PERM1—An Emerging Transcriptional Regulator of Mitochondrial Biogenesis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
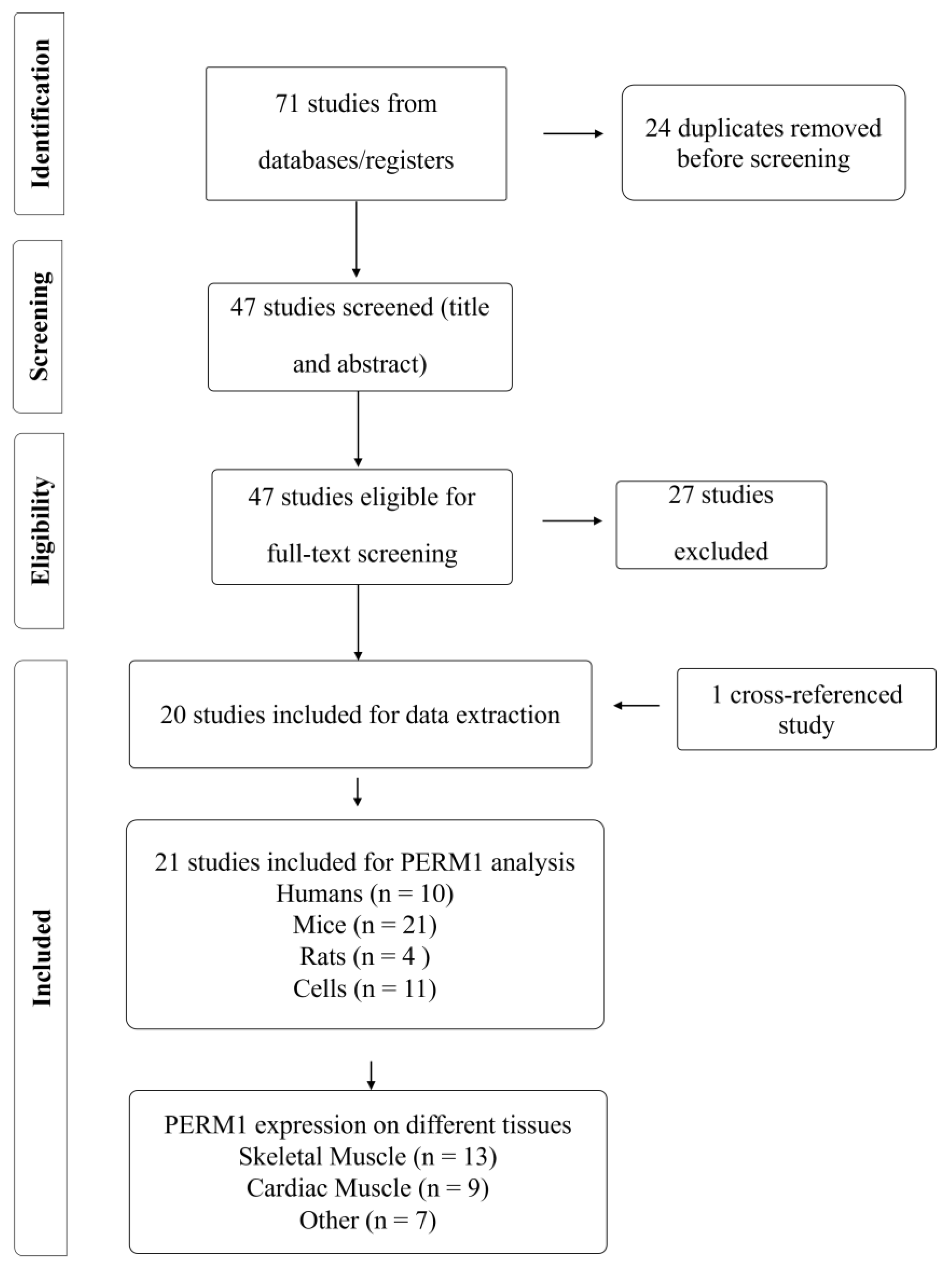
2.3. Selection Process
2.4. Risk of Bias Assessment
2.5. Data Extraction and Data Synthesis
2.6. Bioinformatic Analyses
3. Results
3.1. Risk of Bias
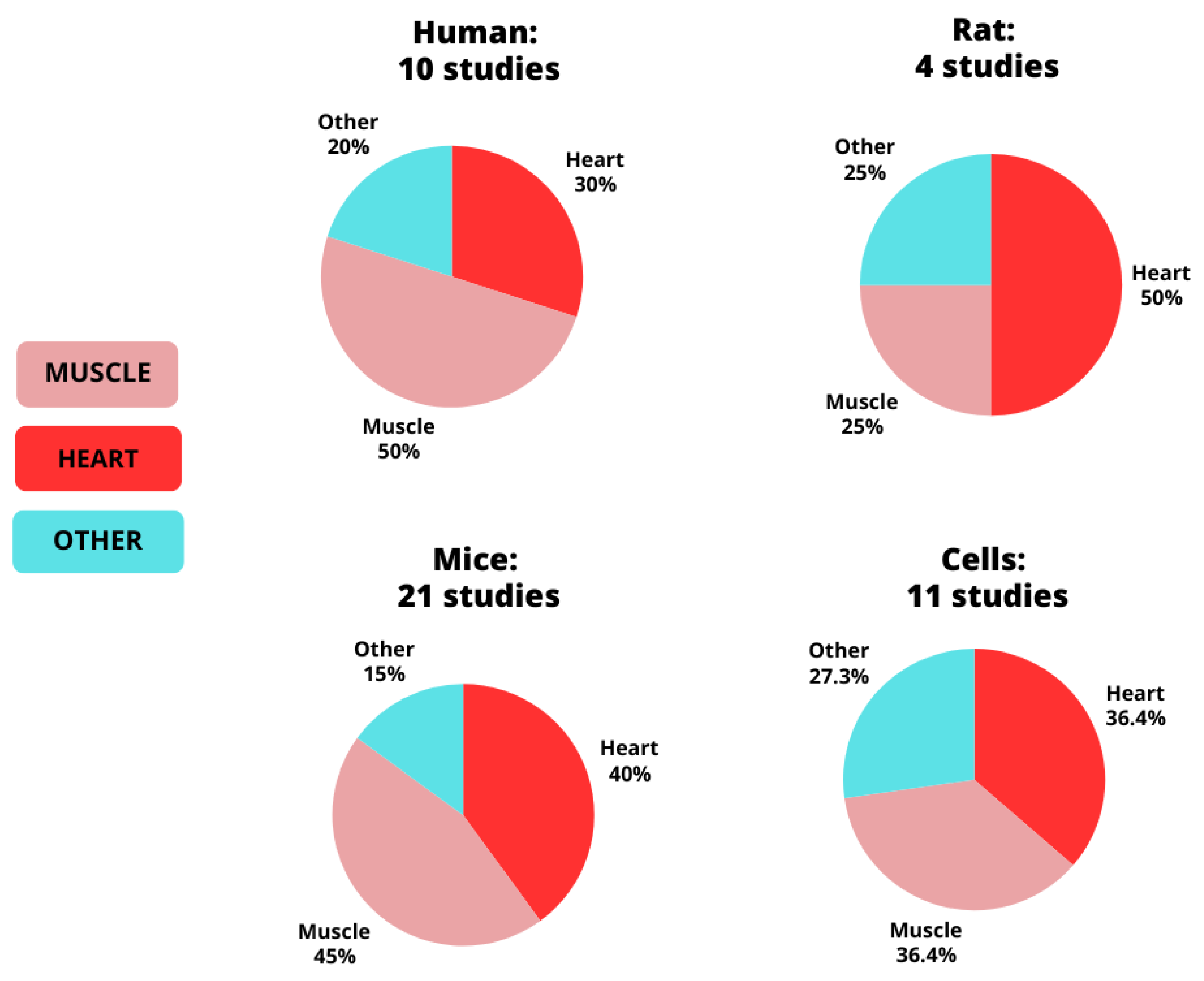
3.2. MetaMEx
4. Discussion
4.1. PERM1 and Skeletal Muscle
4.1.1. Cellular Localization
4.1.2. Signaling/Gene Induction
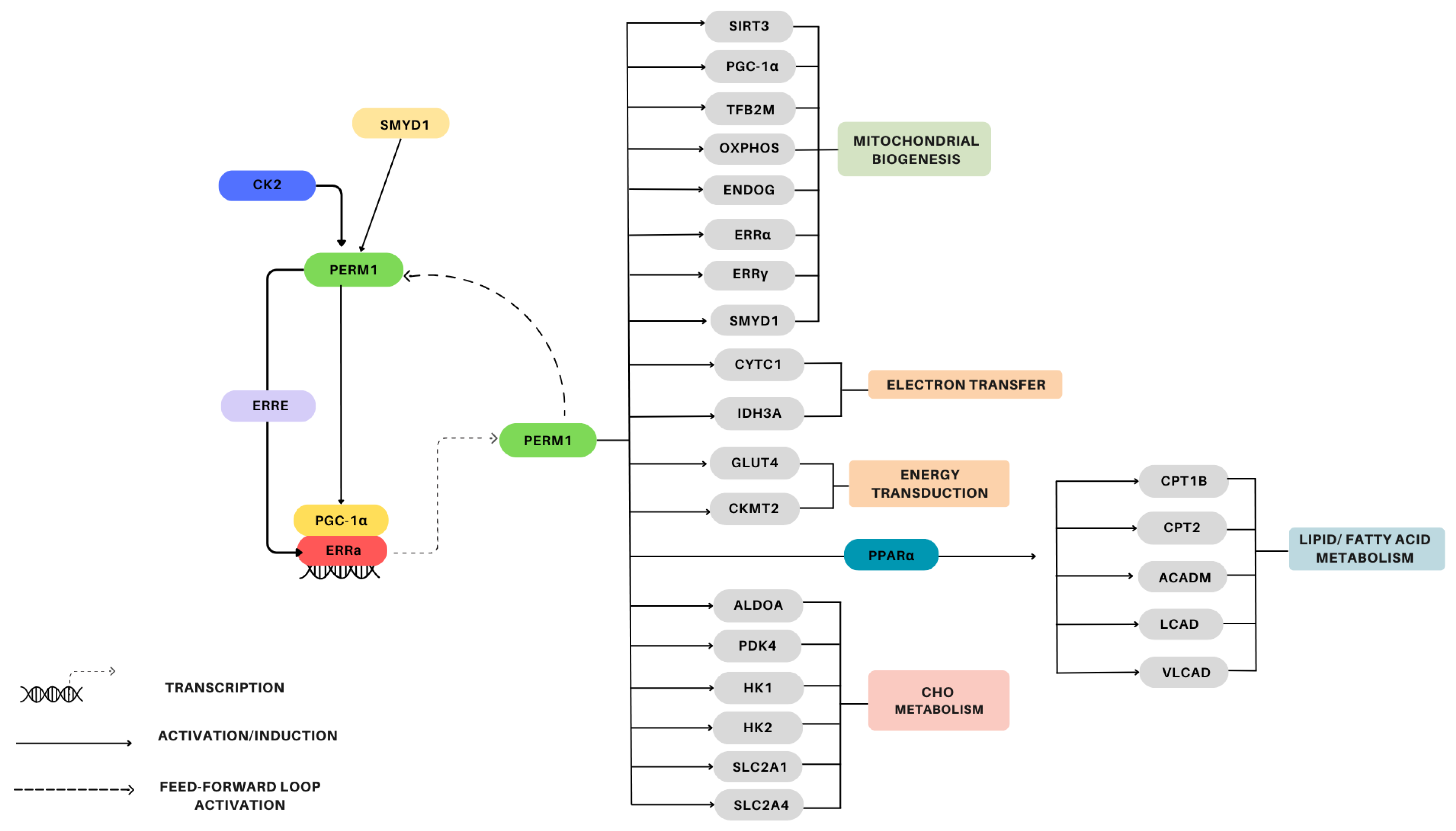
4.1.3. Mitochondrial Biogenesis
4.1.4. PERM1 and Exercise
4.1.5. Physical Inactivity and Disease States
4.2. PERM1 and Heart Tissue
4.2.1. Localization/Structure
4.2.2. Regulation
4.2.3. Energy/Substrate Metabolism
4.2.4. Mitochondrial Biogenesis
4.2.5. Diseases and Pressure Overload
4.3. PERM1 and Other Tissues
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hashim Islam, J.T.B.; Cesare Granata Brendon, J.; Gurd, B.J. Exercise-Induced Mitochondrial Biogenesis: Molecular Regulation, Impact of Training, and Influence on Exercise Performance. In The Routledge Handbook on Biochemistry of Exercise; Routledge: London, UK, 2020; pp. 143–163. [Google Scholar]
- Hawley, J.A.; Hargreaves, M.; Zierath, J.R. Signalling mechanisms in skeletal muscle: Role in substrate selection and muscle adaptation. Essays Biochem. 2006, 42, 1–12. [Google Scholar] [PubMed]
- Hood, D.A.; Tryon, L.D.; Carter, H.N.; Kim, Y.; Chen, C.C. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem. J. 2016, 473, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.F.; Hamilton, K.L. A perspective on the determination of mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E496–E499. [Google Scholar] [CrossRef]
- Hood, D.A. Invited Review: Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 2001, 90, 1137–1157. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef]
- Ballmann, C.; Tang, Y.; Bush, Z.; Rowe, G.C. Adult expression of PGC-1α and -1β in skeletal muscle is not required for endurance exercise-induced enhancement of exercise capacity. Am. J. Physiol.—Endocrinol. Metab. 2016, 311, E928–E938. [Google Scholar] [CrossRef] [PubMed]
- Leick, L.; Wojtaszewski, J.F.P.; Johansen, S.T.; Kiilerich, K.; Comes, G.; Hellsten, Y.; Hidalgo, J.; Pilegaard, H. PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am. J. Physiol.—Endocrinol. Metab. 2008, 294, E463–E474. [Google Scholar] [CrossRef]
- Wende, A.R.; Schaeffer, P.J.; Parker, G.J.; Zechner, C.; Han, D.H.; Chen, M.M.; Hancock, C.R.; Lehman, J.J.; Huss, J.M.; McClain, D.A.; et al. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J. Biol. Chem. 2007, 282, 36642–36651. [Google Scholar] [CrossRef]
- Cho, Y.; Hazen, B.C.; Gandra, P.G.; Ward, S.R.; Schenk, S.; Russell, A.P.; Kralli, A. Perm1 enhances mitochondrial biogenesis, oxidative capacity, and fatigue resistance in adult skeletal muscle. FASEB J. 2016, 30, 674–687. [Google Scholar] [CrossRef]
- Cho, Y.; Hazen, B.C.; Russell, A.P.; Kralli, A. Peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1)- and estrogen-related receptor (ERR)-induced regulator in muscle 1 (Perm1) is a tissue-specific regulator of oxidative capacity in skeletal muscle cells. J. Biol. Chem. 2013, 288, 25207–25218. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Tachibana, S.; Hazen, B.C.; Moresco, J.J.; Yates, J.R., 3rd; Kok, B.; Saez, E.; Ross, R.S.; Russell, A.P.; Kralli, A. Perm1 regulates CaMKII activation and shapes skeletal muscle responses to endurance exercise training. Mol. Metab. 2019, 23, 88–97. [Google Scholar] [CrossRef]
- Drake, J.C.; Wilson, R.J.; Yan, Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016, 30, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Fu, T.; Kelly, D.P.; Vega, R.B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 2018, 28, 969–980. [Google Scholar] [CrossRef]
- Taylor, D.F.; Bishop, D.J. Transcription Factor Movement and Exercise-Induced Mitochondrial Biogenesis in Human Skeletal Muscle: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 1517. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Tetzlaff, J.M.; Brennan, S.E.; Glanville, J.; Hróbjartsson, A.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Weeks, L.; Sterne, J.A.C.; Cochrane Bias Methods GroupCochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Preobrazenski, N.; McCaig, A.; Turner, A.; Kushner, M.; Pacitti, L.; Mendolia, P.; MacDonald, B.; Storoschuk, K.; Bouck, T.; Zaza, Y.; et al. Risk of bias in exercise science: A systematic review of 340 studies. iScience 2024, 27, 109010. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Gabriel, B.M.; Dollet, L.; Smith, J.A.B.; Sardón Puig, L.; Botella, J.; Bishop, D.J.; Krook, A.; Zierath, J.R. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 2020, 11, 470. [Google Scholar] [CrossRef]
- Schild, M.; Ruhs, A.; Beiter, T.; Zügel, M.; Hudemann, J.; Reimer, A.; Krumholz-Wagner, I.; Wagner, C.; Keller, J.; Eder, K.; et al. Basal and exercise induced label-free quantitative protein profiling of m. vastus lateralis in trained and untrained individuals. J. Proteom. 2015, 122, 119–132. [Google Scholar] [CrossRef]
- Brinkmann, C.; Kuckertz, A.; Schiffer, T.; Bloch, W.; Predel, H.G.; Brixius, K. Endurance training alters YKL40, PERM1, and HSP70 skeletal muscle protein contents in men with type 2 diabetes mellitus. Endocr. Res. 2019, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rundqvist, H.C.; Montelius, A.; Osterlund, T.; Norman, B.; Esbjornsson, M.; Jansson, E. Acute sprint exercise transcriptome in human skeletal muscle. PLoS ONE 2019, 14, e0223024. [Google Scholar] [CrossRef]
- Muller, S.; Perdikari, A.; Dapito, D.H.; Sun, W.; Wollscheid, B.; Balaz, M.; Wolfrum, C. ESRRG and PERM1 Govern Mitochondrial Conversion in Brite/Beige Adipocyte Formation. Front. Endocrinol. 2020, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Aravamudhan, S.; Turk, C.; Bock, T.; Keufgens, L.; Nolte, H.; Lang, F.; Krishnan, R.K.; König, T.; Hammerschmidt, P.; Schindler, N.; et al. Phosphoproteomics of the developing heart identifies PERM1—An outer mitochondrial membrane protein. J. Mol. Cell Cardiol. 2021, 154, 41–59. [Google Scholar] [CrossRef]
- Bock, T.; Turk, C.; Aravamudhan, S.; Keufgens, L.; Bloch, W.; Rozsivalova, D.H.; Romanello, V.; Nogara, L.; Blaauw, B.; Trifunovic, A.; et al. PERM1 interacts with the MICOS-MIB complex to connect the mitochondria and sarcolemma via ankyrin B. Nat. Commun. 2021, 12, 4900. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Tian, T.; Zhu, S.; Song, K. Molecular mechanism of the ESRRG-PERM1-CKMT2 signal axis in ovariectomized female rats with OSAHS. Transpl. Immunol. 2022, 74, 101631. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ochiai, H.; Sakuma, H.; Mori, T.; Yazawa, M.; Oka, A.; Kishi, K. Muscle Fiber Composition Changes after Selective Nerve Innervation. Int. J. Mol. Sci. 2022, 23, 7856. [Google Scholar] [CrossRef]
- Elzamzami, F.D.; Samal, A.; Arun, A.S.; Dharmaraj, T.; Prasad, N.R.; Rendon-Jonguitud, A.; DeVine, L.; Walston, J.D.; Cole, R.N.; Wilson, K.L. Native lamin A/C proteomes and novel partners from heart and skeletal muscle in a mouse chronic inflammation model of human frailty. Front. Cell Dev. Biol. 2023, 11, 1240285. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, N.; Ye, H.; Miao, J.; Xia, B.; Yang, Y.; Peng, H.; Xu, S.; Wu, T.; Tao, C.; et al. Glucose restriction enhances oxidative fiber formation: A multi-omic signal network involving AMPK and CaMK2. iScience 2024, 27, 108590. [Google Scholar] [CrossRef]
- Oka, S.I.; Sabry, A.D.; Horiuchi, A.K.; Cawley, K.M.; O’Very, S.A.; Zaitsev, M.A.; Shankar, T.S.; Byun, J.; Mukai, R.; Xu, X.; et al. Perm1 regulates cardiac energetics as a downstream target of the histone methyltransferase Smyd1. PLoS ONE. 2020, 15, e0234913. [Google Scholar] [CrossRef]
- Cho, Y.; Tachibana, S.; Lam, K.; Arita, Y.; Khosrowjerdi, S.; Zhang, O. Perm1 promotes cardiomyocyte mitochondrial biogenesis and protects against hypoxia/reoxygenation-induced damage in mice. J. Biol. Chem. 2021, 297, 100825. [Google Scholar]
- Oka, S.I.; Sreedevi, K.; Shankar, T.S.; Yedla, S.; Arowa, S.; James, A.; Stone, K.G.; Olmos, K.; Sabry, A.D.; Horiuchi, A.; et al. PERM1 regulates energy metabolism in the heart via ERRalpha/PGC-1alpha axis. Front. Cardiovasc. Med. 2022, 9, 1033457. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Oka, S.I.; Xu, X.; Chen, C.F.; Tung, C.Y.; Chang, Y.Y.; Mourad, Y.; Vehra, O.; Ivessa, A.; Yehia, G.; et al. PERM1 regulates genes involved in fatty acid metabolism in the heart by interacting with PPARalpha and PGC-1alpha. Sci. Rep. 2022, 12, 14576. [Google Scholar]
- Tachibana, S.; Yu, N.-K.; Li, R.; Fernandez-Costa, C.; Liang, A.; Choi, J.; Jung, D.; Xiao, C.; Kralli, A.; Yates, J.R.; et al. Perm1 Protects the Heart From Pressure Overload–Induced Dysfunction by Promoting Oxidative Metabolism. Circulation 2023, 147, 916–919. [Google Scholar] [CrossRef]
- Timbergen, M.J.M.; Boers, R.; Vriends, A.L.M.; Boers, J.; van IJcken, W.F.J.; Lavrijsen, M.; Grünhagen, D.J.; Verhoef, C.; Sleijfer, S.; Smits, R.; et al. Differentially Methylated Regions in Desmoid-Type Fibromatosis: A Comparison Between CTNNB1 S45F and T41A Tumors. Front. Oncol. 2020, 10, 565031. [Google Scholar]
- Di Pierro, E.; Perrone, M.; Franco, M.; Granata, F.; Duca, L.; Lattuada, D.; De Luca, G.; Graziadei, G. Mitochondrial DNA Copy Number Drives the Penetrance of Acute Intermittent Porphyria. Life 2023, 13, 1923. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, H.; Qian, Y.; Zhao, S.; Wang, H.; Wang, Y.; Yu, T. The lncRNA lnc_AABR07044470.1 promotes the mitochondrial-damaged inflammatory response to neuronal injury via miR-214-3p/PERM1 axis in acute ischemic stroke. Mol. Biol. Rep. 2024, 51, 412. [Google Scholar] [CrossRef]
- Russell, A.P.; Wada, S.; Vergani, L.; Hock, M.B.; Lamon, S.; Léger, B.; Ushida, T.; Cartoni, R.; Wadley, G.D.; Hespel, P.; et al. Disruption of skeletal muscle mitochondrial network genes and miRNAs in amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 49, 107–117. [Google Scholar] [CrossRef]
- Warren, J.S.; Tracy, C.M.; Miller, M.R.; Makaju, A.; Szulik, M.W.; Oka, S.-I.; Yuzyuk, T.N.; Cox, J.E.; Kumar, A.; Lozier, B.K.; et al. Histone methyltransferase Smyd1 regulates mitochondrial energetics in the heart. Proc. Natl.. Acad. Sci. USA 2018, 115, E7871–E7880. [Google Scholar] [CrossRef]
- Schulz, K.F.; Chalmers, I.; Hayes, R.J.; Altman, D.G. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995, 273, 408–412. [Google Scholar] [CrossRef]
- Moher, D.; Pham, B.; Jones, A.; Cook, D.J.; Jadad, A.R.; Moher, M.; Tugwell, P.; Klassen, T.P. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998, 352, 609–613. [Google Scholar] [CrossRef]
- Munafò, M.R.; Nosek, B.A.; Bishop, D.V.M.; Button, K.S.; Chambers, C.D.; Percie du Sert, N.; manifesto for reproducible. A manifesto for reproducible science. Nat. Hum. Behav. 2017, 1, 0021. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. 1500 scientists lift the lid on reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [PubMed]


| Author | Model/Intervention | Key Findings |
|---|---|---|
| Cho et al., 2013 [12] | C2C12 cells |
|
| Mice |
| |
| Human |
| |
| [21] | Human |
|
| [11] | Muscle specific PERM1 gain-of-function in TA of mice |
|
| Primary Myotubes |
| |
| [22] | Human |
|
| [23] | Human |
|
| [13] | Mice |
|
| PERM1 knockdown in mice |
| |
| ||
| Mice—Diet induced Obesity |
| |
| Duchenne muscular dystrophy (human) |
| |
| [24] | Mice |
|
| [25] | C2C12 cells |
|
| Mice |
| |
| [26] | PERM1 knockdown in mice |
|
| C2C12 cells |
| |
| [27] | Ovariectomized Rats |
|
| [28] | Rat |
|
| [29] | Mice |
|
| [30] | Mice |
|
| Author | Model/Intervention | Key Findings |
|---|---|---|
| [12] | Mice |
|
| Oka et al., 2020 [31] | PERM1 gain-of-function in cardiomyocytes |
|
| PERM1 knockdown in cardiomyocytes |
| |
| SMYD1 knockdown in neonatal rat ventricular myocytes |
| |
| PERM1 knockdown in neonatal rat ventricular myocytes |
| |
| PERM1 gain-of-function in neonatal rat ventricular myocytes |
| |
| Neonatal rat ventricular myocytes treated with phenylephrine (hypertrophic stress/pressure overload model) |
| |
| Mouse failing hearts |
| |
| Human failing hearts |
| |
| [24] | Mice |
|
| ||
| [32] | Cardiomyocytes |
|
| Mice |
| |
| Human |
| |
| [25] | Isolated mitochondria from mouse hearts |
|
| Hearts of mice |
| |
| PERM1 knockdown mice |
| |
| Human |
| |
| [33] | Mice |
|
| Cardiomyocytes |
| |
| [34] | Cardiomyocytes |
|
| Mice |
| |
| [29] | Mice |
|
| [35] | Mice |
|
| Author | Model/Intervention | Key Findings |
|---|---|---|
| [12] | Mice |
|
| Cho et al., 2019 [13] | HEK293 cells |
|
| [24] | Adipose |
|
| Immortalized brown preadipocytes |
| |
| Mice adipose tissue |
| |
| [36] | Human desmoid-type fibromatosis (DTF) tumors |
|
| [26] | HEK-293T cells |
|
| [37] | Human white blood cells |
|
| [38] | Neurons |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares Menezes, E.; Wu, Z.; Renwick, J.R.M.; Moran-MacDonald, A.; Gurd, B.J. PERM1—An Emerging Transcriptional Regulator of Mitochondrial Biogenesis: A Systematic Review. Genes 2024, 15, 1305. https://doi.org/10.3390/genes15101305
Soares Menezes E, Wu Z, Renwick JRM, Moran-MacDonald A, Gurd BJ. PERM1—An Emerging Transcriptional Regulator of Mitochondrial Biogenesis: A Systematic Review. Genes. 2024; 15(10):1305. https://doi.org/10.3390/genes15101305
Chicago/Turabian StyleSoares Menezes, Eveline, Zeyu Wu, John R. M. Renwick, Andres Moran-MacDonald, and Brendon J. Gurd. 2024. "PERM1—An Emerging Transcriptional Regulator of Mitochondrial Biogenesis: A Systematic Review" Genes 15, no. 10: 1305. https://doi.org/10.3390/genes15101305
APA StyleSoares Menezes, E., Wu, Z., Renwick, J. R. M., Moran-MacDonald, A., & Gurd, B. J. (2024). PERM1—An Emerging Transcriptional Regulator of Mitochondrial Biogenesis: A Systematic Review. Genes, 15(10), 1305. https://doi.org/10.3390/genes15101305





