Local Genomic Instability of the SpTransformer Gene Family in the Purple Sea Urchin Inferred from BAC Insert Deletions
Abstract
:1. Introduction
2. Methods
2.1. BAC Plasmids, DNA Isolation, and Sequencing
2.2. BAC Insert Assembly from Sequencing Reads, Alignments, and Dot Plots
2.3. Southern Blots and Riboprobes
3. Results
3.1. Sea Urchin Genomic DNA Harboring the SpTrf Gene Family Is Unstable
3.2. Deletions to the BAC Inserts Are Positioned in a Variety of Locations
3.3. BAC Insert Sequencing and Assembly Verifies Gene Loss and Identifies the Edges of Deletions
3.4. BAC Deletions Are Flanked by STRs
3.5. Some BAC Inserts Are Deleted Prior to Analysis
4. Discussion
4.1. Instability of BAC Inserts When Hosted by E. coli
4.2. Genomic Instability of the SpTrf Gene Family May Underlie Variation in Gene Family Structure and Expression in Sea Urchin Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckley, K.M.; Rast, J.P. Diversity of animal immune receptors and the origins of recognition complexity in the deuterostomes. Dev. Comp. Immunol. 2015, 49, 179–189. [Google Scholar] [CrossRef]
- Buckley, K.M.; Dooley, H. Immunological diversity is a cornerstone of organismal defense and allorecognition across metazoa. J. Immunol. 2022, 208, 203–211. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Guo, X.; Litman, G.W.; Dishaw, L.J.; Zhang, G. Massive expansion and functional divergence of innate immune genes in a protostome. Sci. Rep. 2015, 5, 8693. [Google Scholar] [CrossRef]
- Oren, M.; Barela Hudgell, M.A.; Golconda, P.; Lun, C.M.; Smith, L.C. Genomic instability and shared mechanisms for gene diversification in two distant immune gene families: The echinoid 185/333 and the plant NBS-LRR. In The Evolution of the Immune System: Conservation and Diversification; Malagoli, D., Ed.; Elsevier-Academic Press: London, UK, 2016; pp. 295–310. [Google Scholar]
- Myers, S.; Spencer, C.; Auton, A.; Bottolo, L.; Freeman, C.; Donnelly, P.; McVean, G. The distribution and causes of meiotic recombination in the human genome. Biochem. Soc. Trans. 2006, 34, 526–530. [Google Scholar] [CrossRef]
- Balzano, E.; Pelliccia, F.; Giunta, S. Genome (in)stability at tandem repeats. Semin. Cell Dev. Biol. 2021, 113, 97–112. [Google Scholar] [CrossRef]
- Bzymek, M.; Lovett, S.T. Instability of repetitive DNA sequences: The role of replication in multiple mechanisms. Proc. Natl. Acad. Sci. USA 2001, 98, 8319–8325. [Google Scholar] [CrossRef]
- Armengol, L.; Pujana, M.A.; Cheung, J.; Scherer, S.W.; Estivill, X. Enrichment of segmental duplications in regions of breaks of synteny between the human and mouse genomes suggest their involvement in evolutionary rearrangements. Hum. Mol. Genet. 2003, 12, 2201–2208. [Google Scholar] [CrossRef]
- Koszul, R.; Dujon, B.; Fischer, G. Stability of large segmental duplications in the yeast genome. Genetics 2006, 172, 2211–2222. [Google Scholar] [CrossRef]
- San Mauro, D.; Gower, D.J.; Zardoya, R.; Wilkinson, M. A hotspot of gene order rearrangement by tandem duplication and random loss in the vertebrate mitochondrial genome. Mol. Biol. Evol. 2006, 23, 227–234. [Google Scholar] [CrossRef]
- Zhao, H.; Bourque, G. Recovering genome rearrangements in the mammalian phylogeny. Genome Res. 2009, 19, 934–942. [Google Scholar] [CrossRef]
- Myers, S.; Bottolo, L.; Freeman, C.; McVean, G.; Donnelly, P. A fine-scale map of recombination rates and hotspots across the human genome. Science 2005, 310, 321–324. [Google Scholar] [CrossRef]
- Traherne, J.A.; Martin, M.; Ward, R.; Ohashi, M.; Pellett, F.; Gladman, D.; Middleton, D.; Carrington, M.; Trowsdale, J. Mechanisms of copy number variation and hybrid gene formation in the KIR immune gene complex. Hum. Mol. Genet. 2010, 19, 737–751. [Google Scholar] [CrossRef]
- Lecompte, O.; Ripp, R.; Puzos-Barbe, V.; Duprat, S.; Heilig, R.; Dietrich, J.; Thierry, J.-C.; Poch, O. Genome evolution at the genus level: Comparison of three complete genomes of hyperthermophilic archaea. Genome Res. 2001, 11, 981–993. [Google Scholar] [CrossRef]
- Eichler, E.E.; Sankoff, D. Structural dynamics of eukaryotic chromosome evolution. Science 2003, 301, 793–797. [Google Scholar] [CrossRef]
- Peng, Q.; A Pevzner, P.; Tesler, G. The fragile breakage versus random breakage models of chromosome evolution. PLOS Comput. Biol. 2006, 2, e14. [Google Scholar] [CrossRef]
- Kikuta, H.; Laplante, M.; Navratilova, P.; Komisarczuk, A.Z.; Engström, P.G.; Fredman, D.; Akalin, A.; Caccamo, M.; Sealy, I.; Howe, K.; et al. Genomic regulatory blocks encompass multiple neighboring genes and maintain conserved synteny in vertebrates. Genome Res. 2007, 17, 545–555. [Google Scholar] [CrossRef]
- Mongin, E.; Dewar, K.; Blanchette, M. Long-range regulation is a major driving force in maintaining genome integrity. BMC Evol. Biol. 2009, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Buckley, K.M.; Smith, L.C. Extraordinary diversity among members of the large gene family, 185/333, from the purple sea urchin, Strongylocentrotus purpuratus. BMC Mol. Biol. 2007, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.O.; Wilkins, A.G.; Cooke, G.M.; Raftos, D.A.; Nair, S.V. Characterization of the highly variable immune response gene family, He185/333, in the sea urchin, Heliocidaris erythrogramma. PLoS ONE 2014, 9, e62079. [Google Scholar] [CrossRef] [PubMed]
- Wang, U.; Ding, J.; Liu, Y.; Liu, X.; Chang, Y. Isolation of immune-relating 185/333-1 gene from sea urchin (Strongylocentrotus intermedius) and its expression analysis. J. Ocean Univ. China 2016, 15, 163–170. [Google Scholar] [CrossRef]
- Yakovenko, I.; Donnyo, A.; Ioscovich, O.; Rosental, B.; Oren, M. The diverse transformer (Trf) protein family in the sea urchin Paracentrotus lividus acts through a collaboration between cellular and humoral immune effector arms. Int. J. Mol. Sci. 2021, 22, 6639. [Google Scholar] [CrossRef]
- Nair, S.V.; Del Valle, H.; Gross, P.S.; Terwilliger, D.P.; Smith, L.C. Macroarray analysis of coelomocyte gene expression in response to LPS in the sea urchin. Identification of unexpected immune diversity in an invertebrate. Physiol. Genom. 2005, 22, 33–47. [Google Scholar] [CrossRef]
- Terwilliger, D.P.; Buckley, K.M.; Brockton, V.; Ritter, N.J.; Smith, L.C. Distinctive expression patterns of 185/333 genes in the purple sea urchin, Strongylocentrotus purpuratus: An unexpectedly diverse family of transcripts in response to LPS, β-1,3-glucan, and dsRNA. BMC Mol. Biol. 2007, 8, 16. [Google Scholar] [CrossRef]
- Terwilliger, D.P.; Buckley, K.M.; Mehta, D.; Moorjani, P.G.; Smith, L. Unexpected diversity displayed in cDNAs expressed by the immune cells of the purple sea urchin, Strongylocentrotus purpuratus. Physiol. Genom. 2006, 26, 134–144. [Google Scholar] [CrossRef]
- Brockton, V.; Henson, J.H.; Raftos, D.A.; Majeske, A.J.; Kim, Y.-O.; Smith, L.C. Localization and diversity of 185/333 proteins from the purple sea urchin–unexpected protein-size range and protein expression in a new coelomocyte type. J. Cell Sci. 2008, 121, 339–348. [Google Scholar] [CrossRef]
- Golconda, P.; Buckley, K.M.; Reynolds, C.R.; Romanello, J.P.; Smith, L.C. The axial organ and the pharynx are sites of hematopoiesis in the sea urchin. Front. Immunol. 2019, 10, 870. [Google Scholar] [CrossRef]
- Dheilly, N.M.; Haynes, P.A.; Bove, U.; Nair, S.V.; Raftos, D.A. Comparative proteomic analysis of a sea urchin (Heliocidaris erythrogramma) antibacterial response revealed the involvement of apextrin and calreticulin. J. Invertebr. Pathol. 2011, 106, 223–229. [Google Scholar] [CrossRef]
- Majeske, A.J.; Oren, M.; Sacchi, S.; Smith, L.C. Single sea urchin phagocytes express messages of a single sequence from the diverse Sp185/333 gene family in response to bacterial challenge. J. Immunol. 2014, 193, 5678–5688. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.S.; Schrankel, C.S.; Brown, K.J.; Smith, L.C. Extraordinary diversity of immune response proteins among sea urchins: Nickel-isolated Sp185/333 proteins show broad variations in size and charge. PLoS ONE 2015, 10, e0138892. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.C.H.; Buckley, K.M.; Schrankel, C.S.; Schuh, N.W.; Hibino, T.; Solek, C.M.; Bae, K.; Wang, G.; Rast, J.P. Perturbation of gut bacteria induces a coordinated cellular immune response in the purple sea urchin larva. Immunol. Cell Biol. 2016, 94, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Barela Hudgell, M.A.; Smith, L.C. Sequence diversity, locus structure, and evolutionary history of the SpTransformer genes in the sea urchin genome. Front. Immunol. 2021, 12, 744783. [Google Scholar] [CrossRef]
- Buckley, K.; Munshaw, S.; Kepler, T.; Smith, L. The 185/333 gene family is a rapidly diversifying host-defense gene cluster in the purple sea urchin Strongylocentrotus purpuratus. J. Mol. Biol. 2008, 379, 912–928. [Google Scholar] [CrossRef]
- Smith, L.C. Innate immune complexity in the purple sea urchin: Diversity of the Sp185/333 system. Front. Immunol. 2012, 3, 70. [Google Scholar] [CrossRef] [PubMed]
- Oren, M.; Barela Hudgell, M.A.; D’allura, B.; Agronin, J.; Gross, A.; Podini, D.; Smith, L.C. Short tandem repeats, segmental duplications, gene deletion, and genomic instability in a rapidly diversified immune gene family. BMC Genom. 2016, 17, 900. [Google Scholar] [CrossRef]
- I Arshinoff, B.; A Cary, G.; Karimi, K.; Foley, S.; Agalakov, S.; Delgado, F.; Lotay, V.S.; Ku, C.J.; Pells, T.J.; Beatman, T.R.; et al. Echinobase: Leveraging an extant model organism database to build a knowledgebase supporting research on the genomics and biology of echinoderms. Nucleic Acids Res. 2022, 50, D970–D979. [Google Scholar] [CrossRef]
- Sodergren, E.; Weinstock, G.M.; Davidson, E.H.; Cameron, R.A.; Gibbs, R.A.; Angerer, R.C.; Angerer, L.M.; Arnone, M.I.; Burgess, D.R.; Coffman, J.A.; et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar] [CrossRef]
- A Miller, C.; Buckley, K.M.; Easley, R.L.; Smith, L.C. An Sp185/333 gene cluster from the purple sea urchin and putative microsatellite-mediated gene diversification. BMC Genom. 2010, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Buckley, K.M.; Dong, P.; Cameron, R.A.; Rast, J.P. Bacterial artificial chromosomes as recombinant reporter constructs to investigate gene expression and regulation in echinoderms. Brief. Funct. Genom. 2018, 17, 362–371. [Google Scholar] [CrossRef]
- Cameron, R.A.; Mahairas, G.; Rast, J.P.; Martinez, P.; Biondi, T.R.; Swartzell, S.; Wallace, J.C.; Poustka, A.J.; Livingston, B.T.; Wray, G.A.; et al. A sea urchin genome project: Sequence scan, virtual map, and additional resources. Proc. Natl. Acad. Sci. USA 2000, 97, 9514–9518. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.A.; Rast, J.P.; Brown, C.T. Genomic resources for the study of sea urchin development. Methods Cell Biol. 2004, 74, 733–757. [Google Scholar] [CrossRef]
- Oren, M.; Rosental, B.; Hawley, T.S.; Kim, G.-Y.; Agronin, J.; Reynolds, C.R.; Grayfer, L.; Smith, L.C. Individual sea urchin coelomocytes undergo somatic immune gene diversification. Front. Immunol. 2019, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Rhie, A.; Walenz, B.P.; Dilthey, A.T.; Bickhart, D.M.; Kingan, S.B.; Hiendleder, S.; Williams, J.L.; Smith, T.P.L.; Phillippy, A.M. De novo assembly of haplotype-resolved genomes with trio binning. Nat. Biotechnol. 2018, 36, 1174–1182. [Google Scholar] [CrossRef]
- Nurk, S.; Walenz, B.P.; Rhie, A.; Vollger, M.R.; Logsdon, G.A.; Grothe, R.; Miga, K.H.; Eichler, E.E.; Phillippy, A.M.; Koren, S. HiCanu: Accurate assembly of segmental duplications, satellites, and allelic variants from high-fidelity long reads. Genome Res. 2020, 30, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Noé, L.; Kucherov, G. YASS: Enhancing the sensitivity of DNA similarity search. Nucleic Acids Res. 2005, 33, W540–W543. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Li, H. New strategies to improve minimap2 alignment accuracy. Bioinformatics 2021, 37, 4572–4574. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Turner, D.; Mesirov, J.P. igv.js: An embeddable JavaScript implementation of the Integrative Genomics Viewer (IGV). Bioinformatics 2022, 39, btac830. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Wenger, A.M.; Zehir, A.; Mesirov, J.P. Variant Review with the Integrative Genomics Viewer (IGV). Cancer Res. 2017, 77, 31–34. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatas, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Smith, L.C.; Shih, C.-S.; Dachenhausen, S.G. Coelomocytes express SpBf, a homologue of factor B, the second component in the sea urchin complement system. J. Immunol. 1998, 161, 6784–6793. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kabir, M.A.; Kravets, A.; Andaluz, E.; Larriba, G.; Rustchenko, E. Chromosome instability and unusual features of some widely used strains of Candida albicans. Yeast 2008, 25, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Korlach, J.; Biosciences, P. Understanding accuracy in SMRT sequencing. Pac. Biosci. 2013, 2013, 1–9. [Google Scholar]
- Aguilera, A.; García-Muse, T. Causes of genome instability. Annu. Rev. Genet. 2013, 47, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Piazza, A.; Bermejo, R.; Kriegsman, B.; Colosio, A.; Teulade-Fichou, M.-P.; Foiani, M.; Nicolas, A. G-quadruplex-induced instability during leading-strand replication. EMBO J. 2011, 30, 4033–4046. [Google Scholar] [CrossRef]
- Kruisselbrink, E.; Guryev, V.; Brouwer, K.; Pontier, D.B.; Cuppen, E.; Tijsterman, M. Mutagenic capacity of endogenous G4 DNA underlies genome instability in FANCJ-defective, C. elegans. Curr. Biol. 2008, 18, 900–905. [Google Scholar] [CrossRef]
- Smith, A.C.; Morran, L.T.; Hickman, M.A. Host Defense mechanisms induce genome instability leading to rapid evolution in an opportunistic fungal pathogen. Infect. Immun. 2022, 90, e0032821. [Google Scholar] [CrossRef]
- Dunn, M.J.; Anderson, M.Z. To repeat or not to repeat: Repetitive sequences regulate genome stability in Candida albicans. Genes 2019, 10, 866. [Google Scholar] [CrossRef]
- Bichara, M.; Wagner, J.; Lambert, I. Mechanisms of tandem repeat instability in bacteria. Mutat. Res. Mol. Mech. Mutagen. 2006, 598, 144–163. [Google Scholar] [CrossRef]
- Rast, J.P.; Pancer, Z.; Davidson, E.H. New approaches towards an understanding of deuterostome immunity. Curr. Top. Microbiol. Immunol. 2000, 248, 3–16. [Google Scholar] [CrossRef]
- Majeske, A.J.; Oleksyk, T.K.; Smith, L.C. The Sp185/333 immune response genes and proteins are expressed in cells dispersed within all major organs of the adult purple sea urchin. Innate Immun. 2013, 19, 569–587. [Google Scholar] [CrossRef]
- Zhang, N.; Harrex, A.L.; Holland, B.R.; Fenton, L.E.; Cannon, R.D.; Schmid, J. Sixty alleles of the ALS7 open reading frame in Candida albicans: ALS7 is a hypermutable contingency locus. Genome Res. 2003, 13, 2005–2017. [Google Scholar] [CrossRef] [PubMed]
- Chevanne, D.; Saupe, S.J.; Clavé, C.; Paoletti, M. WD-repeat instability and diversification of the Podospora anserina hnwd non-self recognition gene family. BMC Evol. Biol. 2010, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Bruijnesteijn, J.; de Groot, N.G.; Bontrop, R.E. The genetic mechanisms driving diversification of the KIR gene cluster in primates. Front. Immunol. 2020, 11, 582804. [Google Scholar] [CrossRef] [PubMed]
- Uhrberg, M. The KIR gene family: Life in the fast lane of evolution. Eur. J. Immunol. 2005, 35, 10–15. [Google Scholar] [CrossRef]
- Martin, A.M.; Kulski, J.K.; Gaudieri, S.; Witt, C.S.; Freitas, E.M.; Trowsdale, J.; Christiansen, F.T. Comparative genomic analysis, diversity and evolution of two KIR haplotypes A and B. Gene 2004, 335, 121–131. [Google Scholar] [CrossRef]
- Wilson, M.J.; Torkar, M.; Haude, A.; Milne, S.; Jones, T.; Sheer, D.; Beck, S.; Trowsdale, J. Plasticity in the organization and sequences of human KIR/ILT gene families. Proc. Natl. Acad. Sci. USA 2000, 97, 4778–4783. [Google Scholar] [CrossRef]
- Smith, L.C. Diversification of innate immune genes: Lessons from the purple sea urchin. Dis. Model. Mech. 2010, 3, 274–279. [Google Scholar] [CrossRef]
- Gadgil, R.; Barthelemy, J.; Lewis, T.; Leffak, M. Replication stalling and DNA microsatellite instability. Biophys. Chem. 2017, 225, 38–48. [Google Scholar] [CrossRef]
- Schwartz, M.; Zlotorynski, E.; Goldberg, M.; Ozeri, E.; Rahat, A.; le Sage, C.; Chen, B.P.; Chen, D.J.; Agami, R.; Kerem, B. Homologous recombination and nonhomologous end-joining repair pathways regulate fragile site stability. Genes Dev. 2005, 19, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, J.; Adhikari, S.; Balasubramanian, S. The structure and function of DNA G-quadruplexes. Trends Chem. 2020, 2, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.R.; Singh, S.; Hambarde, S.; Griffin, W.C.; Gao, J.; Chib, S.; Yu, Y.; Ira, G.; Raney, K.D.; Kim, N. Yeast Sub1 and human PC4 are G-quadruplex binding proteins that suppress genome instability at co-transcriptionally formed G4 DNA. Nucleic Acids Res. 2017, 45, 5850–5862. [Google Scholar] [CrossRef] [PubMed]
- van Wietmarschen, N.; Merzouk, S.; Halsema, N.; Spierings, D.C.J.; Guryev, V.; Lansdorp, P.M. BLM helicase suppresses recombination at G-quadruplex motifs in transcribed genes. Nat. Commun. 2018, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Masai, H.; Tanaka, T. G-quadruplex DNA and RNA: Their roles in regulation of DNA replication and other biological functions. Biochem. Biophys. Res. Commun. 2020, 531, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Rawal, P.; Kummarasetti, V.B.R.; Ravindran, J.; Kumar, N.; Halder, K.; Sharma, R.; Mukerji, M.; Das, S.K.; Chowdhury, S. Genome-wide prediction of G4 DNA as regulatory motifs: Role in Escherichia coli global regulation. Genome Res. 2006, 16, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.J.; Węgrzyn, G.; Arluison, V.; Sinden, R.R. Genomic instability of G-quadruplex sequences in Escherichia coli: Roles of DinG, RecG, and RecQ helicases. Genes 2023, 14, 1720. [Google Scholar] [CrossRef]
- Paull, T.T. RNA–DNA hybrids and the convergence with DNA repair. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 371–384. [Google Scholar] [CrossRef]
- García-Muse, T.; Aguilera, A. R loops: From physiological to pathological roles. Cell 2019, 179, 604–618. [Google Scholar] [CrossRef]
- Ortega, P.; Gómez-González, B.; Aguilera, A. Rpd3L and Hda1 histone deacetylases facilitate repair of broken forks by promoting sister chromatid cohesion. Nat. Commun. 2019, 10, 5178. [Google Scholar] [CrossRef]
- Gómez-González, B.; Aguilera, A. Break-induced RNA–DNA hybrids (BIRDHs) in homologous recombination: Friend or foe? Embo Rep. 2023, 24, e57801. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Jinks-Robertson, S. Transcription as a source of genome instability. Nat. Rev. Genet. 2012, 13, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Gómez-González, B.; Aguilera, A. Transcription-mediated replication hindrance: A major driver of genome instability. Genes Dev. 2019, 33, 1008–1026. [Google Scholar] [CrossRef] [PubMed]
- Canela, A.; Maman, Y.; Jung, S.; Wong, N.; Callen, E.; Day, A.; Kieffer-Kwon, K.-R.; Pekowska, A.; Zhang, H.; Rao, S.S.P.; et al. Genome organization drives chromosome fragility. Cell 2017, 170, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Ortega, P.; Gómez-González, B.; Aguilera, A. Heterogeneity of DNA damage incidence and repair in different chromatin contexts. DNA Repair. 2021, 107, 103210. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.C.; Lun, C.M. The SpTransformer gene family (formerly Sp185/333) in the purple sea urchin and the functional diversity of the anti-pathogen rSpTransformer-E1 protein. Front. Immunol. 2017, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Guerra, A.; Aze, A.; Morales, J.; Mulner-Lorillon, O.; Cosson, B.; Cormier, P.; Bradham, C.; Adams, N.; Robertson, A.J.; Marzluff, W.F.; et al. The genomic repertoire for cell cycle control and DNA metabolism in S. purpuratus. Dev. Biol. 2006, 300, 238–251. [Google Scholar] [CrossRef]
- Reinardy, H.C.; Bodnar, A.G. Profiling DNA damage and repair capacity in sea urchin larvae and coelomocytes exposed to genotoxicants. Mutagenesis 2015, 30, 829–839. [Google Scholar] [CrossRef]
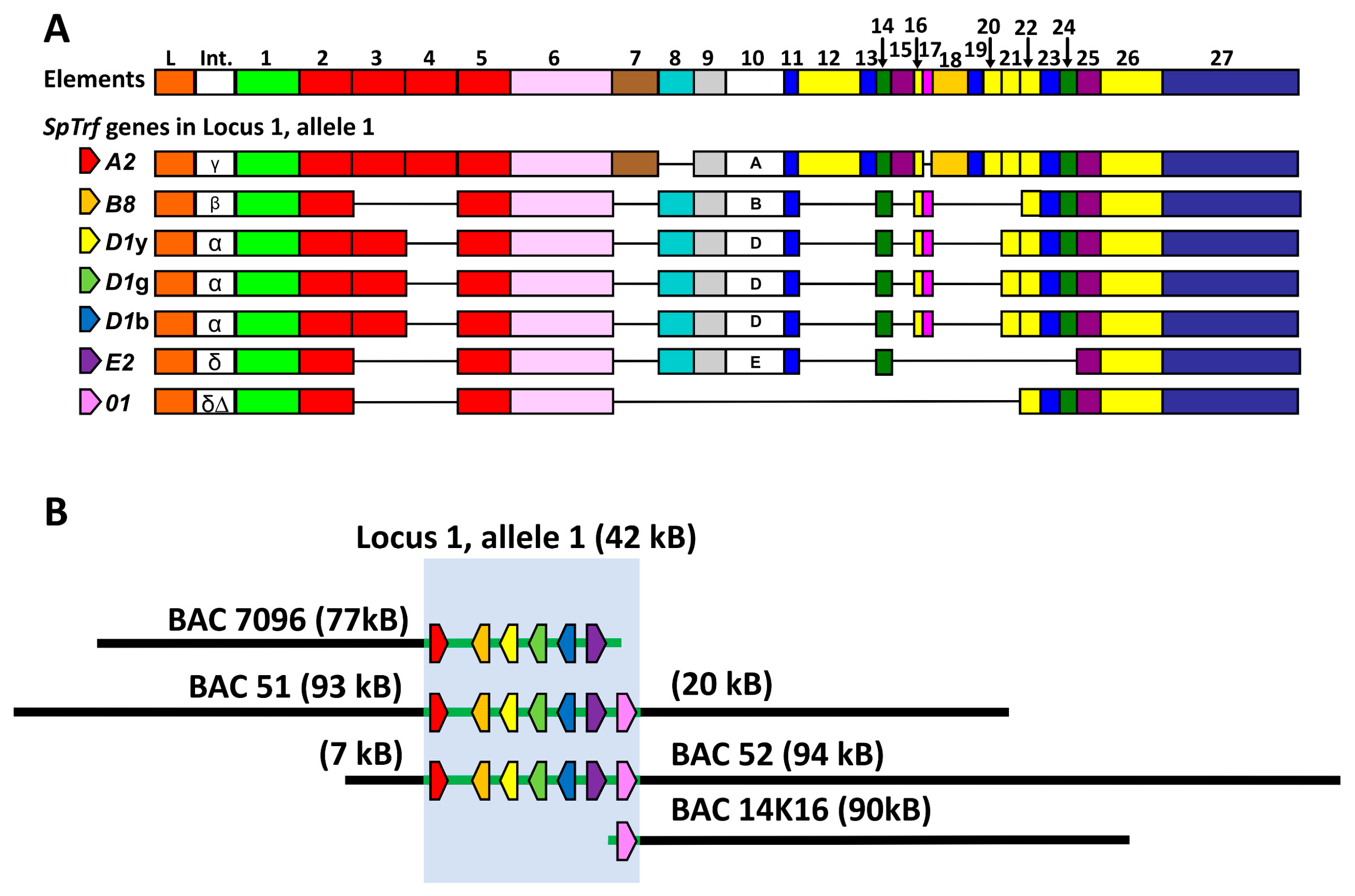

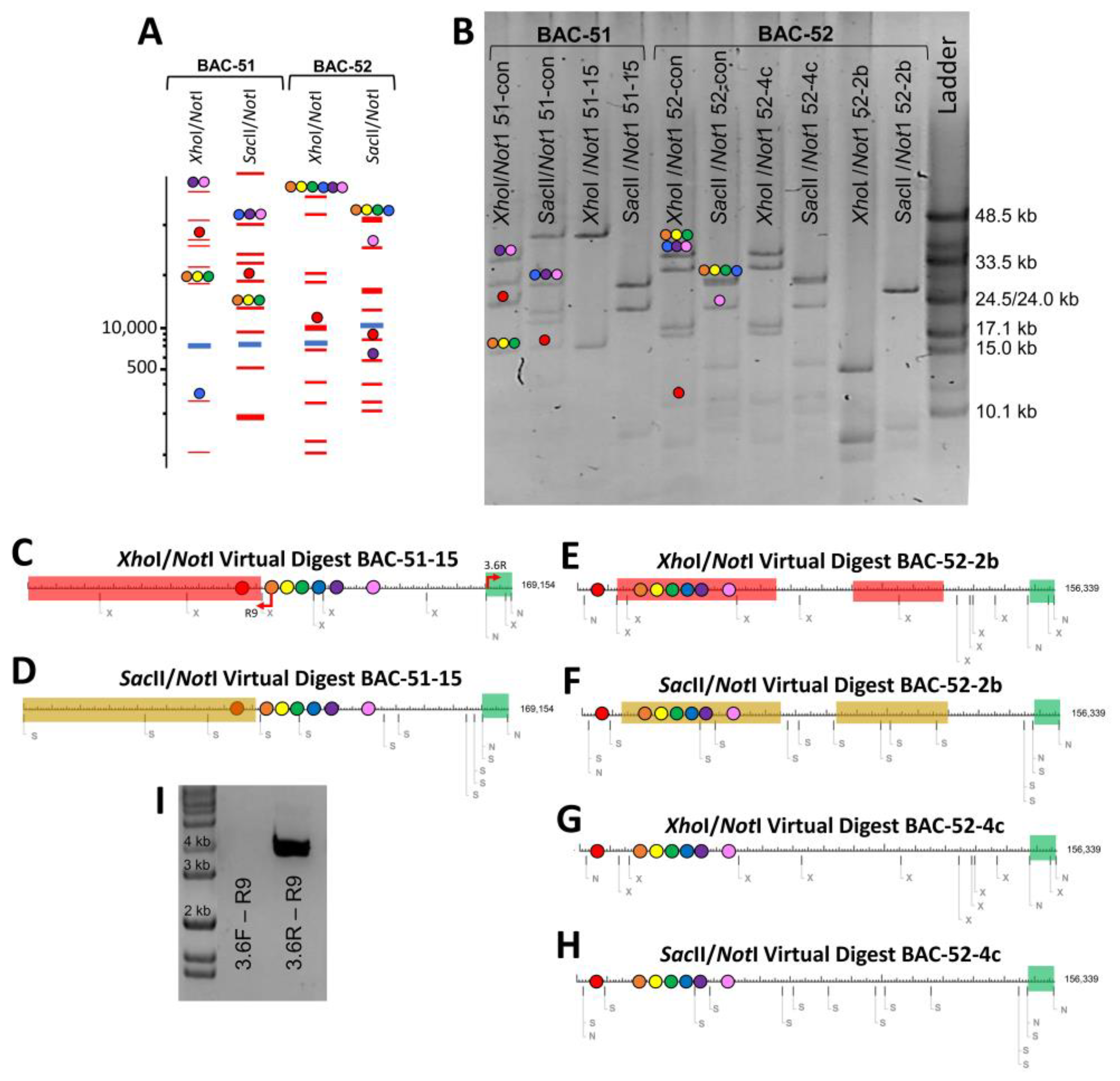
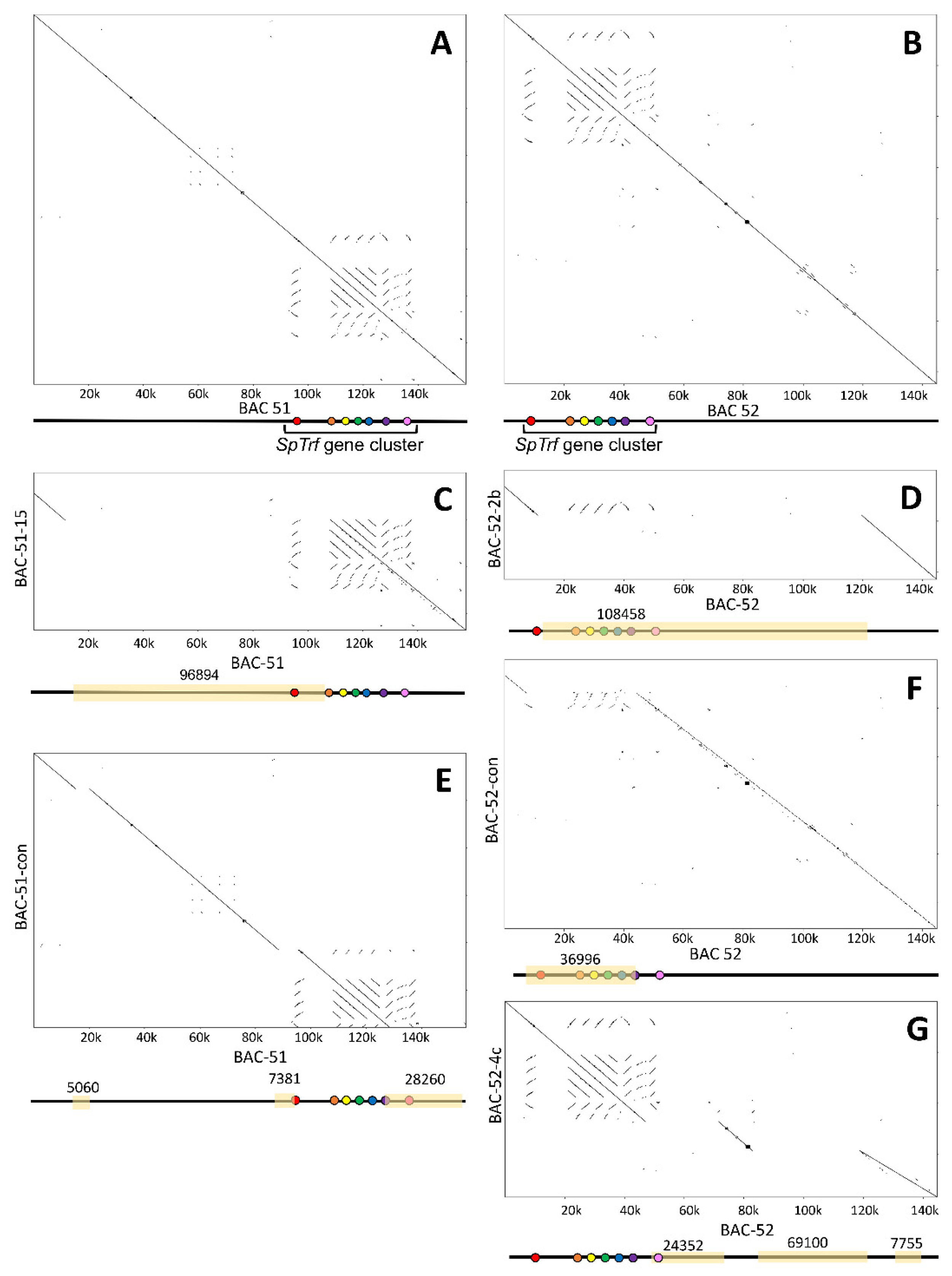

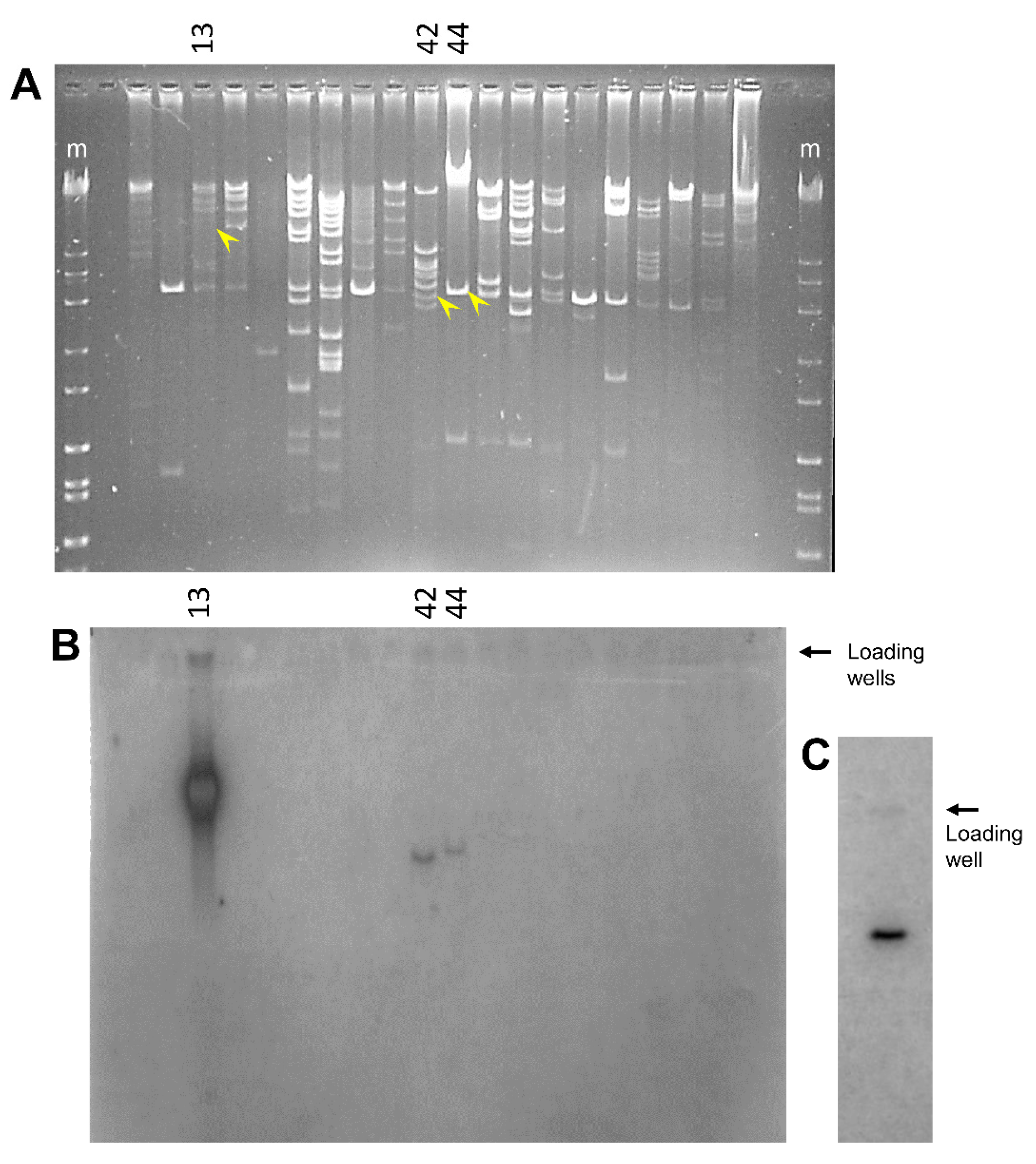
| BAC Insert Screens | SpTrf Gene Copies in BAC Inserts | |||||
|---|---|---|---|---|---|---|
| BAC Clone Name 1 | GenBank Accession Number | Colonies Screened for BAC Insert Size | BACs with Deletions | Colonies Screened | Gene Copies Expected | Gene Copies in 8 BACs with Short Inserts |
| BAC-7096 | BK007096 | 40 | 4 (10%) | 8 | 3 | 3, 2, 2, 3, 3, 3, 3, 3 |
| BAC-51 | KU668451 | 34 | 1 (3%) | 8 | 4 | 4, 4, 4, 3, 4, 4, 4, 4 |
| BAC-52 | KU668452 | 40 | 3 (7.5%) | 8 | 4 | 4, 0, 4, 0, 4, 1, 4, 2 |
| BAC-67 | PP082967 | 40 | 0 (0%) | 8 | 1 | 1, 1, 1, 1, 1, 1, 1, 1 |
| BAC Clone Name 1 | Accession Number | Growth Period (Days) | BAC Insert Size; PFGE 2, Sequence | SpTrf Genes in Locus 1 Allele 1 | Analyses 3 to Identify SpTrf Gene Complement |
|---|---|---|---|---|---|
| BAC-51 | KU668451 | na 4 | Full-length 157,542 bp | All genes present | NotI digest Gene amplicons |
| BAC-51-con 5 | na 6 | 1 | Full-length 115,850 bp 7 | All genes present | NotI digest Gene amplicons Virtual digests Insert sequence |
| BAC-51-15 | PP082968 | 10 | Short 67,180 bp 8 | Deletion of A2 only | NotI digest Gene amplicons Virtual digests Insert sequence |
| BAC-52 | KU668452 | na 4 | Full-length 144,728 bp | All genes present | Gene amplicons |
| BAC-52-con 5 | na 6 | 1 | Full-length 124,749 bp 7 | All genes present | NotI digest Gene amplicons Virtual digests Insert sequence |
| BAC-52-4c | na 6 | 10 | Full-length 76,739 bp 7 | All genes deleted 9 | NotI digest Gene amplicons Insert sequence |
| BAC-52-2b | PP082969 | 10 | Short 36,374bp 8 | All genes deleted except A2 | NotI digest Gene amplicons Virtual digests Insert sequence |
| BAC-52-19 | na | 10 | Short Not sequenced | All genes deleted | NotI digest Gene amplicons |
| BAC-52-4 | na | 10 | Short Not sequenced | All genes present | NotI digest Gene amplicons Virtual digests |
| BAC | Deletion | Local Sequence at Locations of Deletions 2 |
|---|---|---|
| 51 vs. 51-15 | First, 5′ end | CTCTCTCTCTCCCTTTCTCTCTCTCTCTCTCTCTCTCTCTCTCTCTCTCT//CCTCTACCTCTCTACTGTAT |
| First, 3′ end | CTCTCACTTTCTCCTTTCTCTCTCTCTCTCTCTCTCTCT//ATCTATCTCTATCTCTACC | |
| Second, 5′ end | CTCACAGTGTGAAAATGATTTCACAGTGTG//ACCAAACTGTGATAAATAGATTTTCACAGTGTG | |
| Second, 3′ end | TTTACAGTATACCAGAAGTCATTCTTCCCCGATTC//CATCATGCTTTGGAACTTGCTACCTGCTGGA | |
| 52 vs. 52-2b | First, 5′ end | TTTCTTTTTTGCTCAACGGGGGGGGGGG//TAGTGCATGCGCGGATCCAGGGGAGGCCCCCCGCCCCCCAAAA |
| First, 3′ end | TACGCAGGATTTTTTCAAGTGGGGGGGGGGGG//GGGTTTAACATTTTTAAATCGGGCCGAAAATTTCGCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barela Hudgell, M.A.; Momtaz, F.; Jafri, A.; Alekseyev, M.A.; Smith, L.C. Local Genomic Instability of the SpTransformer Gene Family in the Purple Sea Urchin Inferred from BAC Insert Deletions. Genes 2024, 15, 222. https://doi.org/10.3390/genes15020222
Barela Hudgell MA, Momtaz F, Jafri A, Alekseyev MA, Smith LC. Local Genomic Instability of the SpTransformer Gene Family in the Purple Sea Urchin Inferred from BAC Insert Deletions. Genes. 2024; 15(2):222. https://doi.org/10.3390/genes15020222
Chicago/Turabian StyleBarela Hudgell, Megan A., Farhana Momtaz, Abiha Jafri, Max A. Alekseyev, and L. Courtney Smith. 2024. "Local Genomic Instability of the SpTransformer Gene Family in the Purple Sea Urchin Inferred from BAC Insert Deletions" Genes 15, no. 2: 222. https://doi.org/10.3390/genes15020222






