn. Mag. 2016, 12, 128–133. [Google Scholar]
- Yang, X.; Yang, L.; Zheng, H. Hypolipidemic and antioxidant effects of mulberry (Moms alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.; Abbasi, A.; Fu, X.; Liu, R.; Li, C. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Funct. 2016, 7, 530–539. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Zhang, C.; Qi, X.; Zhao, S.; Tao, Y.; Yang, G.; Lee, T.; Wang, X.; Cai, Q.; Li, D.; et al. Draft genome sequence of the mulberry tree Morus notabilis. Nat. Commun. 2013, 4, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Li, D.; Tian, Y.; Zeng, Q.; He, N. Chromosome restructuring and number change during the evolution of Morus notabilis and Morus alba. Hortic. Res. 2022, 9, 30–43. [Google Scholar] [CrossRef]
- Jiao, F.; Luo, G.; Dai, X.; Liu, H.; Yu, G.; Han, S.; Lu, X.; Su, C.; Chen, Q.; Song, Q.; et al. Chromosome-level reference genome and population genomic analysis provide insights into the evolution and improvement of domesticated mulberry (Morus alba). Mol. Plant 2020, 13, 1001–1012. [Google Scholar] [CrossRef]
- Jain, M.; Bansal, J.; Rajkumar, M.S.; Sharma, N.; Khurana, J.P.; Khurana, P. Draft genome sequence of Indian mulberry (Morus indica) provides a resource for functional and translational genomics. Genomics 2022, 114, 110346. [Google Scholar] [CrossRef]
- Dai, F.; Zhuo, X.; Luo, G.; Wang, Z.; Xu, Y.; Wang, D.; Zhong, J.; Lin, S.; Chen, L.; Li, Z.; et al. Genomic Resequencing Unravels the Genetic Basis of Domestication, Expansion, and Trait Improvement in Morus Atropurpurea. Adv. Sci. 2023, 10, 2300039. [Google Scholar] [CrossRef]
- Yin, X.; Xie, X.; Xia, X.; Yu, J.; Ferguson, I.B.; Giovannoni, J.J.; Chen, K. Involvement of an ethylene response factor in chlorophyll degradation during citrus fruit degreening. Plant J. 2016, 86, 403–412. [Google Scholar] [CrossRef]
- Dai, H.; Li, W.; Han, G.; Yang, Y.; Ma, Y.; Li, H.; Zhang, Z. Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci. Hortic. 2013, 164, 202–208. [Google Scholar] [CrossRef]
- Sun, X.; Wang, P.; Jia, X.; Huo, L.; Che, R.; Ma, F. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018, 16, 545–557. [Google Scholar] [CrossRef]
- Jia, Z.; Gou, J.; Sun, Y.; Yuan, L.; Tian, Q.; Yang, X.; Pei, Y.; Luo, K. Enhanced resistance to fungal pathogens in transgenic Populus tomentosa Carr. by overexpression of an nsLTP-like antimicrobial protein gene from motherwort (Leonurus japonicus). Tree Physiol. 2010, 30, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, W.; Ran, L.; Dou, L.; Yao, S.; Hu, J.; Fan, D.; Li, C.; Luo, K. R2R3-MYB transcription factor MYB6 promotes anthocyanin and proanthocyanidin biosynthesis but inhibits secondary cell wall formation in Populus tomentosa. Plant J. 2019, 99, 733–751. [Google Scholar] [CrossRef] [PubMed]
- Machii, M. Leaf disc transformation of mulberry plant (Morus alba L.) by Agrobacterium Ti plasmid. J. Seric. Sci. Jpn. 1990, 59, 105–110. [Google Scholar]
- Das, M.; Chauhan, H.; Chhibbar, A.; Mohd, Q.; Haq, R.; Khurana, P. High-efficiency transformation and selective tolerance against biotic and abiotic stress in mulberry, Morus indica cv. K2, by constitutive and inducible expression of tobacco osmotin. Transgenic Res. 2011, 20, 231–246. [Google Scholar] [CrossRef]
- Saeed, B.; Das, M.; Khurana, P. Overexpression of β-carotene hydroxylase1 (BCH1) in Indian mulberry, Morus indica cv. K2, confers tolerance against UV, high temperature and high irradiance stress induced oxidative damage. Plant Cell Tissue Organ Cult. 2015, 120, 1003–1014. [Google Scholar] [CrossRef]
- Sajeevan, R.; Nataraja, K.; Shivashankara, K.; Pallavi, N.; Gurumurthy, D.; Shivanna, M. Expression of Arabidopsis SHN1 in indian mulberry (Morus indica L.) increases leaf surface wax content and reduces post-harvest water loss. Front. Plant Sci. 2017, 8, 418–430. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Khurana, P. Agrobacterium tumefaciens-mediated transformation of Indian mulberry, Morus indica cv. K2: A time-phased screening strategy. Plant Cell Rep. 2003, 21, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Chitra, D.; Chinthapalli, B.; Padmaja, G. Efficient Regeneration System for Genetic Transformation of Mulberry (Morus indica L. Cultivar S-36) Using in Vitro Derived Shoot Meristems. Am. J. Plant Sci. 2014, 5, 41631. [Google Scholar]
- Negi, N.; Khurana, P. A salicylic acid inducible mulberry WRKY transcription factor, MiWRKY53 is involved in plant defence response. Plant Cell Rep. 2021, 40, 2151–2171. [Google Scholar] [CrossRef] [PubMed]
- Takata, N.; Eriksson, M.E. A simple and efficient transient transformation for hybrid aspen (Populus tremula × P. tremuloides). Plant Methods 2012, 8, 30. [Google Scholar] [CrossRef]
- Mo, R.; Huang, Y.; Yang, S.; Zhang, Q.; Luo, Z. Development of Agrobacterium-mediated transient transformation in persimmon (Diospyros kaki Thunb.). Sci. Hortic. 2015, 192, 29–37. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, N.; Yang, S.; Zhang, Q.; Luo, Z. Development of Agrobacterium-mediated transient ihpRNA-induced gene silencing in persimmon (Diospyros kaki Thunb.). Hortic. Sci. Technol. 2016, 34, 314–323. [Google Scholar]
- Zottini, M.; Barizza, E.; Costa, A.; Formentin, E.; Ruberti, C.; Carimi, F.; Schiavo, F.L. Agroinfiltration of grapevine leaves for fast transient assays of gene expression and for long-term production of stable transformed cells. Plant Cell Rep. 2008, 27, 845–853. [Google Scholar] [CrossRef]
- Min, T.; Yin, X.; Shi, Y.; Luo, Z.; Yao, Y.; Grierson, D.; Ferguson, I.; Chen, K. Ethylene-responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de-astringency. J. Exp. Bot. 2012, 63, 6393–6405. [Google Scholar] [CrossRef] [PubMed]
- Urso, S.; Zottini, M.; Ruberti, C.; Schiavo, F.L.; Stanca, A.M.; Cattivelli, L.; Valè, G. An Agrobacterium tumefaciens-mediated gene silencing system for functional analysis in grapevine. Plant Cell Tissue Organ Cult. 2013, 114, 49–60. [Google Scholar] [CrossRef]
- Ji, X.; Zheng, L.; Liu, Y.; Nie, X.; Liu, S.; Wang, Y. A transient transformation system for the functional characterization of genes involved in stress response. Plant Mol. Biol. Rep. 2014, 32, 732–739. [Google Scholar] [CrossRef]
- Wu, S.; Yang, X.; Liu, L.; Jiang, T.; Wu, H.; Su, C.; Qian, Y.; Jiao, F. Agrobacterium-mediated transient MaFT expression in mulberry (Morus alba L.) leaves. Biosci. Biotech. Bioch. 2015, 79, 1266–1271. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Dominic, K.; Wang, T.; Fan, T.; Hu, F.; Wang, Y.; Zhang, L.; Li, L.; Zhao, W. Mulberry (Morus alba). MmSK gene enhances tolerance to drought stress in transgenic mulberry. Plant Physiol. Biochem. 2018, 132, 603–611. [Google Scholar]
- Wroblewski, T.; Tomczak, A.; Michelmore, R. Optimization of Agrobacterium mediated transient assays of gene expression in lettuce, tomato, and Arabidopsis. Plant Biotechnol. J. 2005, 3, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, A.; Breitler, J.C.; Siré, C.; Meynard, D.; Gantet, P.; Guiderdoni, E. An in planta, Agrobacterium-mediated transient gene expression method for inducing gene silencing in rice (Oryza sativa L.) leaves. Rice 2012, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.D.; Huang, X.H.; Wu, M.M.; Wang, Y.; Chang, Y.X.; Liu, K.; Zhang, J.; Zhang, Y.; Zhang, F.L.; Yi, L.M.; et al. A rapid, highly efficient and economical method of Agrobacterium-mediated in planta transient transformation in living onion epidermis. PLoS ONE 2014, 9, e83556. [Google Scholar] [CrossRef]
- Mo, R.; Yang, S.; Zhang, Q.; Xu, L.; Luo, Z. Vacuum infiltration enhances the Agrobacterium-mediated transient transformation for gene functional analysis in persimmon (Diospyros kaki Thunb.). Sci. Hortic. 2019, 251, 174–180. [Google Scholar] [CrossRef]
- Trick, H.N.; Finer, J.J. SAAT: Sonication-assisted Agrobacterium-mediated transformation. Transgenic Res. 1997, 6, 329–337. [Google Scholar] [CrossRef]
- Trick, H.N.; Finer, J.J. Sonication-assisted Agrobacterium-mediated transformation of soybean (Glycine max [L.] Merrill) embryogenic suspension culture tissue. Plant Cell Rep. 1998, 17, 482–488. [Google Scholar] [CrossRef] [PubMed]
- King, J.L.; Finer, J.J.; McHale, L.K. Development and optimization of agroinfiltration for soybean. Plant Cell Rep. 2015, 34, 133–140. [Google Scholar] [CrossRef]
- Rohela, G.K.; Jogam, P.; Shabnam, A.A.; Shukla, P.; Abbagani, S.; Ghosh, M.K. In vitro regeneration and assessment of genetic fidelity of acclimated plantlets by using ISSR markers in PPR-1 (Morus sp.): An economically important plant. Sci. Hortic. 2018, 241, 313–321. [Google Scholar] [CrossRef]
- Luo, Y.; Han, Y.; Wei, W.; Han, Y.; Yuan, J.; He, N. Transcriptome and metabolome analyses reveal the efficiency of in vitro regeneration by TDZ pretreatment in mulberry. Sci. Hortic. 2023, 310, 111678. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Zeng, Q.; Wang, S.; Luo, Y.; Huang, Y.; Xin, Y.; He, N. Abnormal expression of bHLH3 disrupts a flavonoid homeostasis network, causing differences in pigment composition among mulberry fruits. Hortic. Res. 2020, 7, 83. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, N.; Li, J.; Jin, Q.; Zhu, Z.; Dong, Z.; Li, Y.; Zhang, C.; Yu, C. Transcriptomic Analysis Provides Insights into Anthocyanin Accumulation in Mulberry Fruits. Horticulturae 2022, 8, 920. [Google Scholar] [CrossRef]
- Hughes, N.M.; Morley, C.B.; Smith, W.K. Coordination of anthocyanin decline and photosynthetic maturation in juvenile leaves of three deciduous tree species. New Phytol. 2007, 175, 675–685. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generations of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Vander Gheynst, J.S.; Guo, H.; Simmons, C.W. Response surface studies that elucidate the role of infiltration conditions on Agrobacterium tumefaciens-mediated transient transgene expression in harvested switchgrass (Panicum virgatum). Biomass Bioenerg. 2008, 32, 372–379. [Google Scholar]
- Simmons, C.W.; Nitin, N.; VanderGheynst, J.S. Rapid, in situ detection of Agrobacterium tumefaciens attachment to leaf tissue. Biotechnol. Progr. 2012, 28, 1321–1328. [Google Scholar] [CrossRef]
- Clapham, D.; Ekberg, I.; Eriksson, G.; Hood, E.E.; Norell, L. Within-population variation in susceptibility to Agrobacterium tumefaciens A281 in Picea abies (L.). Karst. Theor. Appl. Genet. 1990, 79, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Malik, T.; Husnain, T.; Riazuddin, S. Strain and cultivar specificity in the Agrobacterium-chickpea interaction. Plant Cell Rep. 1994, 13, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Boase, M.R.; Bradley, J.M.; Borst, N.K. An improved method for transformation of regal pelargonium (Pelargonium × domesticum Dubonnet) by Agrabacterium tumefaciens. Plant Sci. 1998, 139, 59–69. [Google Scholar] [CrossRef]
- Sreeramanan, S.; Maziah, M.; Abdullah, M.P.; Sariah, M.; Xavier, R. Transient expression of gusA and gfp gene in Agrobacterium-mediated banana transformation using single tiny meristematic bud. Asian J. Plant Sci. 2006, 5, 468–480. [Google Scholar]
- Chopra, R.; Aparna; Saini, R. Use of sonication and vacuum infiltration for Agrobacterium-mediated transformation of an Indian lentil (Lens culinaris Medik.) cultivar. Sci. Hortic. 2012, 143, 127–134. [Google Scholar] [CrossRef]
- Oliveira, M.; Febres, V.; Costa, M.; Moore, G.; Otoni, W. High-efficiency Agrobacterium-mediated transformation of citrus via sonication and vacuum infiltration. Plant Cell Rep. 2009, 28, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Wu, E.; Liu, S.; Chang, S.; Tzeng, K.; Kado, C. Characterization and host range of five tumorigenic Agrobacterium tumefaciens strains and possible application in plant transient transformation assays. Plant Pathol. 2013, 62, 1384–1397. [Google Scholar] [CrossRef]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Shuai, Q.; Chen, H.; Fan, L.; Zeng, Q.; He, N. Cloning and expression analyses of the anthocyanin biosynthetic genes in mulberry plants. Mol. Genet. Genom. 2014, 289, 783–793. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, R.; Zhang, N.; Qiu, C.; Huang, S.; Wei, W.; Zhang, C.; Liu, D.; Lin, Q. Refinement and Enhancement of Agrobacterium-Mediated Transient Transformation for Functional Gene Examination in Mulberry (Morus L.). Genes 2024, 15, 1277. https://doi.org/10.3390/genes15101277
Mo R, Zhang N, Qiu C, Huang S, Wei W, Zhang C, Liu D, Lin Q. Refinement and Enhancement of Agrobacterium-Mediated Transient Transformation for Functional Gene Examination in Mulberry (Morus L.). Genes. 2024; 15(10):1277. https://doi.org/10.3390/genes15101277
Chicago/Turabian StyleMo, Rongli, Na Zhang, Changyu Qiu, Sheng Huang, Wei Wei, Chaohua Zhang, Dan Liu, and Qiang Lin. 2024. "Refinement and Enhancement of Agrobacterium-Mediated Transient Transformation for Functional Gene Examination in Mulberry (Morus L.)" Genes 15, no. 10: 1277. https://doi.org/10.3390/genes15101277






