Hmo1 Promotes Efficient Transcription Elongation by RNA Polymerase I in Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Native Elongating Transcript Sequencing (NET-seq)
2.2. NET-seq Data Processing and Analysis
2.3. NET-seq Data Visualization
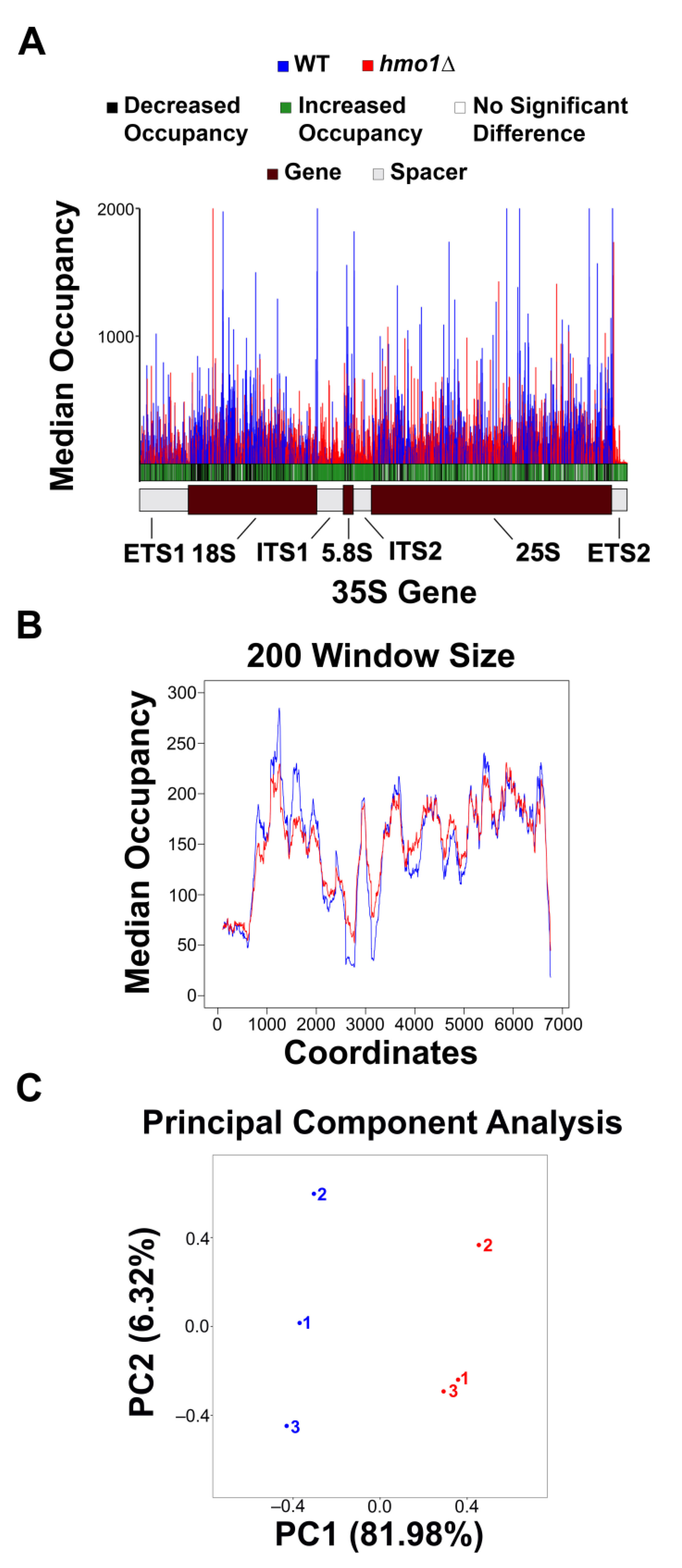
3. Results
3.1. Pol I Occupancy Is Increased in hmo1Δ Yeast
3.2. Pol I Is Sensitive to T/G-Rich Regions in hmo1Δ Yeast
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hernandez, N. TBP, a universal eukaryotic transcription factor? Genes Dev. 1993, 7, 1291–1308. [Google Scholar] [CrossRef]
- Spiker, S.M.; Mardian, J.K.; Isenberg, I. Chromosomal HMG Proteins Occur in Three Eukaryotic Kingdoms. Biochem. Biophys. Res. Commun. 1978, 82, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hock, R.; Furusawa, T.; Ueda, T.; Bustin, M. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007, 17, 72–79. [Google Scholar] [CrossRef]
- Agresti, A.; Bianchi, M.E. HMGB proteins and gene expression. Curr. Opin. Genet. Dev. 2003, 13, 170–178. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y. High mobility group proteins and their post-translational modifications. Biochim. Biophys. Acta 2008, 1784, 1159–1166. [Google Scholar] [CrossRef]
- Catez, F.; Hock, R. Binding and interplay of HMG proteins on chromatin: Lessons from live cell imaging. Biochim. Biophys. Acta 2010, 1799, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.K.; Kundu, A.; Chaudhuri, S. High mobility group proteins: The multifaceted regulators of chromatin dynamics. Nucleus 2018, 61, 213–226. [Google Scholar] [CrossRef]
- Stros, M.; Launholt, D.; Grasser, K.D. The HMG-box: A versatile protein domain occurring in a wide variety of DNA-binding proteins. Cell. Mol. Life Sci. 2007, 64, 2590–2606. [Google Scholar] [CrossRef]
- Stros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta 2010, 1799, 101–113. [Google Scholar] [CrossRef]
- Gadal, O.; Labarre, S.; Boschiero, C.; Thuriaux, P. Hmo1, an HMG-box protein, belongs to the yeast ribosomal DNA transcription system. EMBO J. 2002, 21, 5498–5507. [Google Scholar] [CrossRef]
- Albert, B.; Colleran, C.; Leger-Silvestre, I.; Berger, A.B.; Dez, C.; Normand, C.; Perez-Fernandez, J.; McStay, B.; Gadal, O. Structure-function analysis of Hmo1 unveils an ancestral organization of HMG-Box factors involved in ribosomal DNA transcription from yeast to human. Nucleic Acids Res. 2013, 41, 10135–10149. [Google Scholar] [CrossRef] [PubMed]
- Sanij, E.; Hannan, R.D. The role of UBF in regulating the structure and dynamics of transcriptionally active rDNA chromatin. Epigenetics 2009, 4, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Zatsepina, O.V.; Voit, R.; Grummt, I.; Spring, H.; Semenov, M.V.; Trendelenburg, M.F. The RNA polymerase I-specific transcription initiation factor UBF is associated with transcriptionally active and inactive ribosomal genes. Chromosoma 1993, 102, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Green, M.R. The RNA polymerase I transcription factor, upstream binding factor, interacts directly with the TATA box-binding protein. J. Biol. Chem. 1994, 269, 30140–30146. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.P.; Learned, R.M.; Jantzen, H.M.; Tjian, R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science 1988, 241, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.B.; Wade, J.T.; Struhl, K. An HMG protein, Hmo1, associates with promoters of many ribosomal protein genes and throughout the rRNA gene locus in Saccharomyces cerevisiae. Mol. Cell. Biol. 2006, 26, 3672–3679. [Google Scholar] [CrossRef]
- Lu, J.; Kobayashi, R.; Brill, S.J. Characterization of a high mobility group 1/2 homolog in yeast. J. Biol. Chem. 1996, 271, 33678–33685. [Google Scholar] [CrossRef]
- Achar, Y.J.; Adhil, M.; Choudhary, R.; Gilbert, N.; Foiani, M. Negative supercoil at gene boundaries modulates gene topology. Nature 2020, 577, 701–705. [Google Scholar] [CrossRef]
- Pommier, Y.; Sun, Y.; Huang, S.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell. Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef]
- Stros, M.; Bacikova, A.; Polanska, E.; Stokrova, J.; Strauss, F. HMGB1 interacts with human topoisomerase IIalpha and stimulates its catalytic activity. Nucleic Acids Res. 2007, 35, 5001–5013. [Google Scholar] [CrossRef]
- Clarke, A.M.; Huffines, A.K.; Edwards, Y.J.K.; Petit, C.M.; Schneider, D.A. Defining the Influence of the A12.2 Subunit on Transcription Elongation and Termination by RNA Polymerase I In Vivo. Genes 2021, 12, 1939. [Google Scholar] [CrossRef] [PubMed]
- Huffines, A.K.; Edwards, Y.J.K.; Schneider, D.A. Spt4 Promotes Pol I Processivity and Transcription Elongation. Genes 2021, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Huffines, A.K.; Engel, K.L.; French, S.L.; Zhang, Y.; Viktorovskaya, O.V.; Schneider, D.A. Rate of transcription elongation and sequence-specific pausing by RNA polymerase I directly influence rRNA processing. J. Biol. Chem. 2022, 298, 102730. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.Q.; Huffines, A.K.; Laiho, M.; Schneider, D.A. The small-molecule BMH-21 directly inhibits transcription elongation and DNA occupancy of RNA polymerase I in vivo and in vitro. J. Biol. Chem. 2022, 298, 101450. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.M.; Engel, K.L.; Giles, K.E.; Petit, C.M.; Schneider, D.A. NETSeq reveals heterogeneous nucleotide incorporation by RNA polymerase I. Proc. Natl. Acad. Sci. USA 2018, 115, E11633–E11641. [Google Scholar] [CrossRef] [PubMed]
- Churchman, L.S.; Weissman, J.S. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 2011, 469, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Scull, C.E.; Clarke, A.M.; Lucius, A.L.; Schneider, D.A. Downstream sequence-dependent RNA cleavage and pausing by RNA polymerase I. J. Biol. Chem. 2020, 295, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Turowski, T.W.; Petfalski, E.; Goddard, B.D.; French, S.L.; Helwak, A.; Tollervey, D. Nascent Transcript Folding Plays a Major Role in Determining RNA Polymerase Elongation Rates. Mol. Cell 2020, 79, 488–503. [Google Scholar] [CrossRef]
- Allison, D.F.; Wang, G.G. R-loops: Formation, function, and relevance to cell stress. Cell Stress 2019, 3, 38–46. [Google Scholar] [CrossRef]
- El Hage, A.; French, S.L.; Beyer, A.L.; Tollervey, D. Loss of Topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes Dev. 2010, 24, 1546–1558. [Google Scholar] [CrossRef]
- Stolz, R.; Sulthana, S.; Hartono, S.R.; Malig, M.; Benham, C.J.; Chedin, F. Interplay between DNA sequence and negative superhelicity drives R-loop structures. Proc. Natl. Acad. Sci. USA 2019, 116, 6260–6269. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C.; Luke, B. Regulatory R-loops as facilitators of gene expression and genome stability. Nat. Rev. Mol. Cell Biol. 2020, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Belotserkovskii, B.P.; Tornaletti, S.; D’Souza, A.D.; Hanawalt, P.C. R-loop generation during transcription: Formation, processing and cellular outcomes. DNA Repair 2018, 71, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Tous, C.; Aguilera, A. Impairment of transcription elongation by R-loops in vitro. Biochem. Biophys. Res. Commun. 2007, 360, 428–432. [Google Scholar] [CrossRef]
- Santos-Pereira, J.M.; Aguilera, A. R loops: New modulators of genome dynamics and function. Nat. Rev. Genet. 2015, 16, 583–597. [Google Scholar] [CrossRef]
- Dammann, R.; Lucchini, R.; Koller, T.; Sogo, J.M. Transcription in the yeast rRNA gene locus: Distribution of the active gene copies and chromatin structure of their flanking regulatory sequences. Mol. Cell. Biol. 1995, 15, 5294–5303. [Google Scholar] [CrossRef]
- Merz, K.; Hondele, M.; Goetze, H.; Gmelch, K.; Stoeckl, U.; Griesenbeck, J. Actively transcribed rRNA genes in S. cerevisiae are organized in a specialized chromatin associated with the high-mobility group protein Hmo1 and are largely devoid of histone molecules. Genes Dev. 2008, 22, 1190–1204. [Google Scholar] [CrossRef]
- Sanij, E.; Poortinga, G.; Sharkey, K.; Hung, S.; Holloway, T.P.; Quin, J.; Robb, E.; Wong, L.H.; Thomas, W.G.; Stefanovsky, V.; et al. UBF levels determine the number of active ribosomal RNA genes in mammals. J. Cell Biol. 2008, 183, 1259–1274. [Google Scholar] [CrossRef]
- Tafur, L.; Sadian, Y.; Hoffmann, N.A.; Jakobi, A.J.; Wetzel, R.; Hagen, W.J.H.; Sachse, C.; Muller, C.W. Molecular Structures of Transcribing RNA Polymerase, I. Mol. Cell 2016, 64, 1135–1143. [Google Scholar] [CrossRef]
- Gadal, O.; Mariotte-Labarre, S.; Chedin, S.; Quemeneur, E.; Carles, C.; Sentenac, A.; Thuriaux, P. A34.5, a nonessential component of yeast RNA polymerase I, cooperates with subunit A14 and DNA topoisomerase I to produce a functional rRNA synthesis machine. Mol. Cell. Biol. 1997, 17, 1787–1795. [Google Scholar] [CrossRef]
- Merkl, P.E.; Pilsl, M.; Fremter, T.; Schwank, K.; Engel, C.; Langst, G.; Milkereit, P.; Griesenbeck, J.; Tschochner, H. RNA polymerase I (Pol I) passage through nucleosomes depends on Pol I subunits binding its lobe structure. J. Biol. Chem. 2020, 295, 4782–4795. [Google Scholar] [CrossRef]


| Sample | Forward Primer | Reverse Primer |
|---|---|---|
| WT 1 | CAAGCAGAAGACGGCATACGAGATttctgcctTCCGACGATCATTGATGGTGCC | AATGATACGGCGACCACCGAGATCTACACtagatcgcCGTCTCTTCTGCGGATGACTCG |
| WT 2 | CAAGCAGAAGACGGCATACGAGATgctcaggaTCCGACGATCATTGATGGTGCC | AATGATACGGCGACCACCGAGATCTACACtagatcgcCGTCTCTTCTGCGGATGACTCG |
| WT 3 | CAAGCAGAAGACGGCATACGAGATaggagtccTCCGACGATCATTGATGGTGCC | AATGATACGGCGACCACCGAGATCTACACtagatcgcCGTCTCTTCTGCGGATGACTCG |
| hmo1Δ 1 | CAAGCAGAAGACGGCATACGAGATcatgcctaTCCGACGATCATTGATGGTGCC | AATGATACGGCGACCACCGAGATCTACACtagatcgcCGTCTCTTCTGCGGATGACTCG |
| hmo1Δ 2 | CAAGCAGAAGACGGCATACGAGATgtagagagTCCGACGATCATTGATGGTGCC | AATGATACGGCGACCACCGAGATCTACACtagatcgcCGTCTCTTCTGCGGATGACTCG |
| hmo1Δ 3 | CAAGCAGAAGACGGCATACGAGATcctctctgTCCGACGATCATTGATGGTGCC | AATGATACGGCGACCACCGAGATCTACACtagatcgcCGTCTCTTCTGCGGATGACTCG |
| Software | Version |
|---|---|
| Anaconda | 5.3.1 |
| BEDTools | 2.28.0 |
| car | 3.1-2 |
| carData | 3.0-5 |
| cba | 0.2-23 |
| cowplot | 1.1.1 |
| cutadapt | 3.4 |
| dae | 3.2.19 |
| DiffLogo | 2.14.0 |
| dplyr | 1.1.3 |
| extrafont | 0.19 |
| ez | 4.4-0 |
| FastQC | 0.11.7 |
| forcats | 1.0.0 |
| fqtrim | 0.9.7 |
| ggforce | 0.4.1 |
| ggfortify | 0.4.16 |
| ggplot2 | 3.4.4 |
| ggpubr | 0.6.0 |
| ggseqlogo | 0.1 |
| hexbin | 1.28.3 |
| matrixStats | 0.63.0 |
| pastecs | 1.3.21 |
| plyr | 1.8.8 |
| proxy | 0.4-25 |
| purrr | 1.0.1 |
| R | 4.0.2 |
| rclone | 1.48.0 |
| RcppRoll | 0.3.0 |
| readr | 2.1.4 |
| RStudio | 1.3.959 |
| SAMTools | 1.6 |
| scales | 1.3.0 |
| seqLogo | 1.56.0 |
| STAR | 2.7.1a |
| statmod | 1.4.36 |
| stringr | 1.5.1 |
| tibble | 3.2.1 |
| tidyr | 1.3.0 |
| tweedie | 2.3.5 |
| zoo | 1.8-12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huffines, A.K.; Schneider, D.A. Hmo1 Promotes Efficient Transcription Elongation by RNA Polymerase I in Saccharomyces cerevisiae. Genes 2024, 15, 247. https://doi.org/10.3390/genes15020247
Huffines AK, Schneider DA. Hmo1 Promotes Efficient Transcription Elongation by RNA Polymerase I in Saccharomyces cerevisiae. Genes. 2024; 15(2):247. https://doi.org/10.3390/genes15020247
Chicago/Turabian StyleHuffines, Abigail K., and David A. Schneider. 2024. "Hmo1 Promotes Efficient Transcription Elongation by RNA Polymerase I in Saccharomyces cerevisiae" Genes 15, no. 2: 247. https://doi.org/10.3390/genes15020247
APA StyleHuffines, A. K., & Schneider, D. A. (2024). Hmo1 Promotes Efficient Transcription Elongation by RNA Polymerase I in Saccharomyces cerevisiae. Genes, 15(2), 247. https://doi.org/10.3390/genes15020247






