Is the Relationship Between Cardiovascular Disease and Alzheimer’s Disease Genetic? A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Evidence of Overall Shared Genetic Risk
3.1.1. Presenilin (PSEN) Genes
3.1.2. APOE
3.2. Shared Mechanisms of AD and CVD
3.2.1. Altered Lipid Levels and Metabolism
| Topic | Area of Focus | Articles |
|---|---|---|
| Lipid genetics | AD | correlation [95], association [57,84,226,227,241], MR [229,230,231,237,238,242,243], PRS [244], pleiotropy [228,245], gene [232], systems [246] |
| CVD | correlation [95], GWAS [216,219,220], gene [215,247], MR [237,238], gene [248], association [217] | |
| Plasma lipid levels | AD | [209,225,244,249] |
| CVD | [56,218,221,236] |
3.2.2. Blood Pressure Regulation
| Topic | Area of Focus | Articles |
|---|---|---|
| Blood pressure | AD | GWAS [68,69,71,72,73,74,75,76,273], methylation [70], MR [77,165,229,231,274], association [275,280] |
| Hypertension (ACE) | CVD [281,282], AD [85,241,283,284,285,286,287,288,289,290] | |
| Lower cerebral blood flow | [82,291,292,293,294,295] | |
| Impaired cardiac function | AD | [194,269,296,297,298,299,300] |
Hypertension
Reduced Cerebral Blood Flow
3.2.3. Blood–Brain Barrier Impairment
| Topic | Area of Focus | Articles |
|---|---|---|
| Blood–brain barrier function | General | systems [83,212,332], association [336], serum [337], RNAseq [333,338], CSF [339,340,341], microarray [342] |
| Endothelial and pericyte cell dysregulation | RNAseq [207,210,333,343,344], systems [271,324,345,346] | |
| Dysregulated angiogenesis | [208,246,344,346,347] | |
| Aβ buildup and clearance | heart [13,18,200,262,348], brain [23,28,29,163,234,246,261,312,324,347,349,350,351,352] | |
| Tau buildup and clearance | heart [35], brain [212,341,347,351] | |
| Circulation of Aβ | [15,319,353,354] | |
| Circulation of tau | [197,201,314,353,355,356] | |
| Circulation of metabolites and proteins in AD | plasma [198,272,357,358,359,360,361,362], CSF [265], BDNF [363], homocysteine [364,365], uric acid [366] | |
| Circulation of metabolites and proteins in CVDs | BDNF [363], homocysteine [364,365], uric acid [366], plasma [272,360,366] |
Endothelial and Pericyte Cell Dysregulation
Circulation of Metabolites and Protein Deposits
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2024: MONITORING Health for the SDGs, Sustainable Development Goals; World Health Organization: Genève, Switzerland, 2024.
- Gaziano, T.; Reddy, K.S.; Paccaud, F. Disease Control Priorities in Developing Countries; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- O’Donnell, C.J.; Nabel, E.G. Cardiovascular genomics, personalized medicine, and the national heart, lung, and blood institute. Circ. Cardiovasc. Genet. 2008, 1, 51–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zdravkovic, S.; Wienke, A.; Pedersen, N.L.; Marenberg, M.E.; Yashin, A.I.; De Faire, U. Heritability of death from coronary heart disease: A 36-year follow-up of 20 966 Swedish twins. J. Intern. Med. 2002, 252, 247–254. [Google Scholar] [CrossRef] [PubMed]
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 425, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Slowikowski, S.P.M.; Westaway, D.; Mount, H.T.J. Interactions between beta-amyloid and central cholinergic neurons: Implications for Alzheimer’s disease. J. Psychiatry Neurosci. 2004, 29, 427–441. [Google Scholar]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef]
- Gatz, M.; Reynolds, C.A.; Fratiglioni, L.; Johansson, B.; Mortimer, J.A.; Berg, S.; Fiske, A.; Pedersen, N.L. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 2006, 63, 168–174. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Hofman, A.; Ott, A.; Breteler, M.M.B.; Bots, M.L.; Slooter, A.J.C.; van Harskamp, F.; van Duijn, C.N.; Van Broeckhoven, C.; Grobbee, D.E. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet 1997, 349, 151–154. [Google Scholar] [CrossRef]
- Levine, D.A.; Galecki, A.T.; Langa, K.M.; Unverzagt, F.W.; Kabeto, M.U.; Giordani, B.; Wadley, V.G. Trajectory of cognitive decline after incident stroke. JAMA 2015, 314, 41. [Google Scholar] [CrossRef]
- Troncone, L.; Luciani, M.; Coggins, M.; Wilker, E.H.; Ho, C.-Y.; Codispoti, K.E.; Frosch, M.P.; Kayed, R.; Del Monte, F. Aβ amyloid pathology affects the hearts of patients with Alzheimer’s disease: Mind the heart. J. Am. Coll. Cardiol. 2016, 68, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Fitzpatrick, A.L.; Lopez, O.; Jackson, S.; Lyketsos, C.; Jagust, W.; Ives, D.; DeKosky, S.T.; Kuller, L.H. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J. Am. Geriatr. Soc. 2005, 53, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Stamatelopoulos, K.; Sibbing, D.; Rallidis, L.S.; Georgiopoulos, G.; Stakos, D.; Braun, S.; Gatsiou, A.; Sopova, K.; Kotakos, C.; Varounis, C.; et al. Amyloid-beta (1-40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J. Am. Coll. Cardiol. 2015, 65, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, P.; Borchelt, M.; Smith, J. Relation between cardiovascular and metabolic disease and cognition in very old age: Cross-sectional and longitudinal findings from the Berlin aging study. Health Psychol. 2003, 22, 559–569. [Google Scholar] [CrossRef]
- de Bruijn, R.F.A.G.; Heeringa, J.; Wolters, F.J.; Franco, O.H.; Stricker, B.H.C.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015, 72, 1288–1294. [Google Scholar] [CrossRef]
- Hong, X.; Bu, L.; Wang, Y.; Xu, J.; Wu, J.; Huang, Y.; Liu, J.; Suo, H.; Yang, L.; Shi, Y.; et al. Increases in the risk of cognitive impairment and alterations of cerebral β-amyloid metabolism in mouse model of heart failure. PLoS ONE 2013, 8, e63829. [Google Scholar] [CrossRef]
- Vishwanath, S.; Hopper, I.; Wolfe, R.; Polekhina, G.; Reid, C.M.; Tonkin, A.M.; Murray, A.M.; Shah, R.C.; Storey, E.; Woods, R.L.; et al. Cognitive trajectories and incident dementia after a cardiovascular event in older adults. Alzheimer’s Dement. 2023, 19, 3670–3678. [Google Scholar] [CrossRef]
- Rusanen, M.; Kivipelto, M.; Levälahti, E.; Laatikainen, T.; Tuomilehto, J.; Soininen, H.; Ngandu, T. Heart diseases and long-term risk of dementia and Alzheimer’s disease: A population-based CAIDE study. J. Alzheimer’s Dis. 2014, 42, 183–191. [Google Scholar] [CrossRef]
- Lee, M.; Hughes, T.M.; George, K.M.; E Griswold, M.; Sedaghat, S.; Simino, J.; Lutsey, P.L. Education and cardiovascular health as effect modifiers of APOE ε4 on dementia: The Atherosclerosis Risk in Communities study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1199–1207. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, C.; Fu, C.; Hao, W.; Ozaki, A.; Shrestha, N.; Virani, S.S.; Mishra, S.R.; Zhu, D. Sex differences in the association between cardiovascular diseases and dementia subtypes: A prospective analysis of 464,616 UK Biobank participants. Biol. Sex. Differ. 2022, 13, 21. [Google Scholar] [CrossRef]
- Sparks, D.L.; Hunsaker, J.C.I.I.I.; Scheff, S.W.; Kryscio, R.J.; Henson, J.L.; Markesbery, W.R. Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol. Aging 1990, 11, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Witt, L.S.; Rotter, J.; Stearns, S.C.; Gottesman, R.F.; Kucharska-Newton, A.M.; Sharrett, A.R.; Wruck, L.M.; Bressler, J.; Sueta, C.A.; Chang, P.P. Heart failure and cognitive impairment in the atherosclerosis risk in communities (ARIC) study. J. Gen. Intern. Med. 2018, 33, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Manemann, S.M.; Knopman, D.S.; Sauver, J.S.; Bielinski, S.J.; Chamberlain, A.M.; Weston, S.A.; Jiang, R.; Roger, V.L. Alzheimer’s disease and related dementias and heart failure: A community study. J. Am. Geriatr. Soc. 2022, 70, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Rondina, J.M.; Squarzoni, P.; Souza-Duran, F.L.; Tamashiro-Duran, J.H.; Scazufca, M.; Menezes, P.R.; Vallada, H.; Lotufo, P.A.; Alves, T.C.d.T.F.; Filho, G.B. Framingham coronary heart disease risk score can be predicted from structural brain images in elderly subjects. Front. Aging Neurosci. 2014, 6, 300. [Google Scholar] [CrossRef] [PubMed]
- Bleckwenn, M.; Kleineidam, L.; Wagner, M.; Jessen, F.; Weyerer, S.; Werle, J.; Wiese, B.; Lühmann, D.; Posselt, T.; König, H.-H.; et al. Impact of coronary heart disease on cognitive decline in Alzheimer’s disease: A prospective longitudinal cohort study in primary care. Br. J. Gen. Pract. 2017, 67, e111–e117. [Google Scholar] [CrossRef]
- Rabin, J.S.; Schultz, A.P.; Hedden, T.; Viswanathan, A.; Marshall, G.A.; Kilpatrick, E.; Klein, H.; Buckley, R.F.; Yang, H.-S.; Properzi, M.; et al. Interactive associations of vascular risk and β-amyloid burden with cognitive decline in clinically normal elderly individuals. JAMA Neurol. 2018, 75, 1124. [Google Scholar] [CrossRef]
- Keuss, S.E.; Coath, W.; Nicholas, J.M.; Poole, T.; Barnes, J.; Cash, D.M.; Lane, C.A.; Parker, T.D.; Keshavan, A.; Buchanan, S.M.; et al. Associations of β-amyloid and vascular burden with rates of neurodegeneration in cognitively normal members of the 1946 British Birth Cohort. Neurology 2022, 99, e129–e141. [Google Scholar] [CrossRef]
- Shen, R.; Guo, X.; Zou, T.; Ma, L. Association of cardiovascular health with cognitive function in US older adults: A population-based cross-sectional study. Dement. Geriatr. Cogn. Disord. 2024, 53, 1–11. [Google Scholar] [CrossRef]
- Tin, A.; Bressler, J.; Simino, J.; Sullivan, K.J.; Mei, H.; Windham, B.G.; Griswold, M.; Gottesman, R.F.; Boerwinkle, E.; Fornage, M.; et al. Genetic risk, midlife Life’s Simple 7, and incident dementia in the Atherosclerosis Risk in Communities study. Neurology 2022, 99, e154–e163. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, G.; Feng, C.; Chen, J.; Gan, W.; Ma, Y.; Hu, Y.; Dhana, K.; Voortman, T.; Shen, J.; et al. Cardiovascular risk burden, dementia risk and brain structural imaging markers: A study from UK Biobank. Gen. Psychiatr. 2024, 37, e101209. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Ntanasi, E.; Ramirez, A.; Grenier-Boley, B.; Lambert, J.-C.; Sakka, P.; Yannakoulia, M.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; et al. Vascular burden and genetic risk in association with cognitive performance and dementia in a population-based study. Cereb. Circ. Cogn. Behav. 2022, 3, 100145. [Google Scholar] [CrossRef]
- Peloso, G.M.; Beiser, A.S.; Satizabal, C.L.; Xanthakis, V.; Vasan, R.S.; Pase, M.P.; Destefano, A.L.; Seshadri, S. Cardiovascular health, genetic risk, and risk of dementia in the Framingham Heart Study. Neurology 2020, 95, e1341–e1350. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, H.; Jiang, J.; Bi, Y.; Sun, Y.; Ou, Y.; Tan, L.; Yu, J. Echocardiographic measures of the left heart and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology in cognitively intact adults: The CABLE study. Alzheimer’s Dement. 2024, 20, 3943–3957. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Le Guen, Y.; Belloy, M.E.; Grenier-Boley, B.; de Rojas, I.; Castillo-Morales, A.; Jansen, I.; Nicolas, A.; Bellenguez, C.; Dalmasso, C.; Küçükali, F.; et al. Association of rare APOE missense variants V236E and R251G with risk of Alzheimer disease. JAMA Neurol. 2022, 79, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef]
- Bellenguez, C.; Kucukali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Jansen, I.E.; van der Lee, S.J.; Gomez-Fonseca, D.; de Rojas, I.; Dalmasso, M.C.; Grenier-Boley, B.; Zettergren, A.; Mishra, A.; Ali, M.; Andrade, V.; et al. Genome-wide meta-analysis for Alzheimer’s disease cerebrospinal fluid biomarkers. Acta Neuropathol. 2022, 144, 821–842. [Google Scholar] [CrossRef]
- de Rojas, I.; Moreno-Grau, S.; Tesi, N.; Grenier-Boley, B.; Andrade, V.; Jansen, I.E.; Pedersen, N.L.; Stringa, N.; Zettergren, A.; Hernández, I.; et al. Common variants in Alzheimer’s disease and risk stratification by polygenic risk scores. Nat. Commun. 2021, 12, 3417. [Google Scholar] [CrossRef]
- Holstege, H.; Hulsman, M.; Charbonnier, C.; Grenier-Boley, B.; Quenez, O.; Grozeva, D.; van Rooij, J.G.J.; Sims, R.; Ahmad, S.; Amin, N.; et al. Exome sequencing identifies rare damaging variants in ATP8B4 and ABCA1 as risk factors for Alzheimer’s disease. Nat. Genet. 2022, 54, 1786–1794. [Google Scholar] [CrossRef]
- Wang, Y.; Sarnowski, C.; Lin, H.; Pitsillides, A.N.; Heard-Costa, N.L.; Choi, S.H.; Wang, D.; Bis, J.C.; Blue, E.E.; Alzheimer’s Disease Neuroimaging Initiative (ADNI); et al. Key variants via the Alzheimer’s Disease Sequencing Project whole genome sequence data. Alzheimer’s Dement. 2024, 20, 3290–3304. [Google Scholar] [CrossRef] [PubMed]
- Chemparathy, A.; Le Guen, Y.; Chen, S.; Lee, E.-G.; Leong, L.; Gorzynski, J.E.; Jensen, T.D.; Ferrasse, A.; Xu, G.; Xiang, H.; et al. APOE loss-of-function variants: Compatible with longevity and associated with resistance to Alzheimer’s disease pathology. Neuron 2024, 112, 1110–1116.e5. [Google Scholar] [CrossRef] [PubMed]
- Escott-Price, V.; Bellenguez, C.; Wang, L.-S.; Choi, S.-H.; Harold, D.; Jones, L.; Holmans, P.; Gerrish, A.; Vedernikov, A.; Richards, A.; et al. Gene-wide analysis detects two new susceptibility genes for Alzheimer’s disease. PLoS ONE 2014, 9, e94661. [Google Scholar] [CrossRef] [PubMed]
- Jun, G.; Ibrahim-Verbaas, C.A.; Vronskaya, M.; Lambert, J.C.; Chung, J.; Naj, A.C.; Kunkle, B.W.; Wang, L.S.; Bis, J.C.; Bel-lenguez, C.; et al. A novel Alzheimer disease locus located near the gene encoding tau protein. Mol. Psychiatry 2016, 21, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; DeStafano, A.L.; Bis, J.C.; Beecham, G.W.; Grenier-Boley, B.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef]
- Beecham, G.W.; Hamilton, K.; Naj, A.C.; Martin, E.R.; Huentelman, M.; Myers, A.J.; Corneveaux, J.J.; Hardy, J.; Vonsattel, J.P.; Younkin, S.G.; et al. Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s disease and related dementias. PLoS Genet. 2014, 10, e1004606. [Google Scholar] [CrossRef]
- Yashin, A.I.; Fang, F.; Kovtun, M.; Wu, D.; Duan, M.; Arbeev, K.; Akushevich, I.; Kulminski, A.; Culminskaya, I.; Zhbannikov, I.; et al. Hidden heterogeneity in Alzheimer’s disease: Insights from genetic association studies and other analyses. Exp. Gerontol. 2018, 107, 148–160. [Google Scholar] [CrossRef]
- Seshadri, S.; Fitzpatrick, A.L.; Ikram, M.A.; DeStefano, A.L.; Gudnason, V.; Boada, M.; Bis, J.C.; Smith, A.V.; Carassquillo, M.M.; Lambert, J.C.; et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 2010, 303, 1832–1840. [Google Scholar] [CrossRef]
- Jakobsdottir, J.; van der Lee, S.J.; Bis, J.C.; Chouraki, V.; Li-Kroeger, D.; Yamamoto, S.; Grove, M.L.; Naj, A.; Vronskaya, M.; Salazar, J.L.; et al. Rare functional variant in TM2D3 is associated with late-onset Alzheimer’s disease. PLoS Genet. 2016, 12, e1006327. [Google Scholar] [CrossRef]
- Bis, J.C.; Jian, X.; Kunkle, B.W.; Chen, Y.; Hamilton-Nelson, K.L.; Bush, W.S.; Salerno, W.J.; Lancour, D.; Ma, Y.; Renton, A.E.; et al. Whole exome sequencing study identifies novel rare and common Alzheimer’s-Associated variants involved in immune response and transcriptional regulation. Mol. Psychiatry 2020, 25, 1859–1875. [Google Scholar] [CrossRef]
- Malik, R.; Chauhan, G.; Traylor, M.; Sargurupremraj, M.; Okada, Y.; Mishra, A.; Rutten-Jacobs, L.; Giese, A.K.; van der Laan, S.W.; Gretarsdottir, S.; et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018, 50, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Haessler, J.W.; Manansala, R.; Wiggins, K.L.; Moscati, A.; Beiser, A.; Heard-Costa, N.L.; Sarnowski, C.; Raffield, L.M.; Chung, J.; et al. Whole-genome sequencing association analyses of stroke and its subtypes in ancestrally diverse populations from Trans-Omics for Precision Medicine project. Stroke 2022, 53, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.L.; Vasan, R.S.; Levy, D.; Andersson, C.; Lin, H. Integrated omics analysis of coronary artery calcifications and myocardial infarction: The Framingham Heart Study. Sci. Rep. 2023, 13, 21581. [Google Scholar] [CrossRef] [PubMed]
- Peloso, G.M.; Auer, P.L.; Bis, J.C.; Voorman, A.; Morrison, A.C.; Stitziel, N.O.; Brody, J.A.; Khetarpal, S.A.; Crosby, J.R.; Fornage, M.; et al. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am. J. Hum. Genet. 2014, 94, 223–232. [Google Scholar] [CrossRef]
- Jones, L.; Holmans, P.A.; Hamshere, M.L.; Harold, D.; Moskvina, V.; Ivanov, D.; Pocklington, A.; Abraham, R.; Hollingworth, P.; Sims, R.; et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS ONE 2010, 5, e13950. [Google Scholar] [CrossRef]
- Wu, H.M.; Goate, A.M.; O’Reilly, P.F. Heterogeneous effects of genetic risk for Alzheimer’s disease on the phenome. Transl. Psychiatry 2021, 11, 406. [Google Scholar] [CrossRef]
- Li, Q.S.; Tian, C.; The 23andMe Research Team; Hinds, D.; Seabrook, G.R. The association of clinical phenotypes to known AD/FTD genetic risk loci and their inter-relationship. PLoS ONE 2020, 15, e0241552. [Google Scholar] [CrossRef]
- Kobayashi, N.; Shinagawa, S.; Niimura, H.; Kida, H.; Nagata, T.; Tagai, K.; Shimada, K.; Oka, N.; Shikimoto, R.; Noda, Y.; et al. Increased blood COASY DNA methylation levels a potential biomarker for early pathology of Alzheimer’s disease. Sci. Rep. 2020, 10, 12217. [Google Scholar] [CrossRef]
- Giedraitis, V.; Kilander, L.; Degerman-Gunnarsson, M.; Sundelöf, J.; Axelsson, T.; Syvänen, A.-C.; Lannfelt, L.; Glaser, A. Genetic analysis of Alzheimer’s disease in the Uppsala Longitudinal Study of Adult Men. Dement. Geriatr. Cogn. Disord. 2009, 27, 59–68. [Google Scholar] [CrossRef]
- Loika, Y.; Loiko, E.; Culminskaya, I.; Kulminski, A.M. Exome-wide association study identified clusters of pleiotropic genetic associations with Alzheimer’s disease and thirteen cardiovascular traits. Genes 2023, 14, 1834. [Google Scholar] [CrossRef]
- Li, X.; Long, J.; He, T.; Belshaw, R.; Scott, J. Integrated genomic approaches identify major pathways and upstream regulators in late onset Alzheimer’s disease. Sci. Rep. 2015, 5, 12393. [Google Scholar] [CrossRef] [PubMed]
- International Genomics of Alzheimer’s Disease Consortium (IGAP). Convergent genetic and expression data implicate immunity in Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, M.D.; Chaffin, M.; Zahid, S.; Ritchie, S.; Rotter, J.I.; Rich, S.S.; Gerszten, R.; Guo, X.; Heckbert, S.; Tracy, R.; et al. Neurocognitive trajectory and proteomic signature of inherited risk for Alzheimer’s disease. PLoS Genet. 2022, 18, e1010294. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Xu, M.; Liao, M.; Jiang, Y.; Jiang, Q.; Feng, R.; Zhang, L.; Ma, G.; Wang, G.; Chen, Z.; et al. Integrating genome-wide association study and brain expression data highlights cell adhesion molecules and purine metabolism in Alzheimer’s disease. Mol. Neurobiol. 2015, 52, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Coronary Artery Disease (C4D) Genetics Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat. Genet. 2011, 43, 339–344. [Google Scholar] [CrossRef]
- Sung, Y.J.; Winkler, T.W.; Fuentes, L.d.L.; Bentley, A.R.; Brown, M.R.; Kraja, A.T.; Schwander, K.; Ntalla, I.; Guo, X.; Franceschini, N.; et al. A large-scale multi-ancestry genome-wide study accounting for smoking behavior identifies multiple significant loci for blood pressure. Am. J. Hum. Genet. 2018, 102, 375–400. [Google Scholar] [CrossRef]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Richard, M.A.; Huan, T.; Ligthart, S.; Gondalia, R.; Jhun, M.A.; Brody, J.A.; Irvin, M.R.; Marioni, R.; Shen, J.; Tsai, P.-C.; et al. DNA methylation analysis identifies loci for blood pressure regulation. Am. J. Hum. Genet. 2017, 101, 888–902. [Google Scholar] [CrossRef]
- Kato, N.; Loh, M.; Takeuchi, F.; Verweij, N.; Wang, X.; Zhang, W.; Kelly, T.N.; Saleheen, D.; Lehne, B.; Leach, I.M.; et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat. Genet. 2015, 47, 1282–1293. [Google Scholar] [CrossRef]
- Feitosa, M.F.; Kraja, A.T.; Chasman, D.I.; Sung, Y.J.; Winkler, T.W.; Ntalla, I.; Guo, X.; Franceschini, N.; Cheng, C.-Y.; Sim, X.; et al. Novel genetic associations for blood pressure identified via gene-alcohol interaction in up to 570K individuals across multiple ancestries. PLoS ONE 2018, 13, e0198166. [Google Scholar] [CrossRef]
- Sung, Y.J.; Fuentes, L.d.L.; Winkler, T.W.; I Chasman, D.; Bentley, A.R.; Kraja, A.T.; Ntalla, I.; Warren, H.R.; Guo, X.; Schwander, K.; et al. A multi-ancestry genome-wide study incorporating gene-smoking interactions identifies multiple new loci for pulse pressure and mean arterial pressure. Hum. Mol. Genet. 2019, 28, 2615–2633. [Google Scholar] [CrossRef] [PubMed]
- Surendran, P.; Feofanova, E.V.; Lahrouchi, N.; Ntalla, I.; Karthikeyan, S.; Cook, J.; Chen, L.; Mifsud, B.; Yao, C.; Kraja, A.T.; et al. Discovery of rare variants associated with blood pressure regulation through meta-analysis of 1.3 million individuals. Nat. Genet. 2020, 52, 1314–1332. [Google Scholar] [CrossRef] [PubMed]
- Wain, L.V.; Vaez, A.; Jansen, R.; Joehanes, R.; van der Most, P.J.; Erzurumluoglu, A.M.; O’Reilly, P.F.; Cabrera, C.P.; Warren, H.R.; Rose, L.M.; et al. Novel blood pressure locus and gene discovery using genome-wide association study and expression data sets from blood and the kidney. Hypertension 2017, 70, e4–e19. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Ehret, G.B.; Rice, K.; Verwoert, G.C.; Launer, L.J.; Dehghan, A.; Glazer, N.L.; Morrison, A.C.; Johnson, A.D.; Aspelund, T.; et al. Genome-wide association study of blood pressure and hypertension. Nat. Genet. 2009, 41, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Keaton, J.M.; Kamali, Z.; Xie, T.; Vaez, A.; Williams, A.; Goleva, S.B.; Ani, A.; Evangelou, E.; Hellwege, J.N.; Yengo, L.; et al. Genome-wide analysis in over 1 million individuals of European ancestry yields improved polygenic risk scores for blood pressure traits. Nat. Genet. 2024, 56, 778–791. [Google Scholar] [CrossRef]
- Neumann, J.T.; Riaz, M.; Bakshi, A.; Polekhina, G.; Thao, L.T.P.; Nelson, M.R.; Woods, R.L.; Abraham, G.; Inouye, M.; Reid, C.M.; et al. Predictive performance of a polygenic risk score for incident ischemic stroke in a healthy older population. Stroke 2021, 52, 2882–2891. [Google Scholar] [CrossRef]
- Wanby, P.; Palmquist, P.; Rydén, I.; Brattström, L.; Carlsson, M. The FABP2 gene polymorphism in cerebrovascular disease. Acta Neurol. Scand. 2004, 110, 355–360. [Google Scholar] [CrossRef]
- Lin, H.; Castro-Diehl, C.; Short, M.I.; Xanthakis, V.; Yola, I.M.; Kwan, A.C.; Mitchell, G.F.; Larson, M.G.; Vasan, R.S.; Cheng, S. Shared genetic and environmental architecture of cardiac phenotypes assessed via echocardiography: The Framingham heart study. Circ. Genom. Precis. Med. 2021, 14, e003244. [Google Scholar] [CrossRef]
- Lin, H.; Kwan, A.C.; Castro-Diehl, C.; Short, M.I.; Xanthakis, V.; Yola, I.M.; Salto, G.; Mitchell, G.F.; Larson, M.G.; Vasan, R.S.; et al. Sex-specific differences in the genetic and environmental effects on cardiac phenotypic variation assessed by echocardiography. Sci. Rep. 2023, 13, 5786. [Google Scholar] [CrossRef]
- Di Stolfo, G.; Mastroianno, S.; Soldato, N.; Massaro, R.S.; De Luca, G.; Seripa, D.; Urbano, M.; Gravina, C.; Greco, A.; Siena, P.; et al. The role of TOMM40 in cardiovascular mortality and conduction disorders: An observational study. J. Clin. Med. 2024, 13, 3177. [Google Scholar] [CrossRef]
- Francis, C.M.; Futschik, M.E.; Huang, J.; Bai, W.; Sargurupremraj, M.; Teumer, A.; Breteler, M.M.B.; Petretto, E.; Ho, A.S.R.; Amouyel, P.; et al. Genome-wide associations of aortic distensibility suggest causality for aortic aneurysms and brain white matter hyperintensities. Nat. Commun. 2022, 13, 4505. [Google Scholar] [CrossRef] [PubMed]
- Broce, I.J.; Tan, C.H.; Fan, C.C.; Jansen, I.; Savage, J.E.; Witoelar, A.; Wen, N.; Hess, C.P.; Dillon, W.P.; Glastonbury, C.M.; et al. Dissecting the genetic relationship between cardiovascular risk factors and Alzheimer’s disease. Acta Neuropathol. 2019, 137, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Bone, W.P.; Siewert, K.M.; Jha, A.; Klarin, D.; Damrauer, S.M.; VA Million Veteran Program; Chang, K.M.; Tsao, P.S.; Assimes, T.L.; Ritchie, M.D.; et al. Multi-trait association studies discover pleiotropic loci between Alzheimer’s disease and cardiometabolic traits. Alzheimers Res. Ther. 2021, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; Smith, A.V.; Aspelund, T.; Betensky, R.A.; Smoller, J.W.; Gudnason, V.; Launer, L.J.; Blacker, D. Genetic overlap between vascular pathologies and Alzheimer’s dementia and potential causal mechanisms. Alzheimer’s Dement. 2019, 15, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Colak, D.; Kaya, N.; Al-Zahrani, J.; Al Bakheet, A.; Muiya, P.; Andres, E.; Quackenbush, J.; Dzimiri, N. Left ventricular global transcriptional profiling in human end-stage dilated cardiomyopathy. Genomics 2009, 94, 20–31. [Google Scholar] [CrossRef]
- Colak, D.; Alaiya, A.A.; Kaya, N.; Muiya, N.P.; AlHarazi, O.; Shinwari, Z.; Andres, E.; Dzimiri, N. Integrated left ventricular global transcriptome and proteome profiling in human end-stage dilated cardiomyopathy. PLoS ONE 2016, 11, e0162669. [Google Scholar] [CrossRef]
- Lee, T.; Lee, H.; The Alzheimer’s Disease Neuroimaging Initiative. Identification of disease-related genes that are common between Alzheimer’s and cardiovascular disease using blood genome-wide transcriptome analysis. Biomedicines 2021, 9, 1525. [Google Scholar] [CrossRef]
- Ray, M.; Ruan, J.; Zhang, W. Variations in the transcriptome of Alzheimer’s disease reveal molecular networks involved in cardiovascular diseases. Genome Biol. 2008, 9, R148. [Google Scholar] [CrossRef]
- Gu, Q.; Xu, F.; Orgil, B.-O.; Khuchua, Z.; Munkhsaikhan, U.; Johnson, J.N.; Alberson, N.R.; Pierre, J.F.; Black, D.D.; Dong, D. Systems genetics analysis defines importance of TMEM43/LUMA for cardiac- and metabolic-related pathways. Physiol. Genom. 2022, 54, 22–35. [Google Scholar] [CrossRef]
- Liu, G.; Yao, L.; Liu, J.; Jiang, Y.; Ma, G.; Genetic and Environmental Risk for Alzheimer’s disease (GERAD1) Consortium; Chen, Z.; Zhao, B.; Li, K. Cardiovascular disease contributes to Alzheimer’s disease: Evidence from large-scale genome-wide association studies. Neurobiol. Aging 2014, 35, 786–792. [Google Scholar] [CrossRef]
- Chen, F.; Dong, X.; Yu, Z.; Zhang, Y.; Shi, Y. The brain-heart axis: Integrative analysis of the shared genetic etiology between neuropsychiatric disorders and cardiovascular disease. J. Affect. Disord. 2024, 355, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Andújar-Vera, F.; García-Fontana, C.; la Torre, R.S.-D.; González-Salvatierra, S.; Martínez-Heredia, L.; Iglesias-Baena, I.; Muñoz-Torres, M.; García-Fontana, B. Identification of potential targets linked to the cardiovascular/Alzheimer’s axis through bioinformatics approaches. Biomedicines 2022, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.; Porter, T.; Adewuyi, E.O.; Laws, S.M. Investigating genetic overlap between Alzheimer’s disease, lipids, and coronary artery disease: A large-scale genome-wide cross trait analysis. Int. J. Mol. Sci. 2024, 25, 8814. [Google Scholar] [CrossRef] [PubMed]
- Grace, C.; Clarke, R.; Goel, A.; Farrall, M.; Watkins, H.; Hopewell, J.C. Lack of genetic support for shared aetiology of Coronary Artery Disease and Late-onset Alzheimer’s disease. Sci. Rep. 2018, 8, 7102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Ren, C.-Y.; Yu, C. Causal relationship between Alzheimer’s disease and unstable angina: A bidirectional Mendelian randomization analysis. Front. Psychiatry 2024, 15, 1435394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Cui, Z.; Liu, F.; Hu, J. Identification of pleiotropic and specific therapeutic targets for cardio-cerebral diseases: A large-scale proteome-wide mendelian randomization and colocalization study. PLoS ONE 2024, 19, e0300500. [Google Scholar] [CrossRef]
- Wang, K.; Lu, Y.; Morrow, D.F.; Xiao, D.; Xu, C.; Alzheimer’s Disease Neuroimaging Initiative. Associations of ARHGAP26 polymorphisms with Alzheimer’s disease and cardiovascular disease. J. Mol. Neurosci. 2022, 72, 1085–1097. [Google Scholar] [CrossRef]
- Murdock, D.G.; Bradford, Y.; Schnetz-Boutaud, N.; Mayo, P.; Allen, M.J.; D’Aoust, L.N.; Liang, X.; Mitchell, S.L.; Zuchner, S.; Small, G.W.; et al. KIAA1462, a coronary artery disease associated gene, is a candidate gene for late onset Alzheimer disease in APOE carriers. PLoS ONE 2013, 8, e82194. [Google Scholar] [CrossRef]
- Li, R.; Song, J.; Zhao, A.; Diao, X.; Zhang, T.; Qi, X.; Guan, Z.; An, Y.; Ren, L.; Wang, C.; et al. Association of APP gene polymorphisms and promoter methylation with essential hypertension in Guizhou: A case-control study. Hum. Genom. 2023, 17, 25. [Google Scholar] [CrossRef]
- Karlsson, I.K.; Ploner, A.; Song, C.; Gatz, M.; Pedersen, N.L.; Hägg, S. Genetic susceptibility to cardiovascular disease and risk of dementia. Transl. Psychiatry 2017, 7, e1142. [Google Scholar] [CrossRef]
- Ngwa, J.S.; Fungwe, T.V.; Ntekim, O.; Allard, J.S.; Johnson, S.M.; Castor, C.; Graham, L.; Nadarajah, S.; Gillum, R.F.; Obisesan, T.O.; et al. Associations of pulse and blood pressure with hippocampal volume by APOE and cognitive phenotype: The Alzheimer’s Disease Neuroimaging Initiative (ADNI). Dement. Geriatr. Cogn. Disord. 2018, 45, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, S.; Hopper, I.; Chowdhury, E.; Wolfe, R.; Freak-Poli, R.; Reid, C.M.; Tonkin, A.M.; Murray, A.M.; Shah, R.C.; Chong, T.T.; et al. Cardiovascular disease risk scores and incident dementia and cognitive decline in older men and women. Gerontology 2024, 70, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Legdeur, N.; van der Lee, S.J.; de Wilde, M.; van der Lei, J.; Muller, M.; Maier, A.B.; Visser, P.J. The association of vascular disorders with incident dementia in different age groups. Alzheimers Res. Ther. 2019, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Preis, S.R.; Beiser, A.; Devine, S.; Hankee, L.; Seshadri, S.; Wolf, P.A.; Au, R. Mid-life cardiovascular risk impacts memory function: The Framingham Offspring study. Alzheimer Dis. Assoc. Disord. 2015, 29, 117–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tosto, G.; Bird, T.D.; Bennett, D.A.; Boeve, B.F.; Brickman, A.M.; Cruchaga, C.; Faber, K.; Foroud, T.M.; Farlow, M.; Goate, A.M.; et al. The role of cardiovascular risk factors and stroke in familial Alzheimer Disease. JAMA Neurol. 2016, 73, 1231–1237. [Google Scholar] [CrossRef]
- Zhukovsky, P.; Tio, E.S.; Coughlan, G.; Bennett, D.A.; Wang, Y.; Hohman, T.J.; Pizzagalli, D.A.; Mulsant, B.H.; Voineskos, A.N.; Felsky, D. Genetic influences on brain and cognitive health and their interactions with cardiovascular conditions and depression. Nat. Commun. 2024, 15, 5207. [Google Scholar] [CrossRef]
- Li, M.; Jiang, C.; Lai, Y.; Wang, Y.; Zhao, M.; Li, S.; Peng, X.; He, L.; Guo, X.; Li, S.; et al. Genetic evidence for causal association between atrial fibrillation and dementia: A Mendelian randomization study. J. Am. Heart Assoc. 2023, 12, e029623. [Google Scholar] [CrossRef]
- Sargurupremraj, M.; Soumaré, A.; Bis, J.C.; Surakka, I.; Jürgenson, T.; Joly, P.; Knol, M.J.; Wang, R.; Yang, Q.; Satizabal, C.L.; et al. Genetic complexities of cerebral small vessel disease, blood pressure, and dementia. JAMA Netw. Open 2024, 7, e2412824. [Google Scholar] [CrossRef]
- Siedlinski, M.; Carnevale, L.; Xu, X.; Carnevale, D.; Evangelou, E.; Caulfield, M.J.; Maffia, P.; Wardlaw, J.; Samani, N.J.; Tomaszewski, M.; et al. Genetic analyses identify brain structures related to cognitive impairment associated with elevated blood pressure. Eur. Heart J. 2023, 44, 2114–2125. [Google Scholar] [CrossRef]
- Malik, R.; Georgakis, M.K.; Neitzel, J.; Rannikmäe, K.; Ewers, M.; Seshadri, S.; Sudlow, C.L.; Dichgans, M. Midlife vascular risk factors and risk of incident dementia: Longitudinal cohort and Mendelian randomization analyses in the UK Biobank. Alzheimer’s Dement. 2021, 17, 1422–1431. [Google Scholar] [CrossRef]
- Zhong, A.; Tan, Y.; Liu, Y.; Chai, X.; Peng, W. There is no direct causal relationship between coronary artery disease and Alzheimer disease: A bidirectional Mendelian randomization study. J. Am. Heart Assoc. 2024, 13, e032814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xian, D.; Feng, J.; Ning, L.; Jiang, T.; Xu, W.; Liu, Y.; Zhao, Q.; Peng, M. Causal relationship between Alzheimer’s disease and cardiovascular disease: A bidirectional Mendelian randomization analysis. Aging 2023, 15, 9022–9040. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, M.A.; Skenteris, N.T.; Hutcheson, J.D.; van der Valk, D.D.; Bremer, J.; Goody, P.; Hjortnaes, J.; Jansen, F.; Bouten, C.V.C.; van den Bogaerdt, A.; et al. Integrative multi-omics analysis in calcific aortic valve disease reveals a link to the formation of amyloid-like deposits. Cells 2020, 9, 2164. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Breteler, M.M.; de Bruyne, M.C.; van Harskamp, F.; Grobbee, D.E.; Hofman, A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke 1997, 28, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; He, W.; Chen, H.; Chen, D. The causal effect of Alzheimer’s disease and family history of Alzheimer’s disease on non-ischemic cardiomyopathy and left ventricular structure and function: A Mendelian randomization study. Front. Genet. 2024, 15, 1379865. [Google Scholar] [CrossRef]
- Chi, J.; Hu, J.; Wu, N.; Cai, H.; Lin, C.; Lai, Y.; Huang, J.; Li, W.; Su, P.; Li, M.; et al. Causal effects for neurodegenerative diseases on the risk of myocardial infarction: A two-sample Mendelian randomization study. Aging 2024, 16, 9944–9958. [Google Scholar] [CrossRef]
- Moussavi Nik, S.H.; Porter, T.; Newman, M.; Bartlett, B.; Khan, I.; Sabale, M.; Eccles, M.; Woodfield, A.; Groth, D.; Dore, V.; et al. Relevance of a truncated PRESENILIN 2 transcript to Alzheimer’s disease and neurodegeneration. J. Alzheimer’s Dis. 2021, 80, 1479–1489. [Google Scholar] [CrossRef]
- Gama Sosa, M.A.; Gasperi, R.D.; Rocher, A.B.; Wang, A.C.-J.; Janssen, W.G.M.; Flores, T.; Perez, G.M.; Schmeidler, J.; Dickstein, D.L.; Hof, P.R.; et al. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer’s disease mutations. Am. J. Pathol. 2010, 176, 353–368. [Google Scholar] [CrossRef]
- Oka, S.; Leon, J.; Sakumi, K.; Ide, T.; Kang, D.; LaFerla, F.M.; Nakabeppu, Y. Human mitochondrial transcriptional factor A breaks the mitochondria-mediated vicious cycle in Alzheimer’s disease. Sci. Rep. 2016, 6, 37889. [Google Scholar] [CrossRef]
- Schoemaker, D.; Velilla-Jimenez, L.; Zuluaga, Y.; Baena, A.; Ospina, C.; Bocanegra, Y.; Alvarez, S.; Ochoa-Escudero, M.; Guzmán-Vélez, E.; Martinez, J.; et al. Global cardiovascular risk profile and cerebrovascular abnormalities in presymptomatic individuals with CADASIL or autosomal dominant Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 82, 841–853. [Google Scholar] [CrossRef]
- Naumenko, N.; Koivumäki, J.T.; Lunko, O.; Tuomainen, T.; Leigh, R.; Rabiee, M.; Laurila, J.; Oksanen, M.; Lehtonen, S.; Koistinaho, J.; et al. Presenilin-1 ΔE9 mutation associated sarcoplasmic reticulum leak alters [Ca2+]i distribution in human iPSC-derived cardiomyocytes. J. Mol. Cell Cardiol. 2024, 193, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Moriizumi, E.; Koseki, H.; Shirasawa, T. Presenilin 1 is essential for cardiac morphogenesis. Dev. Dyn. 2004, 230, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Mohuczy, D.; Qian, K.; Phillips, M.I. Presenilins in the heart: Presenilin-2 expression is increased by low glucose and by hypoxia in cardiac cells. Regul. Pept. 2002, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Asahi, M.; Yamaguchi, O.; Hikoso, S.; Nakayama, H.; Kusakari, Y.; Kawai, M.; Hongo, K.; Higuchi, Y.; Kashiwase, K.; et al. Presenilin 2 regulates the systolic function of heart by modulating Ca2+ signaling. FASEB J. 2005, 19, 2069–2071. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Parks, S.B.; Kushner, J.D.; Nauman, D.; Burgess, D.; Ludwigsen, S.; Partain, J.; Nixon, R.R.; Allen, C.N.; Irwin, R.P.; et al. Mutations of presenilin genes in dilated cardiomyopathy and heart failure. Am. J. Hum. Genet. 2006, 79, 1030–1039. [Google Scholar] [CrossRef]
- Sofer, T.; Kurniansyah, N.; Granot-Hershkovitz, E.; Goodman, M.O.; Tarraf, W.; Broce, I.; Lipton, R.B.; Daviglus, M.; Lamar, M.; Wassertheil-Smoller, S.; et al. A polygenic risk score for Alzheimer’s disease constructed using APOE-region variants has stronger association than APOE alleles with mild cognitive impairment in Hispanic/Latino adults in the U.S. Alzheimers Res. Ther. 2023, 15, 146. [Google Scholar] [CrossRef]
- Riaz, M.; Huq, A.; Ryan, J.; Orchard, S.G.; Tiller, J.; Lockery, J.; Woods, R.L.; Wolfe, R.; Renton, A.E.; Goate, A.M.; et al. Effect of APOE and a polygenic risk score on incident dementia and cognitive decline in a healthy older population. Aging Cell 2021, 20, e13384. [Google Scholar] [CrossRef]
- Ma, Y.; Jun, G.R.; Zhang, X.; Chung, J.; Naj, A.C.; Chen, Y.; Bellenguez, C.; Hamilton-Nelson, K.; Martin, E.R.; Kunkle, B.W.; et al. Analysis of whole-exome sequencing data for Alzheimer disease stratified by APOE genotype. JAMA Neurol. 2019, 76, 1099–1108. [Google Scholar] [CrossRef]
- Karlsson, I.K.; Ploner, A.; Wang, Y.; Gatz, M.; Pedersen, N.L.; Hägg, S. Apolipoprotein E DNA methylation and late-life disease. Int. J. Epidemiol. 2018, 47, 899–907. [Google Scholar] [CrossRef]
- Madrid, L.; Moreno-Grau, S.; Ahmad, S.; González-Pérez, A.; de Rojas, I.; Xia, R.; Martino Adami, P.V.; García-González, P.; Kleineidam, L.; Yang, Q.; et al. Multiomics integrative analysis identifies APOE allele-specific blood biomarkers associated to Alzheimer’s disease etiopathogenesis. Aging 2021, 13, 9277–9329. [Google Scholar] [CrossRef]
- Lumsden, A.L.; Mulugeta, A.; Zhou, A.; Hyppönen, E. Apolipoprotein E (APOE) genotype-associated disease risks: A phenome-wide, registry-based, case-control study utilising the UK Biobank. EBioMedicine 2020, 59, 102954. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.R.; Oulhaj, A.; de Jager, C.A.; Williams, J.H. APOE alleles predict the rate of cognitive decline in Alzheimer disease: A nonlinear model. Neurology 2005, 65, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Rosvall, L.; Rizzuto, D.; Wang, H.-X.; Winblad, B.; Graff, C.; Fratiglioni, L. APOE-related mortality: Effect of dementia, cardiovascular disease and gender. Neurobiol. Aging 2009, 30, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Régy, M.; Dugravot, A.; Sabia, S.; Helmer, C.; Tzourio, C.; Hanseeuw, B.; Singh-Manoux, A.; Dumurgier, J. The role of dementia in the association between APOE4 and all-cause mortality: Pooled analyses of two population-based cohort studies. Lancet Healthy Longev. 2024, 5, e422–e430. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.F.; Pereira, F.V.; Pivi, G.A.K.; Smith, M.C.; Bertolucci, P.H.F. Effects of APOE haplotypes and measures of cardiovascular risk over gender-dependent cognitive and functional changes in one year in Alzheimer’s disease. Int. J. Neurosci. 2018, 128, 472–476. [Google Scholar] [CrossRef]
- Beeri, M.S.; Rapp, M.; Silverman, J.M.; Schmeidler, J.; Grossman, H.T.; Fallon, J.T.; Purohit, D.P.; Perl, D.P.; Siddiqui, A.; Lesser, G.; et al. Coronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriers. Neurology 2006, 66, 1399–1404. [Google Scholar] [CrossRef]
- MacLeod, M.J.; De Lange, R.P.; Breen, G.; Meiklejohn, D.; Lemmon, H.; Clair, D.S. Lack of association between apolipoprotein E genoype and ischaemic stroke in a Scottish population. Eur. J. Clin. Investig. 2001, 31, 570–573. [Google Scholar] [CrossRef]
- Duzenli, S.; Pirim, I.; Gepdiremen, A.; Deniz, O. Apolipoprotein E polymorphism and stroke in a population from eastern Turkey. J. Neurogenet. 2004, 18, 365–375. [Google Scholar] [CrossRef]
- Satizabal, C.L.; Samieri, C.; Davis-Plourde, K.L.; Voetsch, B.; Aparicio, H.J.; Pase, M.P.; Romero, J.R.; Helmer, C.; Vasan, R.S.; Kase, C.S.; et al. APOE and the association of fatty acids with the risk of stroke, coronary heart disease, and mortality. Stroke 2018, 49, 2822–2829. [Google Scholar] [CrossRef]
- Kosunen, O.; Talasniemi, S.; Lehtovirta, M.; Heinonen, O.; Helisalmi, S.; Mannermaa, A.; Paljärvi, L.; Ryynänen, M.; Riekkinen, P.J.S.; Soininen, H. Relation of coronary atherosclerosis and apolipoprotein E genotypes in Alzheimer patients. Stroke 1995, 26, 743–748. [Google Scholar] [CrossRef]
- Selvaraj, S.; Claggett, B.; Johansen, M.C.; Cunningham, J.W.; Gottesman, R.F.; Yu, B.; Boerwinkle, E.; Mosley, T.H.; Shah, A.M.; Soloman, S.D. Apolipoprotein E polymorphism, cardiac remodeling, and heart failure in the ARIC study. J. Card. Fail. 2022, 28, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Corbo, R.M.; Scacchi, R.; Vilardo, T.; Ruggeri, M. Polymorphisms in the apolipoprotein E gene regulatory region in relation to coronary heart disease and their effect on plasma apolipoprotein E. Clin. Chem. Lab. Med. 2001, 39, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Versmissen, J.; Oosterveer, D.M.; Hoekstra, M.; Out, R.; Berbée, J.F.P.; Blommesteijn-Touw, A.C.; van Vark-van der Zee, L.; Vongpromek, R.; Vanmierlo, T.; Defesche, J.C.; et al. Apolipoprotein isoform E4 does not increase coronary heart disease risk in carriers of low-density lipoprotein receptor mutations. Circ. Cardiovasc. Genet. 2011, 4, 655–660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ji, H.; Zhou, C.; Pan, R.; Han, L.; Chen, W.; Xu, X.; Huang, Y.; Huang, T.; Zou, Y.; Duan, S. APOE hypermethylation is significantly associated with coronary heart disease in males. Gene 2019, 689, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.C.; Brousseau, T.; Defosse, V.; Evans, A.; Arveiler, D.; Ruidavets, J.B.; Haas, B.; Cambou, J.P.; Luc, G.; Ducimetière, P.; et al. Independent association of an APOE gene promoter polymorphism with increased risk of myocardial infarction and decreased APOE plasma concentrations-the ECTIM study. Hum. Mol. Genet. 2000, 9, 57–61. [Google Scholar] [CrossRef][Green Version]
- van der Cammen, T.J.; Verschoor, C.J.; van Loon, C.P.; van Harskamp, F.; de Koning, I.; Schudel, W.J.; Slooter, A.J.; Van Broeckhoven, C.; van Duijn, C.M. Risk of left ventricular dysfunction in patients with probable Alzheimer’s disease with APOE*4 allele. J. Am. Geriatr. Soc. 1998, 46, 962–967. [Google Scholar] [CrossRef]
- Kang, J.H.; Logroscino, G.; De Vivo, I.; Hunter, D.; Grodstein, F. Apolipoprotein E, cardiovascular disease and cognitive function in aging women. Neurobiol. Aging 2005, 26, 475–484. [Google Scholar] [CrossRef]
- Kaufman, C.S.; Morris, J.K.; Vidoni, E.D.; Burns, J.M.; Billinger, S.A. Apolipoprotein E4 moderates the association between vascular risk factors and brain pathology. Alzheimer Dis. Assoc. Disord. 2021, 35, 223–229. [Google Scholar] [CrossRef]
- Perna, L.; Mons, U.; Rujescu, D.; Kliegel, M.; Brenner, H. Apolipoprotein E e4 and cognitive function: A modifiable association results from two independent cohort studies. Dement. Geriatr. Cogn. Disord. 2016, 41, 35–45. [Google Scholar] [CrossRef]
- Cambronero, F.E.; Liu, D.; Neal, J.E.; Moore, E.E.; Gifford, K.A.; Terry, J.G.; Nair, S.; Pechman, K.R.; Osborn, K.E.; Hohman, T.J.; et al. APOE genotype modifies the association between central arterial stiffening and cognition in older adults. Neurobiol. Aging 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Couderc, R.; Mahieux, F.; Bailleul, S.; Fenelon, G.; Mary, R.; Fermanian, J. Prevalence of apolipoprotein E phenotypes in ischemic cerebrovascular disease. A case-control study. Stroke 1993, 24, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Libri, I.; Silvestri, C.; Caratozzolo, S.; Alberici, A.; Pilotto, A.; Archetti, S.; Trainini, L.; Borroni, B.; Padovani, A.; Benussi, A. Association of APOE genotype with blood-brain barrier permeability in neurodegenerative disorders. Neurobiol. Aging 2024, 140, 33–40. [Google Scholar] [CrossRef]
- Halliday, M.R.; Rege, S.V.; Ma, Q.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Mur, J.; McCartney, D.L.; Walker, R.M.; Campbell, A.; Bermingham, M.L.; Morris, S.W.; Porteous, D.J.; McIntosh, A.M.; Deary, I.J.; Evans, K.L.; et al. DNA methylation in APOE: The relationship with Alzheimer’s and with cardiovascular health. Alzheimer’s Dement. 2020, 6, e12026. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.C.; Tank, R.; Lyall, L.M.; Ward, J.; Celis-Morales, C.; Strawbridge, R.; Ho, F.; Whelan, C.D.; Gill, J.; Welsh, P.; et al. Alzheimer’s disease susceptibility gene apolipoprotein E (APOE) and blood biomarkers in UK Biobank (N = 395,769). J. Alzheimer’s Dis. 2020, 76, 1541–1551. [Google Scholar] [CrossRef]
- Karjalainen, J.-P.; Mononen, N.; Hutri-Kähönen, N.; Lehtimäki, M.; Juonala, M.; Ala-Korpela, M.; Kähönen, M.; Raitakari, O.; Lehtimäki, T. The effect of apolipoprotein E polymorphism on serum metabolome—A population-based 10-year follow-up study. Sci. Rep. 2019, 9, 458. [Google Scholar] [CrossRef]
- Garcia-Segura, M.E.; Fischer, C.E.; Schweizer, T.A.; Munoz, D.G. APOE ɛ4/ɛ4 is associated with aberrant motor behavior through both Lewy body and cerebral amyloid angiopathy pathology in high Alzheimer’s disease pathological load. J. Alzheimer’s Dis. 2019, 72, 1077–1087. [Google Scholar] [CrossRef]
- Insel, P.S.; Hansson, O.; Mattsson-Carlgren, N. Association between apolipoprotein E ε2 vs ε4, age, and β-amyloid in adults without cognitive impairment. JAMA Neurol. 2021, 78, 229–235. [Google Scholar] [CrossRef]
- Chalmers, K.; Wilcock, G.K.; Love, S. APOE epsilon 4 influences the pathological phenotype of Alzheimer’s disease by favouring cerebrovascular over parenchymal accumulation of A beta protein. Neuropathol. Appl. Neurobiol. 2003, 29, 231–238. [Google Scholar] [CrossRef]
- Hawkes, C.A.; Sullivan, P.M.; Hands, S.; Weller, R.O.; Nicoll, J.A.R.; Carare, R.O. Disruption of arterial perivascular drainage of amyloid-β from the brains of mice expressing the human APOE ε4 allele. PLoS ONE 2012, 7, e41636. [Google Scholar] [CrossRef] [PubMed]
- Schmechel, D.E.; Saunders, A.M.; Strittmatter, W.J.; Crain, B.J.; Hulette, C.M.; Joo, S.H.; Pericak-Vance, D.E.; Goldgaber, D.; Roses, A.D. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 9649–9653. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, J.D.; Aguirre-Acevedo, D.C.; Gaona, C.; Chavez, C.A.; Calmón, G.E.; Silva, E.R.; de Erausquin, G.A.; Gil, M.; Mena, L.J.; Terwilliger, J.D.; et al. Nighttime blood pressure interacts with APOE genotype to increase the risk of incident dementia of the Alzheimer’s type in Hispanics. J. Alzheimer’s Dis. 2020, 77, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, T.; Baba, S.; Ishikawa, K.; Mannami, T.; Fu, Y.; Inamoto, N.; Asai, T.; Fukuda, M.; Higaki, J.; Ogata, J.; et al. Epsilon 4 allele of apolipoprotein E gene associates with lower blood pressure in young Japanese subjects: The Suita Study. J. Hypertens. 2002, 20, 2017–2021. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-W.; Zhu, Y.-X.; Liu, H.-Y.; Liu, J.; Zhu, X.-Q.; Zhou, J.-N.; Liu, R.-Y. Decreased cerebral blood flow velocity in apolipoprotein E ɛ4 allele carriers with mild cognitive impairment. Eur. J. Neurol. 2007, 14, 150–155. [Google Scholar] [CrossRef]
- Bown, C.W.; Liu, D.; Osborn, K.E.; Gupta, D.K.; Mendes, L.A.; Pechman, K.R.; Hohman, T.J.; Wang, T.J.; Gifford, K.A.; Jefferson, A.L. Apolipoprotein E genotype modifies the association between cardiac output and cognition in older adults. J. Am. Heart Assoc. 2019, 8, e011146. [Google Scholar] [CrossRef]
- Wolters, F.J.; de Bruijn, R.F.A.G.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A.; Heart Brain Connection Collaborative Research Group. Cerebral vasoreactivity, apolipoprotein E, and the risk of dementia: A population-based study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 204–210. [Google Scholar] [CrossRef]
- Day, G.S.; Cruchaga, C.; Wingo, T.; Schindler, S.E.; Coble, D.; Morris, J.C. Association of acquired and heritable factors with intergenerational differences in age at symptomatic onset of Alzheimer disease between offspring and parents with dementia. JAMA Netw. Open 2019, 2, e1913491. [Google Scholar] [CrossRef]
- Vélez, J.I.; Lopera, F.; Creagh, P.K.; Piñeros, L.B.; Das, D.; Cervantes-Henríquez, M.L.; Acosta-López, J.E.; Isaza-Ruget, M.A.; Espinosa, L.G.; Easteal, S.; et al. Targeting neuroplasticity, cardiovascular, and cognitive-associated genomic variants in familial Alzheimer’s disease. Mol. Neurobiol. 2019, 56, 3235–3243. [Google Scholar] [CrossRef]
- Emanuele, E.; Peros, E.; Tomaino, C.; Feudatari, E.; Bernardi, L.; Binetti, G.; Maletta, R.; D’Angelo, A.; Montagna, L.; Bruni, A.C.; et al. Apolipoprotein(a) null phenotype is related to a delayed age at onset of Alzheimer’s disease. Neurosci. Lett. 2004, 357, 45–48. [Google Scholar] [CrossRef]
- Tao, Q.; Ang, T.F.A.; DeCarli, C.; Auerbach, S.H.; Devine, S.; Stein, T.D.; Zhang, X.; Massaro, J.; Au, R.; Qiu, W.Q. Association of chronic low-grade inflammation with risk of Alzheimer disease in ApoE4 carriers. JAMA Netw. Open 2018, 1, e183597. [Google Scholar] [CrossRef] [PubMed]
- Bellou, E.; Escott-Price, V. Are Alzheimer’s and coronary artery diseases genetically related to longevity? Front. Psychiatry 2022, 13, 1102347. [Google Scholar] [CrossRef]
- Lee, A.J.; Raghavan, N.S.; Bhattarai, P.; Siddiqui, T.; Sariya, S.; Reyes-Dumeyer, D.; Flowers, X.E.; Cardoso, S.A.L.; De Jager, P.L.; Bennett, D.A.; et al. FMNL2 regulates gliovascular interactions and is associated with vascular risk factors and cerebrovascular pathology in Alzheimer’s disease. Acta Neuropathol. 2022, 144, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, J.; Willenborg, C.; Nahrstaedt, J.; Preuss, M.; König, I.R.; Baumert, J.; Linsel-Nitschke, P.; Gieger, C.; Tennstedt, S.; Belcredi, P.; et al. Genome-wide association study identifies a new locus for coronary artery disease on chromosome 10p11.23. Eur. Heart J. 2011, 32, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, J.; Hot, D.; Hansmannel, F.; Kerdraon, O.; Ferreira, S.; Hubans, C.; Maurage, C.A.; Huot, L.; Bensemain, F.; Laumet, G.; et al. Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer’s disease. Mol. Psychiatry 2009, 14, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Wightman, D.P.; Jansen, I.E.; Savage, J.E.; Shadrin, A.A.; Bahrami, S.; Holland, D.; Rongve, A.; Børte, S.; Winsvold, B.S.; Drange, O.K.; et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 2021, 53, 1276–1282. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.; Finucane, H.K.; Anttila, V.; Gusev, A.; Day, F.R.; Loh, P.-R.; ReproGen Consortium; Psychiatric Genomics Consortium; Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3; Duncan, L.; et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015, 47, 1236–1241. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef]
- Cruts, M.; Van Broeckhoven, C. Molecular genetics of Alzheimer’s disease. Ann. Med. 1998, 30, 560–565. [Google Scholar] [CrossRef]
- Sherrington, R.; Rogaev, E.I.; Liang, Y.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Chi, H.; Lin, C.; Li, G.; Holman, K.; et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995, 375, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Gianni, D.; Li, A.; Tesco, G.; McKay, K.M.; Moore, J.; Raygor, K.; Rota, M.; Gwathmey, J.K.; Dec, G.W.; Aretz, T.; et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation 2010, 121, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.-L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 2018, 98, 1141–1154.e7. [Google Scholar] [CrossRef] [PubMed]
- Kulminski, A.M.; Ukraintseva, S.V.; Arbeev, K.G.; Manton, K.G.; Oshima, J.; Martin, G.M.; Il’yasova, D.; Yashin, A.I. Health-protective and adverse effects of the apolipoprotein E ɛ2 allele in older men. J. Am. Geriatr. Soc. 2008, 56, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Kulminski, A.M.; Arbeev, K.G.; Culminskaya, I.; Arbeeva, L.; Ukraintseva, S.V.; Stallard, E.; Christensen, K.; Schupf, N.; Province, M.A.; Yashin, A.I. Age, gender, and cancer but not neurodegenerative and cardiovascular diseases strongly modulate systemic effect of the Apolipoprotein E4 allele on lifespan. PLoS Genet. 2014, 10, e1004141. [Google Scholar] [CrossRef]
- Rebeck, G.W.; Reiter, J.S.; Strickland, D.K.; Hyman, B.T. Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron 1993, 11, 575–580. [Google Scholar] [CrossRef]
- Tiraboschi, P.; Hansen, L.A.; Masliah, E.; Alford, M.; Thal, L.J.; Corey-Bloom, J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology 2004, 62, 1977–1983. [Google Scholar] [CrossRef]
- Fernández-Calle, R.; Konings, S.C.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; Martinson, I.; Boza-Serrano, A.; Venero, J.L.; Nielsen, H.M.; et al. APOE in the bullseye of neurodegenerative diseases: Impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol. Neurodegener. 2022, 17, 62. [Google Scholar] [CrossRef]
- Cooper, J.G.; Ghodsi, M.; Stukas, S.; Leach, S.; Brooks-Wilson, A.; Wellington, C.L. APOE ε4 carrier status modifies plasma p-tau181 concentrations in cognitively healthy super-seniors. Alzheimers Dement 2024, 20, 4373–4380. [Google Scholar] [CrossRef]
- Fagan, A.M.; Watson, M.; Parsadanian, M.; Bales, K.R.; Paul, S.M.; Holtzman, D.M. Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2002, 9, 305–318. [Google Scholar] [CrossRef]
- Svobodová, H.; Kucera, F.; Stulc, T.; Vrablík, M.; Amartuvshin, B.; Altannavch, T.; Ceska, R. Apolipoprotein E gene polymorphism in the Mongolian population. Folia Biol. 2007, 53, 138–142. [Google Scholar]
- Sanna, G.D.; Nusdeo, G.; Piras, M.R.; Forteleoni, A.; Murru, M.R.; Saba, P.S.; Dore, S.; Sotgiu, G.; Parodi, G.; Ganau, A. Cardiac abnormalities in Alzheimer disease: Clinical relevance beyond pathophysiological rationale and instrumental findings? JACC Heart Fail 2019, 7, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.M.; Roses, A.D. Apolipoprotein E4 allele frequency, ischemic cerebrovascular disease, and Alzheimer’s disease. Stroke 1993, 24, 1416–1417. [Google Scholar] [CrossRef] [PubMed]
- Van Giau, V.; Bagyinszky, E.; An, S.S.A.; Kim, S.Y. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 2015, 11, 1723–1737. [Google Scholar] [CrossRef] [PubMed]
- Luciani, M.; Montalbano, M.; Troncone, L.; Bacchin, C.; Uchida, K.; Daniele, G.; Jacobs Wolf, B.; Butler, H.M.; Kiel, J.; Berto, S.; et al. Big tau aggregation disrupts microtubule tyrosination and causes myocardial diastolic dysfunction: From discovery to therapy. Eur. Heart J. 2023, 44, 1560–1570. [Google Scholar] [CrossRef]
- Dark, H.E.; Paterson, C.; Daya, G.N.; Peng, Z.; Duggan, M.R.; Bilgel, M.; An, Y.; Moghekar, A.; Davatzikos, C.; Resnick, S.M.; et al. Proteomic indicators of health predict Alzheimer’s disease biomarker levels and dementia risk. Ann. Neurol. 2024, 95, 260–273. [Google Scholar] [CrossRef]
- Sanbe, A.; Osinska, H.; Saffitz, J.E.; Glabe, C.G.; Kayed, R.; Maloyan, A.; Robbins, J. Desmin-related cardiomyopathy in transgenic mice: A cardiac amyloidosis. Proc. Natl. Acad. Sci. USA 2004, 101, 10132–10136. [Google Scholar] [CrossRef]
- Subramanian, K.; Gianni, D.; Balla, C.; Assenza, G.E.; Joshi, M.; Semigran, M.J.; Macgillivray, T.E.; Van Eyk, J.E.; Agnetti, G.; Paolocci, N.; et al. Cofilin-2 phosphorylation and sequestration in myocardial aggregates: Novel pathogenetic mechanisms for idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1199–1214. [Google Scholar] [CrossRef]
- Betrie, A.H.; Ayton, S.; Bush, A.I.; Angus, J.A.; Lei, P.; Wright, C.E. Evidence of a cardiovascular function for microtubule-associated protein tau. J. Alzheimer’s Dis. 2017, 56, 849–860. [Google Scholar] [CrossRef]
- Kyrtsos, C.R.; Baras, J.S. Modeling the role of the glymphatic pathway and cerebral blood vessel properties in Alzheimer’s disease pathogenesis. PLoS ONE 2015, 10, e0139574. [Google Scholar] [CrossRef]
- Lee, E.-G.; Chen, S.; Leong, L.; Tulloch, J.; Yu, C.-E. TOMM40 RNA transcription in Alzheimer’s disease brain and its implication in mitochondrial dysfunction. Genes 2021, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; La Rue, A.; Hermann, B.P.; Xu, G.; Koscik, R.L.; Jonaitis, E.M.; Bendlin, B.B.; Hogan, K.J.; Roses, A.D.; Saunders, A.M.; et al. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOEɛ3/ɛ3 genotype. Alzheimer’s Dement. 2011, 7, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-X.; Zhu, Y.-R.; Liu, H.-M.; Chen, S.-L.; Zhang, D.-M. Effect of BIN1 on cardiac dysfunction and malignant arrhythmias. Acta Physiol. 2020, 228, e13429. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, C.; Wei, J.; Shufelt, C.; CHitzeman, T.; Cook-Wiens, G.; Pepine, C.J.; Handberg, E.; Anderson, R.D.; Petersen, J.; Hong, T.; et al. Association of coronary microvascular dysfunction and cardiac bridge integrator 1, a cardiomyocyte dysfunction biomarker. Clin. Cardiol. 2021, 44, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Tsartsalis, S.; Sleven, H.; Fancy, N.; Wessely, F.; Smith, A.M.; Willumsen, N.; Cheung, T.K.D.; Rokicki, M.J.; Chau, V.; Ifie, E.; et al. A single nuclear transcriptomic characterisation of mechanisms responsible for impaired angiogenesis and blood-brain barrier function in Alzheimer’s disease. Nat. Commun. 2024, 15, 2243. [Google Scholar] [CrossRef]
- Stefanova, N.A.; Maksimova, K.Y.; Rudnitskaya, E.A.; Muraleva, N.A.; Kolosova, N.G. Association of cerebrovascular dysfunction with the development of Alzheimer’s disease-like pathology in OXYS rats. BMC Genom. 2018, 19, 75. [Google Scholar] [CrossRef]
- Tokuoka, S.M.; Hamano, F.; Kobayashi, A.; Adachi, S.; Andou, T.; Natsume, T.; Oda, Y. Plasma proteomics and lipidomics facilitate elucidation of the link between Alzheimer’s disease development and vessel wall fragility. Sci. Rep. 2024, 14, 19901. [Google Scholar] [CrossRef]
- Yu, M.; Nie, Y.; Yang, J.; Yang, S.; Li, R.; Rao, V.; Hu, X.; Fang, C.; Li, S.; Song, D.; et al. Integrative multi-omic profiling of adult mouse brain endothelial cells and potential implications in Alzheimer’s disease. Cell Rep. 2023, 42, 113392. [Google Scholar] [CrossRef]
- Singh Angom, R.; Wang, Y.; Wang, E.; Pal, K.; Bhattacharya, S.; Watzlawik, J.O.; Rosenberry, T.L.; Das, P.; Mukhopadhyay, D. VEGF receptor-1 modulates amyloid β 1-42 oligomer-induced senescence in brain endothelial cells. FASEB J. 2019, 33, 4626–4637. [Google Scholar] [CrossRef]
- Aguilar-Pineda, J.A.; Vera-Lopez, K.J.; Shrivastava, P.; Chávez-Fumagalli, M.A.; Nieto-Montesinos, R.; Alvarez-Fernandez, K.L.; Goyzueta Mamani, L.D.; Davila Del-Carpio, G.; Gomez-Valdez, B.; Miller, C.L.; et al. Vascular smooth muscle cell dysfunction contribute to neuroinflammation and Tau hyperphosphorylation in Alzheimer disease. iScience 2021, 24, 102993. [Google Scholar] [CrossRef]
- D’Agostino RBSr Pencina, M.J.; Massaro, J.M.; Coady, S. Cardiovascular disease risk assessment: Insights from Framingham. Glob. Heart 2013, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Akadam-Teker, B.; Kurnaz, O.; Coskunpinar, E.; Daglar-Aday, A.; Kucukhuseyin, O.; Cakmak, H.A.; Teker, E.; Bugra, Z.; Ozturk, O.; Yilmaz-Aydogan, H. The effects of age and gender on the relationship between HMGCR promoter-911 SNP (rs33761740) and serum lipids in patients with coronary heart disease. Gene 2013, 528, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hindorff, L.A.; Lemaitre, R.N.; Smith, N.L.; Bis, J.C.; Marciante, K.D.; Rice, K.M.; Lumley, T.; Enquobahrie, D.A.; Li, G.; Heckbert, S.R.; et al. Common genetic variation in six lipid-related and statin-related genes, statin use and risk of incident nonfatal myocardial infarction and stroke. Pharmacogenet Genom. 2008, 18, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Han, K.; Kim, H.-S.; Park, Y.-M.; Kwon, H.-S.; Yoon, K.-H.; Lee, S.-H. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: A nationwide population-based study. Eur. Heart J. 2017, 38, 3560–3566. [Google Scholar] [CrossRef]
- Vojinovic, D.; Kalaoja, M.; Trompet, S.; Fischer, K.; Shipley, M.J.; Li, S.; Havulinna, A.S.; Perola, M.; Salomaa, V.; Yang, Q.; et al. Association of circulating metabolites in plasma or serum and risk of stroke: Meta-analysis from 7 prospective cohorts. Neurology 2021, 96, e1110–e1123. [Google Scholar] [CrossRef]
- Kanoni, S.; Graham, S.E.; Wang, Y.; Surakka, I.; Ramdas, S.; Zhu, X.; Clarke, S.L.; Bhatti, K.F.; Vedantam, S.; Winkler, T.W.; et al. Implicating genes, pleiotropy, and sexual dimorphism at blood lipid loci through multi-ancestry meta-analysis. Genome Biol. 2022, 23, 268. [Google Scholar] [CrossRef]
- Cadby, G.; Giles, C.; Melton, P.E.; Huynh, K.; Mellett, N.A.; Duong, T.; Nguyen, A.; Cinel, M.; Smith, A.; Olshansky, G.; et al. Comprehensive genetic analysis of the human lipidome identifies loci associated with lipid homeostasis with links to coronary artery disease. Nat. Commun. 2022, 13, 3124. [Google Scholar] [CrossRef]
- Hedman, Å.K.; Mendelson, M.M.; Marioni, R.E.; Gustafsson, S.; Joehanes, R.; Irvin, M.R.; Zhi, D.; Sandling, J.K.; Yao, C.; Liu, C.; et al. Epigenetic patterns in blood associated with lipid traits predict incident coronary heart disease events and are enriched for results from genome-wide association studies. Circ. Cardiovasc. Genet. 2017, 10, e001487. [Google Scholar] [CrossRef]
- Hebert, P.R. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. JAMA 1997, 278, 313. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Crowe, T.; Sasiela, W.J.; Tsai, J.; Orazem, J.; Magorien, R.D.; O’Shaughnessy, C.; Ganz, P.; et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 2005, 352, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L.; Boström, K.; Jungbjer, B. Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathol. 1997, 94, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Tynkkynen, J.; Chouraki, V.; van der Lee, S.J.; Hernesniemi, J.; Yang, Q.; Li, S.; Beiser, A.; Larson, M.G.; Sääksjärvi, K.; Shipley, M.J.; et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: A prospective study in eight cohorts. Alzheimer’s Dement. 2018, 14, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Tindale, L.C.; Leach, S.; Spinelli, J.J.; Brooks-Wilson, A.R. Lipid and Alzheimer’s disease genes associated with healthy aging and longevity in healthy oldest-old. Oncotarget 2017, 8, 20612–20621. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lin, Y.; Li, X.; Driver, J.A.; Liang, L. Shared genetic architecture between metabolic traits and Alzheimer’s disease: A large-scale genome-wide cross-trait analysis. Hum. Genet. 2019, 138, 271–285. [Google Scholar] [CrossRef]
- Desikan, R.S.; Schork, A.J.; Wang, Y.; Thompson, W.K.; Dehghan, A.; Ridker, P.M.; Chasman, D.I.; McEvoy, L.K.; Holland, D.; Chen, C.H.; et al. Polygenic overlap between C-reactive protein, plasma lipids, and Alzheimer disease. Circulation 2015, 131, 2061–2069. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Ou, Y.-N.; Yang, Y.-X.; Wang, Z.-T.; Tan, L.; Yu, J.-T. Associations of cardiovascular risk factors and lifestyle behaviors with neurodegenerative disease: A Mendelian randomization study. Transl. Psychiatry 2023, 13, 267. [Google Scholar] [CrossRef]
- Tan, J.-S.; Hu, M.-J.; Yang, Y.-M.; Yang, Y.-J. Genetic predisposition to low-density lipoprotein cholesterol may increase risks of both individual and familial Alzheimer’s disease. Front. Med. 2021, 8, 798334. [Google Scholar] [CrossRef]
- European Alzheimer’s & Dementia Biobank Mendelian Randomization (EADB-MR) Collaboration; Luo, J.; Thomassen, J.Q.; Bellenguez, C.; Grenier-Boley, B.; de Rojas, I.; Castillo, A.; Parveen, K.; Küçükali, F.; Nicolas, A.; et al. Genetic associations between modifiable risk factors and Alzheimer disease. JAMA Netw. Open 2023, 6, e2313734. [Google Scholar]
- Picard, C.; Poirier, A.; Bélanger, S.; Labonté, A.; Auld, D.; Poirier, J.; PREVENT-AD Research Group. Proprotein convertase subtilisin/kexin type 9 (PCSK9) in Alzheimer’s disease: A genetic and proteomic multi-cohort study. PLoS ONE 2019, 14, e0220254. [Google Scholar] [CrossRef]
- Ko, Y.-A.; Billheimer, J.T.; Lyssenko, N.N.; Kueider-Paisley, A.; Wolk, D.A.; Arnold, S.E.; Leung, Y.Y.; Shaw, L.M.; Trojanowski, J.Q.; Kaddurah-Daouk, R.F.; et al. ApoJ/Clusterin concentrations are determinants of cerebrospinal fluid cholesterol efflux capacity and reduced levels are associated with Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yagi, R.; Ogata, S.; Masuda, T.; Saito, T.; Saido, T.; Ohtsuki, S. Proteomic alterations in the brain and blood-brain barrier during brain Aβ accumulation in an APP knock-in mouse model of Alzheimer’s disease. Fluids Barriers CNS 2023, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Algotsson, A.; Winblad, B. Patients with Alzheimer’s disease may be particularly susceptible to adverse effects of statins. Dement. Geriatr. Cogn. Disord. 2004, 17, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; van der Toorn, J.E.; Sijbrands, E.J.G.; de Rijke, Y.B.; Kavousi, M.; Bos, D. Lipoprotein(a) is associated with a larger systemic burden of arterial calcification. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1102–1109. [Google Scholar] [CrossRef]
- Larsson, S.C.; Gill, D.; Mason, A.; Jiang, T.; Bäck, M.; Butterworth, A.; Burgess, S. Lipoprotein(a) in Alzheimer, atherosclerotic, cerebrovascular, thrombotic, and valvular disease: Mendelian randomization investigation. Circulation 2020, 141, 1826–1828. [Google Scholar] [CrossRef]
- Pan, Y.; Li, H.; Wang, Y.; Meng, X.; Wang, Y. Causal effect of LP(a) [lipoprotein(a)] level on ischemic stroke and Alzheimer disease: A Mendelian randomization study. Stroke 2019, 50, 3532–3539. [Google Scholar] [CrossRef]
- Iwamoto, T.; Watanabe, D.; Umahara, T.; Sakurai, H.; Hanyu, H.; Kanaya, K. Dual inverse effects of lipoprotein(a) on the dementia process in Japanese late-onset Alzheimer’s disease. Psychogeriatrics 2004, 4, 64–71. [Google Scholar] [CrossRef]
- Ray, L.; Khemka, V.K.; Behera, P.; Bandyopadhyay, K.; Pal, S.; Pal, K.; Basu, D.; Chakrabarti, S. Serum homocysteine, dehydroepiandrosterone sulphate and lipoprotein (a) in Alzheimer’s disease and vascular dementia. Aging Dis. 2013, 4, 57–64. [Google Scholar]
- Myllykangas, L.; Polvikoski, T.; Sulkava, R.; Verkkoniemi, A.; Tienari, P.; Niinistö, L.; Kontula, K.; Hardy, J.; Haltia, M.; Pérez-Tur, J. Cardiovascular risk factors and Alzheimer’s disease: A genetic association study in a population aged 85 or over. Neurosci. Lett. 2000, 292, 195–198. [Google Scholar] [CrossRef]
- Williams, D.M.; Finan, C.; Schmidt, A.F.; Burgess, S.; Hingorani, A.D. Lipid lowering and Alzheimer disease risk: A mendelian randomization study. Ann. Neurol. 2020, 87, 30–39. [Google Scholar] [CrossRef]
- Lord, J.; Jermy, B.; Green, R.; Wong, A.; Xu, J.; Legido-Quigley, C.; Dobson, R.; Richards, M.; Proitsi, P. Mendelian randomization identifies blood metabolites previously linked to midlife cognition as causal candidates in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2009808118. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, R.J.; Reus, L.M.; De Witte, W.; Tijms, B.M.; Olde Rikkert, M.; Visser, P.J.; Poelmans, G. Genetic overlap between Alzheimer’s disease and blood lipid levels. Neurobiol. Aging 2021, 108, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tong, T.; Chang, A.; Ang, T.F.A.; Tao, Q.; Auerbach, S.; Devine, S.; Qiu, W.Q.; Mez, J.; Massaro, J.; et al. Midlife lipid and glucose levels are associated with Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Johnson, R.S.; Damodarasamy, M.; MacCoss, M.J.; Keene, C.D.; Banks, W.A.; Reed, M.J. Data-independent acquisition proteomic analysis of the brain microvasculature in Alzheimer’s disease identifies major pathways of dysfunction and upregulation of cytoprotective responses. Fluids Barriers CNS 2024, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Aulchenko, Y.S.; Ripatti, S.; Lindqvist, I.; Boomsma, D.; Heid, I.M.; Pramstaller, P.P.; Penninx, B.W.; Janssens, A.C.; Wilson, J.F.; Spector, T.; et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 2009, 41, 47–55. [Google Scholar]
- Cupido, A.J.; Reeskamp, L.F.; Hingorani, A.D.; Finan, C.; Asselbergs, F.W.; Hovingh, G.K.; Schmidt, A.F. Joint genetic inhibition of PCSK9 and CETP and the association with coronary artery disease: A factorial Mendelian randomization study. JAMA Cardiol. 2022, 7, 955–964. [Google Scholar] [CrossRef]
- Tan, Z.S.; Seshadri, S.; Beiser, A.; Wilson, P.W.F.; Kiel, D.P.; Tocco, M.; D’Agostino, R.B.; Wolf, P.A. Plasma total cholesterol level as a risk factor for Alzheimer disease: The Framingham Study. Arch. Intern. Med. 2003, 163, 1053–1057. [Google Scholar] [CrossRef]
- Davignon, J.; Gregg, R.E.; Sing, C.F. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis 1988, 8, 1–21. [Google Scholar] [CrossRef]
- de Bruijn, R.F.A.G.; Ikram, M.A. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014, 12, 130. [Google Scholar] [CrossRef]
- Garcia, A.R.; Finch, C.; Gatz, M.; Kraft, T.; Rodriguez, D.E.; Cummings, D.; Charifson, M.; Buetow, K.; A Beheim, B.; Allayee, H.; et al. APOE4 is associated with elevated blood lipids and lower levels of innate immune biomarkers in a tropical Amerindian subsistence population. eLife 2021, 10, e68231. [Google Scholar] [CrossRef]
- Ong, W.-Y.; Ng, M.P.-E.; Loke, S.-Y.; Jin, S.; Wu, Y.-J.; Tanaka, K.; Wong, P.T. Comprehensive gene expression profiling reveals synergistic functional networks in cerebral vessels after hypertension or hypercholesterolemia. PLoS ONE 2013, 8, e68335. [Google Scholar] [CrossRef] [PubMed]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Roqueta-Rivera, M.; Esquejo, R.M.; Phelan, P.E.; Sandor, K.; Daniel, B.; Foufelle, F.; Ding, J.; Li, X.; Khorasanizadef, S.; Osborne, T.F. SETDB2 links glucocorticoid to lipid metabolism through Insig2a regulation. Cell Metab. 2016, 24, 474–484. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Canfrán-Duque, A.; Aryal, B.; Tellides, G.; Chang, Y.J.; Suárez, Y.; Osborne, T.F.; Fernández-Hernando, C. Deficiency of histone lysine methyltransferase SETDB2 in hematopoietic cells promotes vascular inflammation and accelerates atherosclerosis. JCI Insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kim, S.H.; Arifuzzaman, S.; Yoon, T.; Chai, J.C.; Lee, Y.S.; Park, K.S.; Jung, K.H.; Chai, Y.G. Transcriptome sequencing reveals that LPS-triggered transcriptional responses in established microglia BV2 cell lines are poorly representative of primary microglia. J. Neuroinflamm. 2016, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Costet, P.; Krempf, M.; Cariou, B. PCSK9 and LDL cholesterol: Unravelling the target to design the bullet. Trends Biochem. Sci. 2008, 33, 426–434. [Google Scholar] [CrossRef]
- Courtemanche, H.; Bigot, E.; Pichelin, M.; Guyomarch, B.; Boutoleau-Bretonnière, C.; Le May, C.; Derkinderen, P.; Cariou, B. PCSK9 concentrations in cerebrospinal fluid are not specifically increased in Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 62, 1519–1525. [Google Scholar] [CrossRef]
- Redberg, R.F.; Prasad, V. Evolocumab in patients with cardiovascular disease. N. Engl. J. Med. 2017, 377, 786–787. [Google Scholar]
- Jonas, M.C.; Costantini, C.; Puglielli, L. PCSK9 is required for the disposal of non-acetylated intermediates of the nascent membrane protein BACE1. EMBO Rep. 2008, 9, 916–922. [Google Scholar] [CrossRef]
- Greco, S.; Zaccagnini, G.; Fuschi, P.; Voellenkle, C.; Carrara, M.; Sadeghi, I.; Bearzi, C.; Maimone, B.; Castelvecchio, S.; Stellos, K.; et al. Increased BACE1-AS long noncoding RNA and β-amyloid levels in heart failure. Cardiovasc. Res. 2017, 113, 453–463. [Google Scholar] [CrossRef]
- Taylor, H.A.; Simmons, K.J.; Clavane, E.M.; Trevelyan, C.J.; Brown, J.M.; Przemyłska, L.; Watt, N.T.; Matthews, L.C.; Meakin, P.J. PTPRD and DCC are novel BACE1 substrates differentially expressed in Alzheimer’s disease: A data mining and bioinformatics study. Int. J. Mol. Sci. 2022, 23, 4568. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Tijms, B.M.; Gobom, J.; Reus, L.; Jansen, I.; Hong, S.; Dobricic, V.; Kilpert, F.; Kate, M.T.; Barkhof, F.; Tsolaki, M.; et al. Pathophysiological subtypes of Alzheimer’s disease based on cerebrospinal fluid proteomics. Brain 2020, 143, 3776–3792. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, M.A.; Modarresi, F.; Khalil, A.M.; Wood, D.E.; Sahagan, B.G.; Morgan, T.E.; Finch, C.E.; St Laurent, G., 3rd; Kenny, P.J.; Wahlestedt, C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat. Med. 2008, 14, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, M.A.; Zhang, M.; Huang, J.; Modarresi, F.; Van der Brug, M.P.; Nalls, M.A.; Cookson, M.R.; St-Laurent, G., 3rd; Wahlestedt, C. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 2010, 11, R56. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Chen, J.; Chi, H.; Yu, Z.; Wang, J.; Chen, C. Attenuated ability of BACE1 to cleave the amyloid precursor protein via silencing long noncoding RNA BACE1-AS expression. Mol. Med. Rep. 2014, 10, 1275–1281. [Google Scholar] [CrossRef]
- Agsten, M.; Hessler, S.; Lehnert, S.; Volk, T.; Rittger, A.; Hartmann, S.; Raab, C.; Kim, D.Y.; Groemer, T.W.; Schwake, M.; et al. BACE1 modulates gating of KCNQ1 (Kv7.1) and cardiac delayed rectifier KCNQ1/KCNE1 (IKs). J. Mol. Cell. Cardiol. 2015, 89, 335–348. [Google Scholar] [CrossRef]
- Adewuyi, E.O.; O’Brien, E.K.; Nyholt, D.R.; Porter, T.; Laws, S.M. A large-scale genome-wide cross-trait analysis reveals shared genetic architecture between Alzheimer’s disease and gastrointestinal tract disorders. Commun. Biol. 2022, 5, 691. [Google Scholar] [CrossRef]
- Aung, H.H.; Tsoukalas, A.; Rutledge, J.C.; Tagkopoulos, I. A systems biology analysis of brain microvascular endothelial cell lipotoxicity. BMC Syst. Biol. 2014, 8, 80. [Google Scholar] [CrossRef]
- Chen, L.; Sun, X.; Wang, Z.; Lu, Y.; Chen, M.; He, Y.; Xu, H.; Zheng, L. The impact of plasma vitamin C levels on the risk of cardiovascular diseases and Alzheimer’s disease: A Mendelian randomization study. Clin. Nutr. 2021, 40, 5327–5334. [Google Scholar] [CrossRef]
- Levy, D.; DeStefano, A.L.; Larson, M.G.; O’Donnell, C.J.; Lifton, R.P.; Gavras, H.; Cupples, L.A.; Myers, R.H. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension 2000, 36, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Surendran, P.; Drenos, F.; Young, R.; Warren, H.; Cook, J.P.; Manning, A.K.; Grarup, N.; Sim, X.; Barnes, D.R.; Witkowska, K.; et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat. Genet. 2016, 48, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Launer, L.J.; Ross, G.W.; Petrovitch, H.; Masaki, K.; Foley, D.; White, L.R.; Havlik, R.J. Midlife blood pressure and dementia: The Honolulu-Asia aging study. Neurobiol. Aging 2000, 21, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Mangialasche, F.; Richard, E.; Andrieu, S.; Bennett, D.A.; Breteler, M.; Fratiglioni, B.L.; Hooshmand, B.; Khachaturian, A.S.; Schneider, L.S.; et al. Advances in the prevention of Alzheimer’s disease and dementia. J. Intern. Med. 2014, 275, 229–250. [Google Scholar] [CrossRef]
- de Oliveira, F.F.; Bertolucci, P.H.F.; Chen, E.S.; Smith, M.C. Risk factors for age at onset of dementia due to Alzheimer’s disease in a sample of patients with low mean schooling from São Paulo, Brazil. Int. J. Geriatr. Psychiatry 2014, 29, 1033–1039. [Google Scholar] [CrossRef]
- d’Arbeloff, T.; Elliott, M.L.; Knodt, A.R.; Sison, M.; Melzer, T.R.; Ireland, D.; Ramrakha, S.; Poulton, R.; Caspi, A.; Moffitt, T.e.; et al. Midlife cardiovascular fitness is reflected in the brain’s white matter. Front. Aging Neurosci. 2021, 13, 652575. [Google Scholar] [CrossRef]
- Weber, C.M.; Moiz, B.; Clyne, A.M. Brain microvascular endothelial cell metabolism and its ties to barrier function. Vitam. Horm. 2024, 126, 25–75. [Google Scholar]
- Skoog, I.; Lernfelt, B.; Landahl, S.; Palmertz, B.; Andreasson, L.A.; Nilsson, L.; Persson, G.; Odén, A.; Svanborg, A. 15-year longitudinal study of blood pressure and dementia. Lancet 1996, 347, 1141–1145. [Google Scholar] [CrossRef]
- Larkin, J.E.; Frank, B.C.; Gaspard, R.M.; Duka, I.; Gavras, H.; Quackenbush, J. Cardiac transcriptional response to acute and chronic angiotensin II treatments. Physiol. Genom. 2004, 18, 152–166. [Google Scholar] [CrossRef][Green Version]
- Chung, C.-M.; Wang, R.-Y.; Fann, C.S.J.; Chen, J.-W.; Jong, Y.-S.; Jou, Y.-S.; Yang, H.-C.; Kang, C.-S.; Chen, C.-C.; Chang, H.-C.; et al. Fine-mapping angiotensin-converting enzyme gene: Separate QTLs identified for hypertension and for ACE activity. PLoS ONE 2013, 8, e56119. [Google Scholar] [CrossRef][Green Version]
- Kehoe, P.G.; Russ, C.; McIlory, S.; Williams, H.; Holmans, P.; Holmes, C.; Liolitsa, D.; Vahidassr, D.; Powell, J.; McGleenon, B.; et al. Variation in DCP1, encoding ACE, is associated with susceptibility to Alzheimer disease. Nat. Genet. 1999, 21, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Alvarez, V.; Lahoz, C.H.; Martínez, C.; Peña, J.; Sánchez, J.M.; Guisasola, L.M.; Salas-Puig, J.; Morís, G.; Vidal, J.A.; et al. Angiotensin converting enzyme and endothelial nitric oxide synthase DNA polymorphisms and late onset Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1999, 67, 733–736. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.F.; Berretta, J.M.; de Almeida Junior, G.V.; de Almeida, S.S.; Chen, E.S.; Smith, M.C.; Henrique Ferreira Bertolucci, P. Pharmacogenetic analyses of variations of measures of cardiovascular risk in Alzheimer’s dementia. Indian J. Med. Res. 2019, 150, 261–271. [Google Scholar] [PubMed]
- Ryan, D.K.; Karhunen, V.; Su, B.; Traylor, M.; Richardson, T.G.; Burgess, S.; Tzoulaki, I.; Gill, D. Genetic evidence for protective effects of angiotensin-converting enzyme against Alzheimer disease but not other neurodegenerative diseases in European populations. Neurol. Genet. 2022, 8, e200014. [Google Scholar] [CrossRef] [PubMed]
- Ouk, M.; Wu, C.-Y.; Rabin, J.S.; Jackson, A.; Edwards, J.D.; Ramirez, J.; Masellis, M.; Swartz, R.H.; Herrmann, N.; Lanctôt, K.L.; et al. The use of angiotensin-converting enzyme inhibitors vs. angiotensin receptor blockers and cognitive decline in Alzheimer’s disease: The importance of blood-brain barrier penetration and APOE ε4 carrier status. Alzheimers Res. Ther. 2021, 13, 43. [Google Scholar] [CrossRef]
- AbdAlla, S.; Langer, A.; Fu, X.; Quitterer, U. ACE inhibition with captopril retards the development of signs of neurodegeneration in an animal model of Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 16917–16942. [Google Scholar] [CrossRef]
- Wharton, W.; Stein, J.H.; Korcarz, C.; Sachs, J.; Olson, S.R.; Zetterberg, H.; Dowling, M.; Ye, S.; Gleason, C.E.; Underbakke, G.; et al. The effects of ramipril in individuals at risk for Alzheimer’s disease: Results of a pilot clinical trial. J. Alzheimers Dis. 2012, 32, 147–156. [Google Scholar] [CrossRef]
- Ohrui, T.; Tomita, N.; Sato-Nakagawa, T.; Matsui, T.; Maruyama, M.; Niwa, K.; Arai, H.; Sasaki, H. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004, 63, 1324–1325. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Liu, D.; Gupta, D.K.; Pechman, K.R.; Watchmaker, J.M.; Gordon, E.A.; Rane, S.; Bell, S.P.; Mendes, L.A.; Davis, L.T.; et al. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology 2017, 89, 2327–2334. [Google Scholar] [CrossRef]
- Bown, C.W.; Do, R.; Khan, O.A.; Liu, D.; Cambronero, F.E.; Moore, E.E.; Osborn, K.E.; Gupta, D.K.; Pechman, K.R.; Mendes, L.A.; et al. Lower cardiac output relates to longitudinal cognitive decline in aging adults. Front. Psychol. 2020, 11, 569355. [Google Scholar] [CrossRef]
- Clark, L.R.; Zuelsdorff, M.; Norton, D.; Johnson, S.C.; Wyman, M.F.; Hancock, L.M.; Carlsson, C.M.; Asthana, S.; Flowers-Benton, S.; Gleason, C.E.; et al. Association of cardiovascular risk factors with cerebral perfusion in whites and African Americans. J. Alzheimers Dis. 2020, 75, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, A.L.; Cambronero, F.E.; Liu, D.; Moore, E.E.; Neal, J.E.; Terry, J.G.; Nair, S.; Pechman, K.R.; Rane, S.; Davis, L.T.; et al. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation 2018, 138, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Korte, N.; Ilkan, Z.; Pearson, C.L.; Pfeiffer, T.; Singhal, P.; Rock, J.R.; Sethi, H.; Gill, D.; Attwell, D.; Tammaro, P. The Ca2+-gated channel TMEM16A amplifies capillary pericyte contraction and reduces cerebral blood flow after ischemia. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Turdi, S.; Guo, R.; Huff, A.F.; Wolf, E.M.; Culver, B.; Ren, J. Cardiomyocyte contractile dysfunction in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. PLoS ONE 2009, 4, e6033. [Google Scholar] [CrossRef] [PubMed]
- Kresge, H.A.; Liu, D.; Gupta, D.K.; Moore, E.E.; Osborn, K.E.; Acosta, L.M.Y.; Bell, S.P.; Pechman, K.R.; Gifford, K.A.; Mendes, L.A.; et al. Lower left ventricular ejection fraction relates to cerebrospinal fluid biomarker evidence of neurodegeneration in older adults. J. Alzheimer’s Dis. 2020, 74, 965–974. [Google Scholar] [CrossRef]
- Johansen, M.C.; Mosley, T.H.; Knopman, D.S.; Wong, D.F.; Ndumele, C.; Shah, A.M.; Soloman, S.D.; Gottesman, R. Associations between atrial cardiopathy and cerebral amyloid: The ARIC-PET study. J. Am. Heart Assoc. 2020, 9, e018399. [Google Scholar] [CrossRef]
- Cermakova, P.; Lund, L.H.; Fereshtehnejad, S.-M.; Johnell, K.; Winblad, B.; Dahlström, U.; Eriksdotter, M.; Religa, D. Heart failure and dementia: Survival in relation to types of heart failure and different dementia disorders. Eur. J. Heart Fail. 2015, 17, 612–619. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Beiser, A.S.; Himali, J.J.; Seshadri, S.; O’Donnell, C.J.; Manning, W.J.; Wolf, P.A.; Au, R.; Benjamin, E.J. Low cardiac index is associated with incident dementia and Alzheimer disease: The Framingham Heart Study. Circulation 2015, 131, 1333–1339. [Google Scholar] [CrossRef]
- Perrotta, M.; Lembo, G.; Carnevale, D. Hypertension and dementia: Epidemiological and experimental evidence revealing a detrimental relationship. Int. J. Mol. Sci. 2016, 17, 347. [Google Scholar] [CrossRef]
- Mullan, M.; Crawford, F.; Axelman, K.; Houlden, H.; Lilius, L.; Winblad, B.; Lannfelt, L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat. Genet. 1992, 1, 345–347. [Google Scholar] [CrossRef]
- Bader, M.; Peters, J.; Baltatu, O.; Müller, D.N.; Luft, F.C.; Ganten, D. Tissue renin-angiotensin systems: New insights from experimental animal models in hypertension research. J. Mol. Med. 2001, 79, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Nimata, M.; Kishimoto, C.; Shioji, K.; Ishizaki, K.; Kitaguchi, S.; Hashimoto, T.; Nagata, N.; Kawai, C. Upregulation of redox-regulating protein, thioredoxin, in endomyocardial biopsy samples of patients with myocarditis and cardiomyopathies. Mol. Cell Biochem. 2003, 248, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Casademont, J.; Miró, O. Electron transport chain defects in heart failure. Heart Fail. Rev. 2002, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Uchida, S.; Takahashi, S.; Takayama, M.; Nagata, Y.; Suzuki, N.; Kanda, T. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer’s disease. Brain Res. 2010, 1352, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, F.; Berretta, J.M.; Suchi Chen, E.; Cardoso Smith, M.; Ferreira Bertolucci, P.H. Pharmacogenetic effects of angiotensin-converting enzyme inhibitors over age-related urea and creatinine variations in patients with dementia due to Alzheimer disease. Colomb. Med. 2016, 47, 76–80. [Google Scholar] [CrossRef]
- Quitterer, U.; AbdAlla, S. Improvements of symptoms of Alzheimer’s disease by inhibition of the angiotensin system. Pharmacol. Res. 2020, 154, 104230. [Google Scholar] [CrossRef]
- Liu, S.; Ando, F.; Fujita, Y.; Liu, J.; Maeda, T.; Shen, X.; Kikuchi, K.; Matsumoto, A.; Yokomori, M.; Tanabe-Fujimura, C.; et al. A clinical dose of angiotensin-converting enzyme (ACE) inhibitor and heterozygous ACE deletion exacerbate Alzheimer’s disease pathology in mice. J. Biol. Chem. 2019, 294, 9760–9770. [Google Scholar] [CrossRef]
- Hemming, M.L.; Selkoe, D.J. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J. Biol. Chem. 2005, 280, 37644–37650. [Google Scholar] [CrossRef]
- Carnevale, D.; Lembo, G. “Alzheimer-like” pathology in a murine model of arterial hypertension. Biochem. Soc. Trans. 2011, 39, 939–944. [Google Scholar] [CrossRef]
- Hughes, T.M.; Kuller, L.H.; Barinas-Mitchell, E.J.M.; Mackey, R.H.; McDade, E.M.; Klunk, W.E.; Aizenstein, H.J.; Cohen, A.D.; Snitz, B.E.; Mathis, C.A.; et al. Pulse wave velocity is associated with β-amyloid deposition in the brains of very elderly adults. Neurology 2013, 81, 1711–1718. [Google Scholar] [CrossRef]
- Hughes, T.M.; Kuller, L.H.; Barinas-Mitchell, E.J.M.; McDade, E.M.; Klunk, W.E.; Cohen, A.D.; Mathis, C.A.; Dekosky, S.T.; Price, J.C.; Lopez, O.L. Arterial stiffness and β-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014, 71, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Liu, D.; Li, J.; Schimmel, S.J.; Cambronero, F.E.; Terry, J.G.; Nair, S.; Pechman, K.R.; Moore, M.E.; Bell, S.P.; et al. Association of aortic stiffness with biomarkers of neuroinflammation, synaptic dysfunction, and neurodegeneration. Neurology 2021, 97, e329–e340. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.C. How do heart disease and stroke become risk factors for Alzheimer’s disease? Neurol. Res. 2006, 28, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Middelberg, R.P.S.; Ferreira, M.A.R.; Henders, A.K.; Heath, A.C.; Madden, P.A.F.; Montgomery, G.W.; Martin, N.G.; Whitfield, J.B. Genetic variants in LPL, OASL and TOMM40/APOE-C1-C2-C4 genes are associated with multiple cardiovascular-related traits. BMC Med. Genet. 2011, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.; Qiu, C.; Shao, Q.; Li, J. Associations of vascular risk factors, APOE and TOMM40 polymorphisms with cognitive function in dementia-free Chinese older adults: A community-based study. Front. Psychiatry 2021, 12, 617773. [Google Scholar] [CrossRef]
- Saha, O.; Melo de Farias, A.R.; Pelletier, A.; Siedlecki-Wullich, D.; Landeira, B.S.; Gadaut, J.; Carrier, A.; Vreulx, A.; Guyot, K.; Shen, Y.; et al. The Alzheimer’s disease risk gene BIN1 regulates activity-dependent gene expression in human-induced glutamatergic neurons. Mol. Psychiatry 2024, 29, 2634–2646. [Google Scholar] [CrossRef]
- Zhu, F.; Wolters, F.J.; Yaqub, A.; Leening, M.J.G.; Ghanbari, M.; Boersma, E.; Arfan Ikram, M.; Kavousi, M. Plasma amyloid-β in relation to cardiac function and risk of heart failure in general population. JACC Heart Fail. 2023, 11, 93–102. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, J.-H.; Dai, C.-L.; Liu, Q.; Iqbal, K.; Liu, F.; Gong, C. Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral deficits in mice. Front. Aging Neurosci. 2014, 6, 10. [Google Scholar] [CrossRef]
- Song, B.; Ao, Q.; Wang, Z.; Liu, W.; Niu, Y.; Shen, Q.; Zuo, H.; Zhang, X.; Gong, Y. Phosphorylation of tau protein over time in rats subjected to transient brain ischemia. Neural Regen. Res. 2013, 8, 3173–3182. [Google Scholar]
- Casserly, I.; Topol, E. Convergence of atherosclerosis and Alzheimer’s disease: Inflammation, cholesterol, and misfolded proteins. Lancet 2004, 363, 1139–1146. [Google Scholar] [CrossRef]
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Pritchard, H.A.T.; Walsh, K.R.; Strangward, P.; White, C.; Hill-Eubanks, D.; Alakrawi, M.; Hennig, G.W.; Allan, S.M.; Nelson, M.T.; et al. Functionally linked potassium channel activity in cerebral endothelial and smooth muscle cells is compromised in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2204581119. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.W.; Crenshaw, D.G.; Saunders, A.M.; Roses, A.D. Genetic variation at a single locus and age of onset for Alzheimer’s disease. Alzheimer’s Dement. 2010, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Roses, A.D.; Lutz, M.W.; Amrine-Madsen, H.; Saunders, A.M.; Crenshaw, D.G.; Sundseth, S.S.; Huentelman, M.J.; Welsh-Bohmer, K.A.; Reiman, E.M. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2010, 10, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Cruchaga, C.; Nowotny, P.; Kauwe, J.S.K.; Ridge, P.G.; Mayo, K.; Bertelsen, S.; Hinrichs, A.; Fagan, A.M.; Holtzman, D.M.; Morris, J.C.; et al. Association and expression analyses with single-nucleotide polymorphisms in TOMM40 in Alzheimer disease. Arch. Neurol. 2011, 68, 1013–1019. [Google Scholar] [CrossRef]
- Xue-Shan, Z.; Juan, P.; Qi, W.; Zhong, R.; Li-Hong, P.; Zhi-Han, T.; Zhi-Sheng, J.; Gui-Xue, W.; Lu-Shan, L. Imbalanced cholesterol metabolism in Alzheimer’s disease. Clin. Chim. Acta 2016, 456, 107–114. [Google Scholar] [CrossRef]
- McEniery, C.M.; Wallace, S.; Mackenzie, I.S.; McDonnell, B.; Yasmin Newby, D.E.; Cockcroft, J.R.; Wilkinson, I.B. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 2006, 48, 602–608. [Google Scholar] [CrossRef]
- Suhara, T.; Magrané, J.; Rosen, K.; Christensen, R.; Kim, H.-S.; Zheng, B.; McPhie, D.L.; Walsh, K.; Querfurth, H. Abeta42 generation is toxic to endothelial cells and inhibits eNOS function through an Akt/GSK-3beta signaling-dependent mechanism. Neurobiol. Aging 2003, 24, 437–451. [Google Scholar] [CrossRef]
- Liu, W.; Cai, H.; Lin, M.; Zhu, L.; Gao, L.; Zhong, R.; Bi, S.; Xue, Y.; Shang, X. MicroRNA-107 prevents amyloid-beta induced blood-brain barrier disruption and endothelial cell dysfunction by targeting Endophilin-1. Exp. Cell Res. 2016, 343, 248–257. [Google Scholar] [CrossRef]
- Van Skike, C.E.; Jahrling, J.B.; Olson, A.B.; Sayre, N.L.; Hussong, S.A.; Ungvari, Z.; Lechleiter, J.D.; Galvan, V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H693–H703. [Google Scholar] [CrossRef]
- Bersini, S.; Arrojo EDrigo, R.; Huang, L.; Shokhirev, M.N.; Hetzer, M.W. Transcriptional and functional changes of the human microvasculature during physiological aging and Alzheimer disease. Adv. Biosyst. 2020, 4, e2000044. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2011, 13, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Vacher, M.; Porter, T.; Milicic, L.; Bourgeat, P.; Dore, V.; Villemagne, V.L.; Laws, S.M.; Doecke, J.D. A targeted association study of blood-brain barrier gene SNPs and brain atrophy. J. Alzheimer’s Dis. 2022, 86, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- French, S.; Arias, J.; Bolakale-Rufai, I.; Zahra, S.; Rubab Khakwani, K.Z.; Bedrick, E.J.; Serrano, G.E.; Beach, T.G.; Reiman, E.; Weinkauf, C. Serum detection of blood brain barrier injury in subjects with a history of stroke and transient ischemic attack. JVS Vasc. Sci. 2024, 5, 100206. [Google Scholar] [CrossRef]
- Li, M.; Hao, X.; Hu, Z.; Tian, J.; Shi, J.; Ma, D.; Guo, M.; Li, S.; Zuo, C.; Liang, Y.; et al. Microvascular and cellular dysfunctions in Alzheimer’s disease: An integrative analysis perspective. Sci. Rep. 2024, 14, 20944. [Google Scholar] [CrossRef]
- Skoog, I.; Wallin, A.; Fredman, P.; Hesse, C.; Aevarsson, O.; Karlsson, I.; Gottfries, C.G.; Blennow, K. A population study on blood-brain barrier function in 85-year-olds: Relation to Alzheimer’s disease and vascular dementia. Neurology 1998, 50, 966–971. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Van Hulle, C.; Ince, S.; Okonkwo, O.C.; Bendlin, B.B.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Love, S.; Blennow, K.; Zetterberg, H.; et al. Elevated CSF angiopoietin-2 correlates with blood-brain barrier leakiness and markers of neuronal injury in early Alzheimer’s disease. Transl. Psychiatry 2024, 14, 3. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, L.; Liu, X.; Zheng, J.; Liu, Y.; Ruan, X.; Cao, S.; Cai, H.; Li, Z.; Xue, Y. TRA2A-induced upregulation of LINC00662 regulates blood-brain barrier permeability by affecting ELK4 mRNA stability in Alzheimer’s microenvironment. RNA Biol. 2020, 17, 1293–1308. [Google Scholar] [CrossRef]
- Ting, K.K.; Coleman, P.; Kim, H.J.; Zhao, Y.; Mulangala, J.; Cheng, N.C.; Li, W.; Gunatilake, D.; Johnstone, D.M.; Loo, L.; et al. Vascular senescence and leak are features of the early breakdown of the blood-brain barrier in Alzheimer’s disease models. GeroScience 2023, 45, 3307–3331. [Google Scholar] [CrossRef] [PubMed]
- Mäe, M.A.; He, L.; Nordling, S.; Vazquez-Liebanas, E.; Nahar, K.; Jung, B.; Li, X.; Tan, B.C.; Foo, J.C.; Cazenave-Gassiot, A.; et al. Single-cell analysis of blood-brain barrier response to pericyte loss. Circ. Res. 2021, 128, e46–e62. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N.; et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 2022, 603, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qaisar, U.; Yin, X.; Grammas, P. Gene expression profiling in Alzheimer’s disease brain microvessels. J. Alzheimer’s Dis. 2012, 31, 193–205. [Google Scholar] [CrossRef]
- Yang, H.-S.; Yau, W.-Y.W.; Carlyle, B.C.; Trombetta, B.A.; Zhang, C.; Shirzadi, Z.; Schultz, A.P.; Pruzin, J.J.; Fitzpatrick, C.D.; Kirn, D.R.; et al. Plasma VEGFA and PGF impact longitudinal tau and cognition in preclinical Alzheimer’s disease. Brain 2024, 147, 2158–2168. [Google Scholar] [CrossRef]
- Sanbe, A.; Osinska, H.; Villa, C.; Gulick, J.; Klevitsky, R.; Glabe, C.G.; Kayed, R.; Robbins, J. Reversal of amyloid-induced heart disease in desmin-related cardiomyopathy. Proc. Natl. Acad. Sci. USA 2005, 102, 13592–13597. [Google Scholar] [CrossRef]
- Castellano, J.M.; Deane, R.; Gottesdiener, A.J.; Verghese, P.B.; Stewart, F.R.; West, T.; Paoletti, A.C.; Kasper, T.R.; DeMattos, R.B.; Zlokovic, B.V.; et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15502–15507. [Google Scholar] [CrossRef]
- Juul Rasmussen, I.; Tybjærg-Hansen, A.; Rasmussen, K.L.; Nordestgaard, B.G.; Frikke-Schmidt, R. Blood-brain barrier transcytosis genes, risk of dementia and stroke: A prospective cohort study of 74,754 individuals. Eur. J. Epidemiol. 2019, 34, 579–590. [Google Scholar] [CrossRef]
- Coomans, E.M.; van Westen, D.; Binette, A.P.; Strandberg, O.; Spotorno, N.; Serrano, G.E.; Beach, T.G.; Palmqvist, S.; Stomrud, E.; Ossenkoppele, R.; et al. Interactions between vascular burden and amyloid-β pathology on trajectories of tau accumulation. Brain 2024, 147, 949–960. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Hajjar, I.; Yang, Z.; Okafor, M.; Liu, C.; Waligorska, T.; Goldstein, F.C.; Shaw, L.M. Association of plasma and cerebrospinal fluid Alzheimer disease biomarkers with race and the role of genetic ancestry, vascular comorbidities, and neighborhood factors. JAMA Netw. Open 2022, 5, e2235068. [Google Scholar] [CrossRef] [PubMed]
- Stamatelopoulos, K.; Pol, C.J.; Ayers, C.; Georgiopoulos, G.; Gatsiou, A.; Brilakis, E.S.; Khera, A.; Drosatos, K.; de Lemos, J.A.; Stellos, K. Amyloid-beta (1-40) peptide and subclinical cardiovascular disease. J. Am. Coll. Cardiol. 2018, 72, 1060–1061. [Google Scholar] [CrossRef] [PubMed]
- Sarnowski, C.; Ghanbari, M.; Bis, J.C.; Logue, M.; Fornage, M.; Mishra, A.; Ahmad, S.; Beiser, A.S.; Boerwinkle, E.; Bouteloup, V.; et al. Meta-analysis of genome-wide association studies identifies ancestry-specific associations underlying circulating total tau levels. Commun. Biol. 2022, 5, 336. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Huang, H.; Zhao, L.; Verble, D.D.; Nutaitis, A.; Tharwani, S.D.; Brown, A.L.; Zetterberg, H.; Hu, W.; Shin, R.; et al. Baseline results: The association between cardiovascular risk and preclinical Alzheimer’s disease pathology (ASCEND) study. J. Alzheimer’s Dis. 2020, 75, 109–117. [Google Scholar] [CrossRef]
- Walker, K.A.; Chen, J.; Zhang, J.; Fornage, M.; Yang, Y.; Zhou, L.; Grams, M.E.; Tin, A.; Daya, N.; Hoogeveen, R.C.; et al. Large-scale plasma proteomic analysis identifies proteins and pathways associated with dementia risk. Nat. Aging 2021, 1, 473–489. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Walker, K.A.; Zhong, H.; Ghoneim, D.H.; Zhang, Z.; Surendran, P.; Fahle, S.; Butterworth, A.; Ashad Alam, M.; et al. Associations between genetically predicted plasma protein levels and Alzheimer’s disease risk: A study using genetic prediction models. Alzheimers Res. Ther. 2024, 16, 8. [Google Scholar] [CrossRef]
- Ahmad, S.; Milan, M.D.C.; Hansson, O.; Demirkan, A.; Agustin, R.; Sáez, M.E.; Giagtzoglou, N.; Cabrera-Socorro, A.; Bakker, M.H.M.; Ramirez, A.; et al. CDH6 and HAGH protein levels in plasma associate with Alzheimer’s disease in APOE ε4 carriers. Sci. Rep. 2020, 10, 8233. [Google Scholar] [CrossRef]
- Theeke, L.A.; Liu, Y.; Wang, S.; Luo, X.; Navia, R.O.; Xiao, D.; Xu, C.; Wang, K.; Alzheimer and Disease Neuroimaging Initiative. Plasma proteomic biomarkers in Alzheimer’s disease and cardiovascular disease: A longitudinal study. Int. J. Mol. Sci. 2024, 25, 10751. [Google Scholar] [CrossRef]
- Walker, K.A.; Chen, J.; Shi, L.; Yang, Y.; Fornage, M.; Zhou, L.; Schlosser, P.; Surapaneni, A.; Grams, M.E.; Duggan, M.R.; et al. Proteomics analysis of plasma from middle-aged adults identifies protein markers of dementia risk in later life. Sci. Transl. Med. 2023, 15, eadf5681. [Google Scholar] [CrossRef]
- Zhang, A.; Pan, C.; Wu, M.; Lin, Y.; Chen, J.; Zhong, N.; Zhang, R.; Pu, L.; Han, L.; Pan, H. Causal association between plasma metabolites and neurodegenerative diseases. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 134, 111067. [Google Scholar] [CrossRef]
- Li, S.; Weinstein, G.; Zare, H.; Teumer, A.; Völker, U.; Friedrich, N.; Knol, M.J.; Satizabal, C.L.; Petyuk, V.A.; Adams, H.H.H.; et al. The genetics of circulating BDNF: Towards understanding the role of BDNF in brain structure and function in middle and old ages. Brain Commun. 2020, 2, fcaa176. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, S.P.; Dynan, K.B.; Lawson, J.T.; Patterson, C.C.; Passmore, A.P. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke 2002, 33, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Souto, J.C.; Blanco-Vaca, F.; Soria, J.M.; Buil, A.; Almasy, L.; Ordoñez-Llanos, J.; Martín-Campos, J.M.; Lathrop, M.; Stone, W.; Blangero, J.; et al. A genomewide exploration suggests a new candidate gene at chromosome 11q23 as the major determinant of plasma homocysteine levels: Results from the GAIT project. Am. J. Hum. Genet. 2005, 76, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Efstathiadou, A.; Gill, D.; McGrane, F.; Quinn, T.; Dawson, J. Genetically determined uric acid and the risk of cardiovascular and neurovascular diseases: A Mendelian randomization study of outcomes investigated in randomized trials. J. Am. Heart Assoc. 2019, 8, e012738. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, K.; Hosono, T.; Nakamura, T.; Bu, G.; Michikawa, M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 2011, 286, 17536–17542. [Google Scholar] [CrossRef]
- Alata, W.; Ye, Y.; St-Amour, I.; Vandal, M.; Calon, F. Human apolipoprotein E ɛ4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J. Cereb. Blood Flow. Metab. 2015, 35, 86–94. [Google Scholar] [CrossRef]
- Nortley, R.; Korte, N.; Izquierdo, P.; Hirunpattarasilp, C.; Mishra, A.; Jaunmuktane, Z.; Kyrargyri, V.; Pfeiffer, T.; Khennouf, L.; Madry, C.; et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 2019, 365, eaav9518. [Google Scholar] [CrossRef]
- Allen, M.; Carrasquillo, M.M.; Funk, C.; Heavner, B.D.; Zou, F.; Younkin, C.S.; Burgess, J.D.; Chai, H.S.; Crook, J.; Eddy, J.A.; et al. Human whole genome genotype and transcriptome data for Alzheimer’s and other neurodegenerative diseases. Sci. Data 2016, 3, 160089. [Google Scholar] [CrossRef]
- Bennett, D.A.; Schneider, J.A.; Arvanitakis, Z.; Wilson, R.S. Overview and findings from the religious orders study. Curr. Alzheimer Res. 2012, 9, 628–645. [Google Scholar] [CrossRef]
- Spilman, P.; Podlutskaya, N.; Hart, M.J.; Debnath, J.; Gorostiza, O.; Bredesen, D.; Richardson, A.; Strong, R.; Galvan, V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE 2010, 5, e9979. [Google Scholar] [CrossRef]
- Lin, A.-L.; Zheng, W.; Halloran, J.J.; Burbank, R.R.; Hussong, S.A.; Hart, M.J.; Javors, M.; Shih, Y.Y.; Muir, E.; Solano Fonseca, R.; et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 2013, 33, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Qi, X.; Kociok, N.; Skosyrski, S.; Emilio, A.; Ruan, Q.; Grant, M.B.; Saftig, P.; Serneels, L.; Golde, T.; et al. β-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol. Med. 2012, 4, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Kolev, V.; Kacer, D.; Mouta-Bellum, C.; Soldi, R.; Graziani, I.; Kirov, A.; Friesel, R.; Liaw, L.; Small, D.; et al. Novel cross-talk between three cardiovascular regulators: Thrombin cleavage fragment of Jagged1 induces fibroblast growth factor 1 expression and release. Mol. Biol. Cell 2008, 19, 4863–4874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hayashi, S.-I.; Rakugi, H.; Morishita, R. Insight into the role of angiopoietins in ageing-associated diseases. Cells 2020, 9, 2636. [Google Scholar] [CrossRef]
- Procter, T.V.; Williams, A.; Montagne, A. Interplay between brain pericytes and endothelial cells in dementia. Am. J. Pathol. 2021, 191, 1917–1931. [Google Scholar] [CrossRef]
- Miners, J.S.; Kehoe, P.G.; Love, S.; Zetterberg, H.; Blennow, K. CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood-brain barrier dysfunction and disease pathology. Alzheimers Res. Ther. 2019, 11, 81. [Google Scholar] [CrossRef]
- Roffe, C.; Sills, S.; Halim, M.; Wilde, K.; Allen, M.B.; Jones, P.W.; Crome, P. Unexpected nocturnal hypoxia in patients with acute stroke. Stroke 2003, 34, 2641–2645. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef]
- Walsh, R.; Rutland, C.; Thomas, R.; Loughna, S. Cardiomyopathy: A systematic review of disease-causing mutations in myosin heavy chain 7 and their phenotypic manifestations. Cardiology 2010, 115, 49–60. [Google Scholar] [CrossRef]
- Kirk, E.P.; Sunde, M.; Costa, M.; Rankin, S.A.; Wolstein, O.; Castro, M.L.; Butler, T.L.; Hyun, C.; Guo, G.; Otway, R.; et al. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and valvulogenesis and cardiomyopathy. Am. J. Hum. Genet. 2007, 81, 280–291. [Google Scholar] [CrossRef]
- Guo, D.-C.; Regalado, E.S.; Gong, L.; Duan, X.; Santos-Cortez, R.L.P.; Arnaud, P.; Ren, Z.; Cai, B.; Hostetler, E.M.; Moran, R.; et al. LOX mutations predispose to thoracic aortic aneurysms and dissections. Circ. Res. 2016, 118, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, M.; Kakinuma, Y.; Handa, T.; Yamasaki, F.; Sato, T. Donepezil, anti-Alzheimer’s disease drug, prevents cardiac rupture during acute phase of myocardial infarction in mice. PLoS ONE 2011, 6, e20629. [Google Scholar] [CrossRef] [PubMed]
- Inanaga, K.; Ichiki, T.; Miyazaki, R.; Takeda, K.; Hashimoto, T.; Matsuura, H.; Sunagawa, K. Acetylcholinesterase inhibitors attenuate atherogenesis in apolipoprotein E-knockout mice. Atherosclerosis 2010, 213, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Meyers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.M.; Loch, D.C.; Habashi, J.P.; Calderon, J.F.; Chen, Y.; Bedja, D.; van Erp, C.; Gerber, E.E.; Parker, S.J.; Sauls, K.; et al. Angiotensin II-dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J. Clin. Investig. 2014, 124, 448–460. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Finan, C.; Chopade, S.; Ellmerich, S.; Rossor, M.N.; Hingorani, A.D.; Pepys, M.B. Genetic evidence for serum amyloid P component as a drug target in neurodegenerative disorders. Open Biol. 2024, 14, 230419. [Google Scholar] [CrossRef]
- Spotila, L.D.; Jacques, P.F.; Berger, P.B.; Ballman, K.V.; Ellison, R.C.; Rozen, R. Age dependence of the influence of methylenetetrahydrofolate reductase genotype on plasma homocysteine level. Am. J. Epidemiol. 2003, 158, 871–877. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Nho, K.; Ramanan, V.K.; Lai, D.; Foroud, T.M.; Lane, K.; Murrell, J.R.; Gao, S.; Hall, K.S.; Unverzagt, F.W.; et al. Genetic influences on plasma homocysteine levels in African Americans and Yoruba Nigerians. J. Alzheimer’s Dis. 2016, 49, 991–1003. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Li, Y.; Joseph-Mathurin, N.; Gordon, B.A.; Hassenstab, J.; Benzinger, T.L.S.; Buckles, V.; Fagan, A.M.; Perrin, R.J.; Goate, A.M.; et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat. Med. 2020, 26, 398–407. [Google Scholar] [CrossRef]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011, 3, 89ra57. [Google Scholar] [CrossRef]
- Toledo, J.B.; Arnold, S.E.; Raible, K.; Brettschneider, J.; Xie, S.X.; Grossman, M.; Monsell, S.E.; Kukull, W.A.; Trojanowski, J.Q. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013, 136, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Ghebremedhin, E.; Rüb, U.; Yamaguchi, H.; Del Tredici, K.; Braak, H. Two types of sporadic cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 2002, 61, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Martin, T.A.; Gross, D.R.; Hunsaker, J.C., 3rd. Link between heart disease, cholesterol, and Alzheimer’s disease: A review. Microsc. Res. Tech. 2000, 50, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R.; Bernstein, B.W. Actin dynamics and cofilin-actin rods in alzheimer disease. Cytoskeleton 2016, 73, 477–497. [Google Scholar] [CrossRef]
- Cermakova, P.; Eriksdotter, M.; Lund, L.H.; Winblad, B.; Religa, P.; Religa, D. Heart failure and Alzheimer’s disease. J. Intern. Med. 2015, 277, 406–425. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Saffi, M.A.L.; Polanczyk, C.A.; Rabelo-Silva, E.R. Lifestyle interventions reduce cardiovascular risk in patients with coronary artery disease: A randomized clinical trial. Eur. J. Cardiovasc. Nurs. 2014, 13, 436–443. [Google Scholar] [CrossRef]
- Miller, K.L.; Alfaro-Almagro, F.; Bangerter, N.K.; Thomas, D.L.; Yacoub, E.; Xu, J.; Bartsch, A.J.; Jbabdi, S.; Sotiropoulos, S.N.; Andersson, J.L.R.; et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 2016, 19, 1523–1536. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Holliday, J.; Gibson, L.M.; Garratt, S.; Oesingmann, N.; Alfaro-Almagro, F.; Bell, J.D.; Boultwood, C.; Collins, R.; Conroy, M.C.; et al. The UK Biobank imaging enhancement of 100,000 participants: Rationale, data collection, management and future directions. Nat. Commun. 2020, 11, 2624. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 486–510. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; McGee, D.L. Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham study. Diabetes Care 1979, 2, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Palmer, B.W.; Cooke, J.R.; Corey-Bloom, J.; Fiorentino, L.; Natarajan, L.; Liu, L.; Ayalon, L.; He, F.; Loredo, J.S. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: A randomized controlled study. J. Am. Geriatr. Soc. 2008, 56, 2076–2081. [Google Scholar] [CrossRef]
- Cooke, J.R.; Ayalon, L.; Palmer, B.W.; Loredo, J.S.; Corey-Bloom, J.; Natarajan, L.; Liu, L.; Ancoli-Israel, S. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: A preliminary study. J. Clin. Sleep. Med. 2009, 05, 305–309. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease-a critical review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef]
- Sochocka, M.; Zwolińska, K.; Leszek, J. The infectious etiology of Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 996–1009. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Wójtowicz, A.K. Elastin-derived peptides in the central nervous system: Friend or foe. Cell Mol. Neurobiol. 2022, 42, 2473–2487. [Google Scholar] [CrossRef]
- Ma, J.; Wang, B.; Wei, X.; Tian, M.; Bao, X.; Zhang, Y.; Qi, H.; Zhang, Y.; Hu, M. Accumulation of extracellular elastin-derived peptides disturbed neuronal morphology and neuron-microglia crosstalk in aged brain. J. Neurochem. 2024, 168, 1460–1474. [Google Scholar] [CrossRef]
- Ma, C.; Su, J.; Sun, Y.; Feng, Y.; Shen, N.; Li, B.; Liang, Y.; Yang, X.; Wu, H.; Zhang, H.; et al. Significant upregulation of Alzheimer’s β-amyloid levels in a living system induced by extracellular elastin polypeptides. Angew. Chem. Int. Ed. Engl. 2019, 58, 18703–18709. [Google Scholar] [CrossRef] [PubMed]
- Wahart, A.; Hocine, T.; Albrecht, C.; Henry, A.; Sarazin, T.; Martiny, L.; El Btaouri, H.; Maurice, P.; Bennasroune, A.; Romier-Crouzet, B.; et al. Role of elastin peptides and elastin receptor complex in metabolic and cardiovascular diseases. FEBS J. 2019, 286, 2980–2993. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Bush, A.I.; Ceccarelli, A.; Cooper, J.; de Jager, C.A.; Erickson, K.I.; Fraser, G.; Kesler, S.; Levin, S.M.; Lucey, B.; et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol. Aging 2014, 35 (Suppl. 2), S74–S78. [Google Scholar] [CrossRef] [PubMed]
- Primatesta, P.; Falaschetti, E.; Gupta, S.; Marmot, M.G.; Poulter, N.R. Association between smoking and blood pressure: Evidence from the health survey for England. Hypertension 2001, 37, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wilson, P.W.F.; Kannel, W.B. Beyond established and novel risk factors: Lifestyle risk factors for cardiovascular disease. Circulation 2008, 117, 3031–3038. [Google Scholar] [CrossRef]
- Rossor, M.N.; Fox, N.C.; Mummery, C.J.; Schott, J.M.; Warren, J.D. The diagnosis of young-onset dementia. Lancet Neurol. 2010, 9, 793–806. [Google Scholar] [CrossRef]
- Koedam, E.L.G.E.; Lauffer, V.; van der Vlies, A.E.; van der Flier, W.M.; Scheltens, P.; Pijnenburg, Y.A.L. Early-versus late-onset Alzheimer’s disease: More than age alone. J. Alzheimer’s Dis. 2010, 19, 1401–1408. [Google Scholar] [CrossRef]
- Hebert, L.E.; Scherr, P.A.; McCann, J.J.; Beckett, L.A.; Evans, D.A. Is the risk of developing Alzheimer’s disease greater for women than for men? Am. J. Epidemiol. 2001, 153, 132–136. [Google Scholar] [CrossRef]
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences between women and men in incidence rates of dementia and Alzheimer’s disease. J. Alzheimers Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef]
- Appelman, Y.; van Rijn, B.B.; Ten Haaf, M.E.; Boersma, E.; Peters, S.A.E. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015, 241, 211–218. [Google Scholar] [CrossRef]
- Leening, M.J.G.; Ferket, B.S.; Steyerberg, E.W.; Kavousi, M.; Deckers, J.W.; Nieboer, D.; Heeringa, J.; Portegies, M.L.P.; Hofman, A.; Ikram, M.A.; et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: Prospective population based cohort study. BMJ 2014, 349, g5992. [Google Scholar] [CrossRef] [PubMed]
- Jousilahti, P.; Vartiainen, E.; Tuomilehto, J.; Puska, P. Sex, age, cardiovascular risk factors, and coronary heart disease: A prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation 1999, 99, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Carnethon, M.R.; Pu, J.; Howard, G.; Albert, M.A.; Anderson, C.A.M.; Bertoni, A.G.; Mujahid, M.S.; Palaniappan, L.; Taylor, H.A., Jr.; Willis, M.; et al. Cardiovascular health in African Americans: A scientific statement from the American heart association. Circulation 2017, 136, e393–e423. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.C.; Rahal, C. The GWAS Diversity Monitor tracks diversity by disease in real time. Nat. Genet. 2020, 52, 242–243. [Google Scholar] [CrossRef]

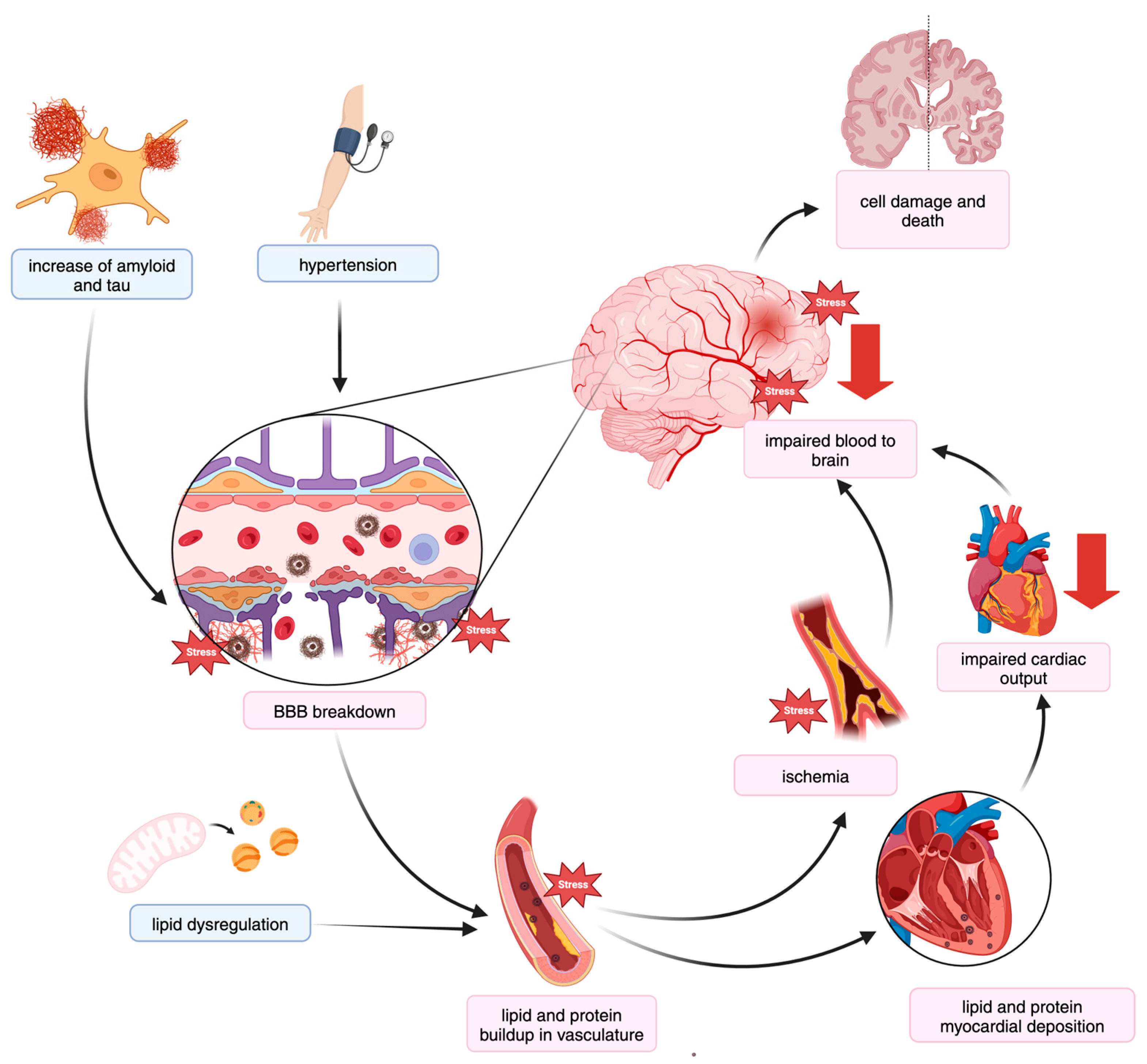
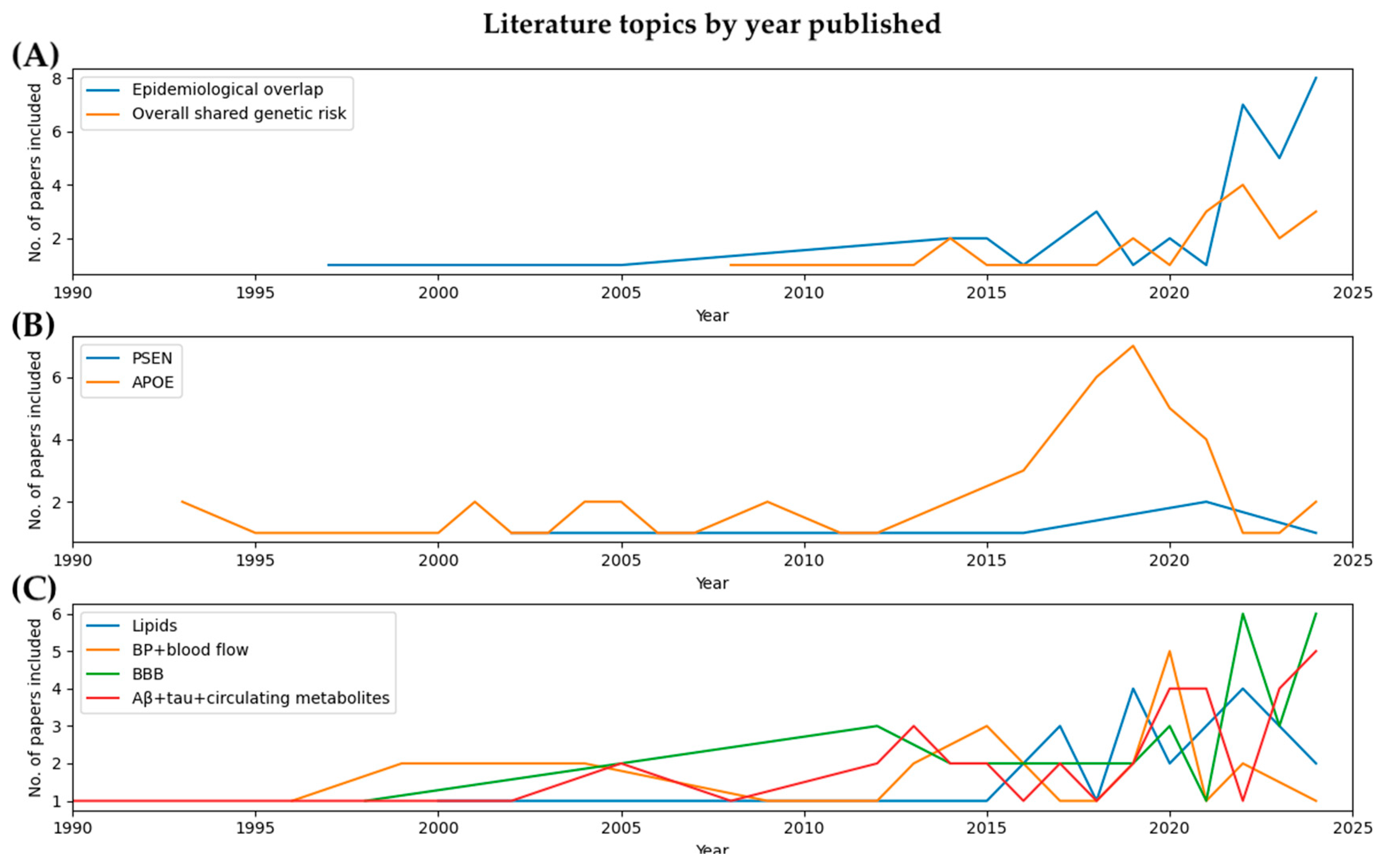
| Topic | Area of Focus | Articles |
|---|---|---|
| General | AD genetics | GWAS [37,38,39,40,41,42,43,44,45,46,47,48,49,50], pheWAS [58,59], methylation [60,61], EWAS [51,52,62], systems [63,64], PRS [65], other [66] |
| CVD genetics | Multi-trait [62], CAD [67], BP [68,69,70,71,72,73,74,75,76,77], stroke [53,54,78], MI [55], CeVD [79], cardiac structure [80,81,82,83] | |
| Overlapping genetics | Association [59,84,85,86], pathways [66], microarray [87,88], MS [88], RNAseq [89,90], systems [89,91,92,93,94], correlation [93,95], MR [96,97,98], gene [99,100,101], | |
| Effect of CVD on AD and dementia | PRS [31,33,34,102], association [19,20,21,26,31,32,103,104,105,106,107,108], MR [97,109,110,111,112,113,114], multi-omics [115], other [14,17,22,24,27,28,116] | |
| Effect of AD on CVD | Other [25,116], MR [97,113,114,117,118], | |
| PSEN | AD | [119,120,121] |
| CVD | [122,123,124,125,126,127] | |
| APOE | AD | GWAS [49,61], PRS [128,129], EWAS [130,131], multi-omics [132], association [133,134], other [135,136,137,138] |
| CVD | CAD [138], stroke [139,140,141], atherosclerosis [142], HF [143], CHD [141,144,145,146], MI [147], LV dysfunction [148], general [133,136,149,150,151,152,153] | |
| Effect on BBB | [154,155,156] | |
| Effect on lipids | [59,141,145,146,157,158,159] | |
| Effect on amyloid | [150,160,161,162,163,164] | |
| Effect on blood pressure | [165,166] | |
| Effect on blood flow | [167,168,169] | |
| AD onset | [170,171,172] | |
| Inflammation | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, A.; Ritchie, M.D. Is the Relationship Between Cardiovascular Disease and Alzheimer’s Disease Genetic? A Scoping Review. Genes 2024, 15, 1509. https://doi.org/10.3390/genes15121509
Moore A, Ritchie MD. Is the Relationship Between Cardiovascular Disease and Alzheimer’s Disease Genetic? A Scoping Review. Genes. 2024; 15(12):1509. https://doi.org/10.3390/genes15121509
Chicago/Turabian StyleMoore, Anni, and Marylyn D. Ritchie. 2024. "Is the Relationship Between Cardiovascular Disease and Alzheimer’s Disease Genetic? A Scoping Review" Genes 15, no. 12: 1509. https://doi.org/10.3390/genes15121509
APA StyleMoore, A., & Ritchie, M. D. (2024). Is the Relationship Between Cardiovascular Disease and Alzheimer’s Disease Genetic? A Scoping Review. Genes, 15(12), 1509. https://doi.org/10.3390/genes15121509






