POPDC1 Variants Cause Atrioventricular Node Dysfunction and Arrhythmogenic Changes in Cardiac Electrophysiology and Intracellular Calcium Handling in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Zebrafish Strains
2.3. Generation of popdc1KO Zebrafish
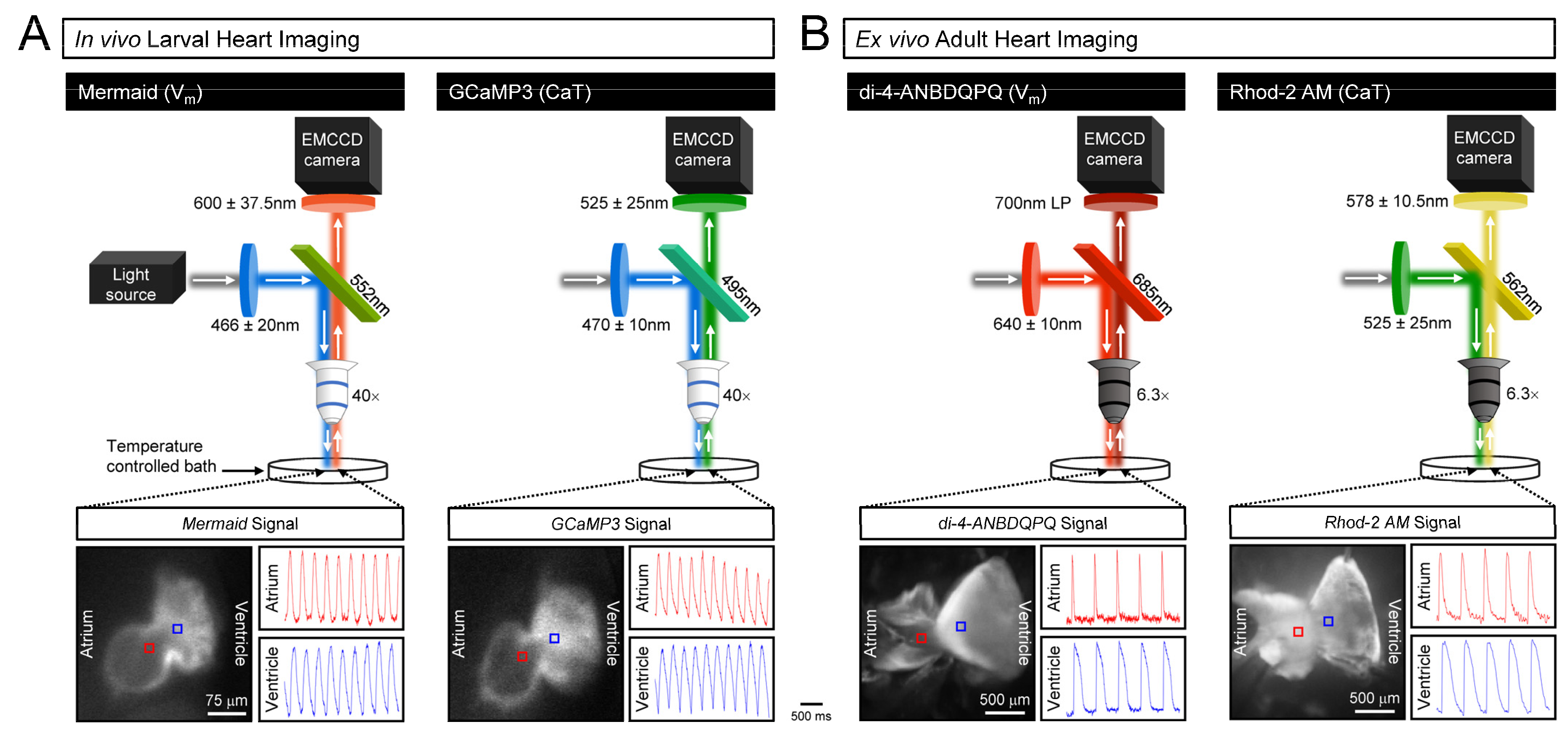
2.4. Larval Zebrafish Preparation and Functional Imaging
2.5. Ex Vivo Adult Heart Preparation and Functional Imaging
2.6. SNS Stress
2.7. AP and Ca2+ Transient Measurements
2.8. Statistical Analysis
3. Results
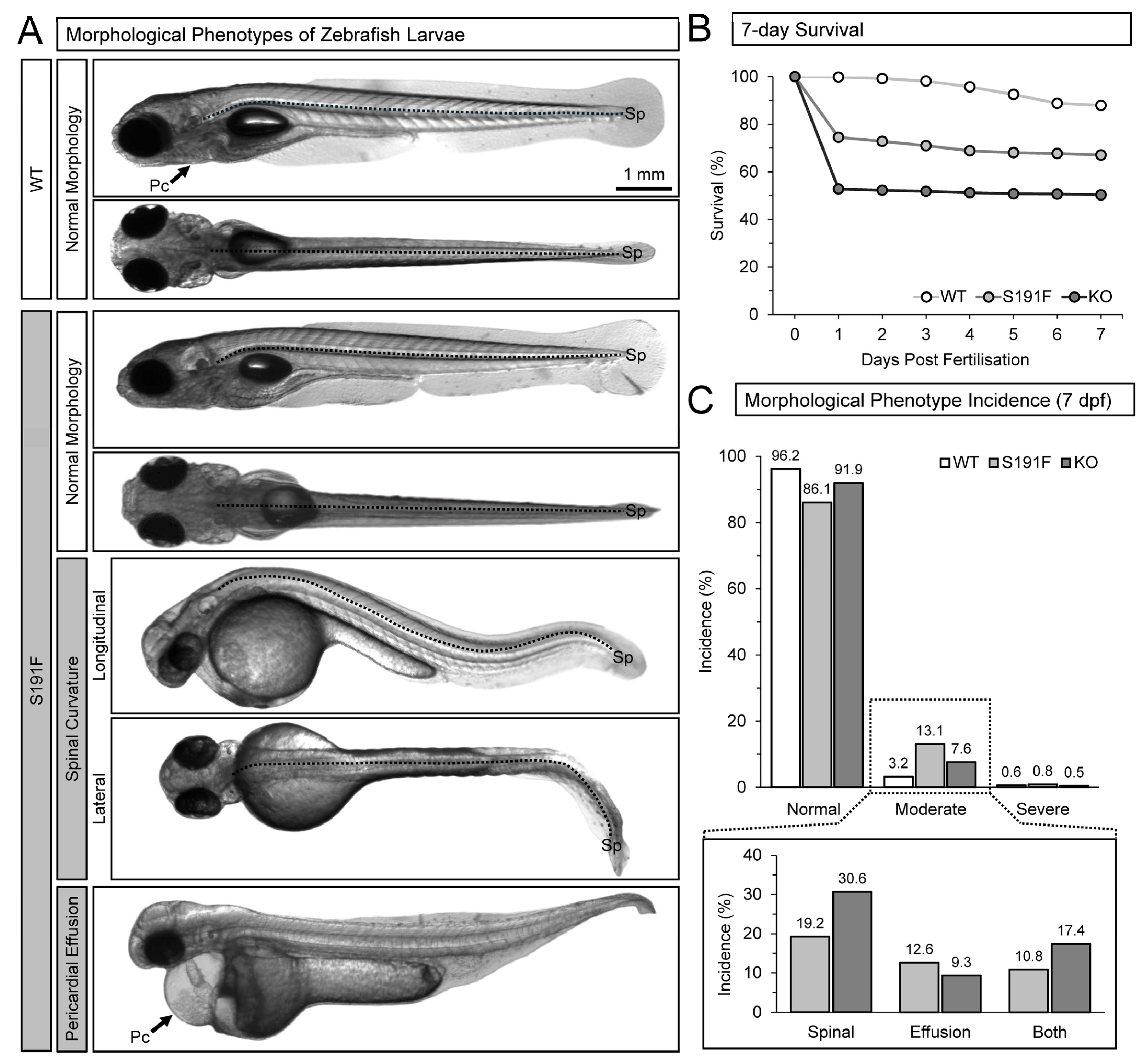
3.1. Effects of popdc1 Dysfunction on Larval Survival and Incidence of Morphological Phenotypes
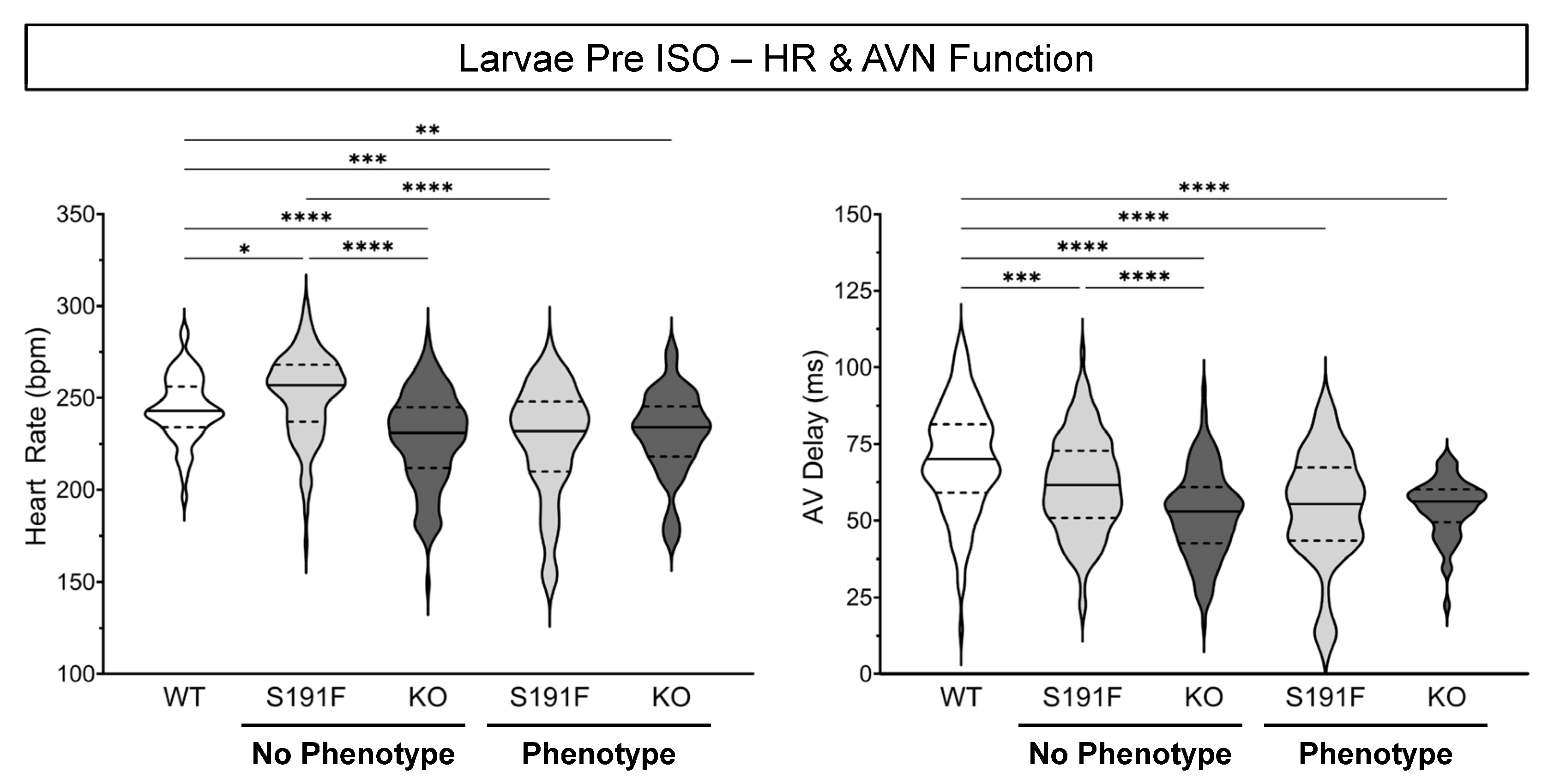
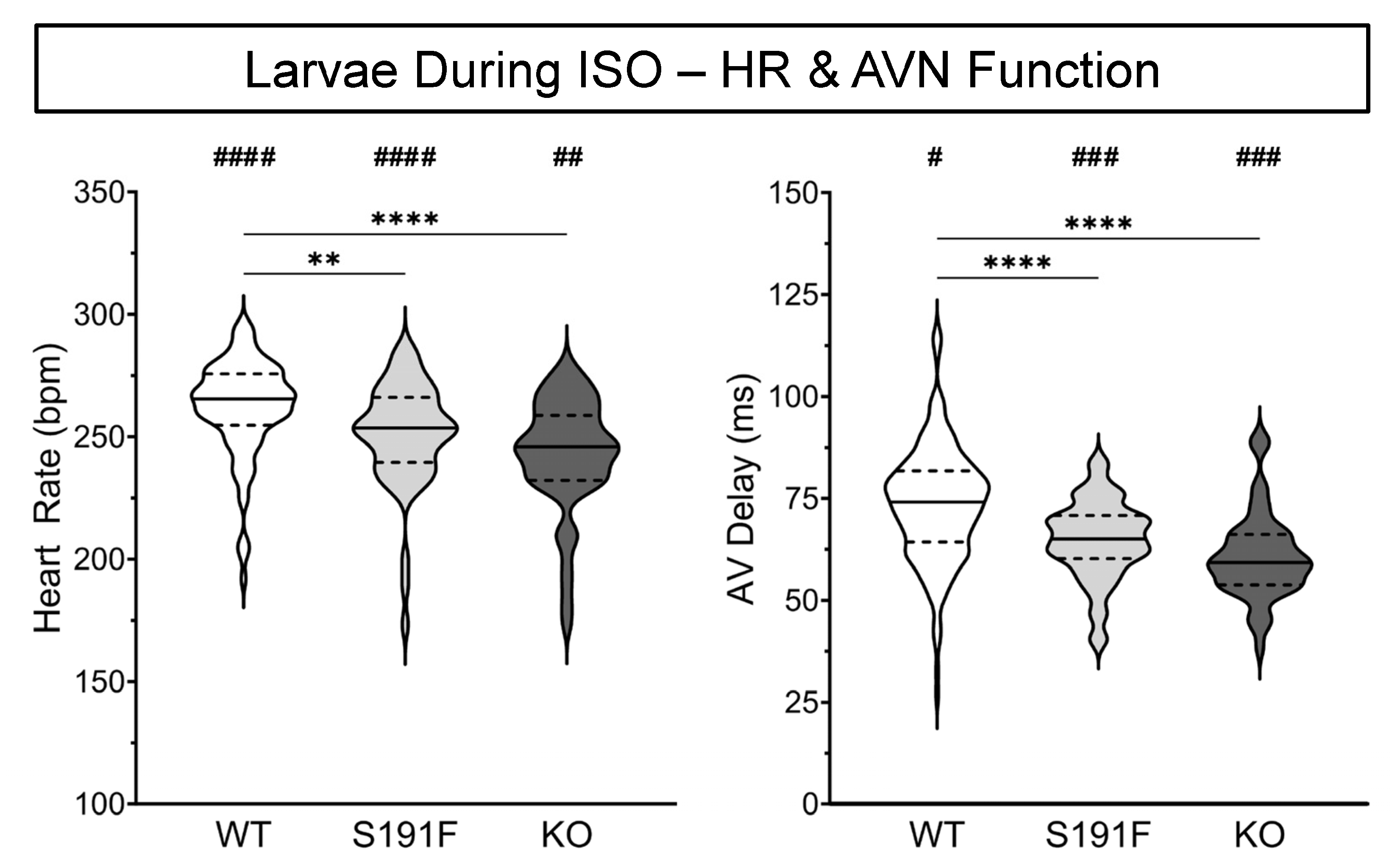
3.2. Effects of popdc1 Dysfunction on HR and AVN Function in Larval Zebrafish
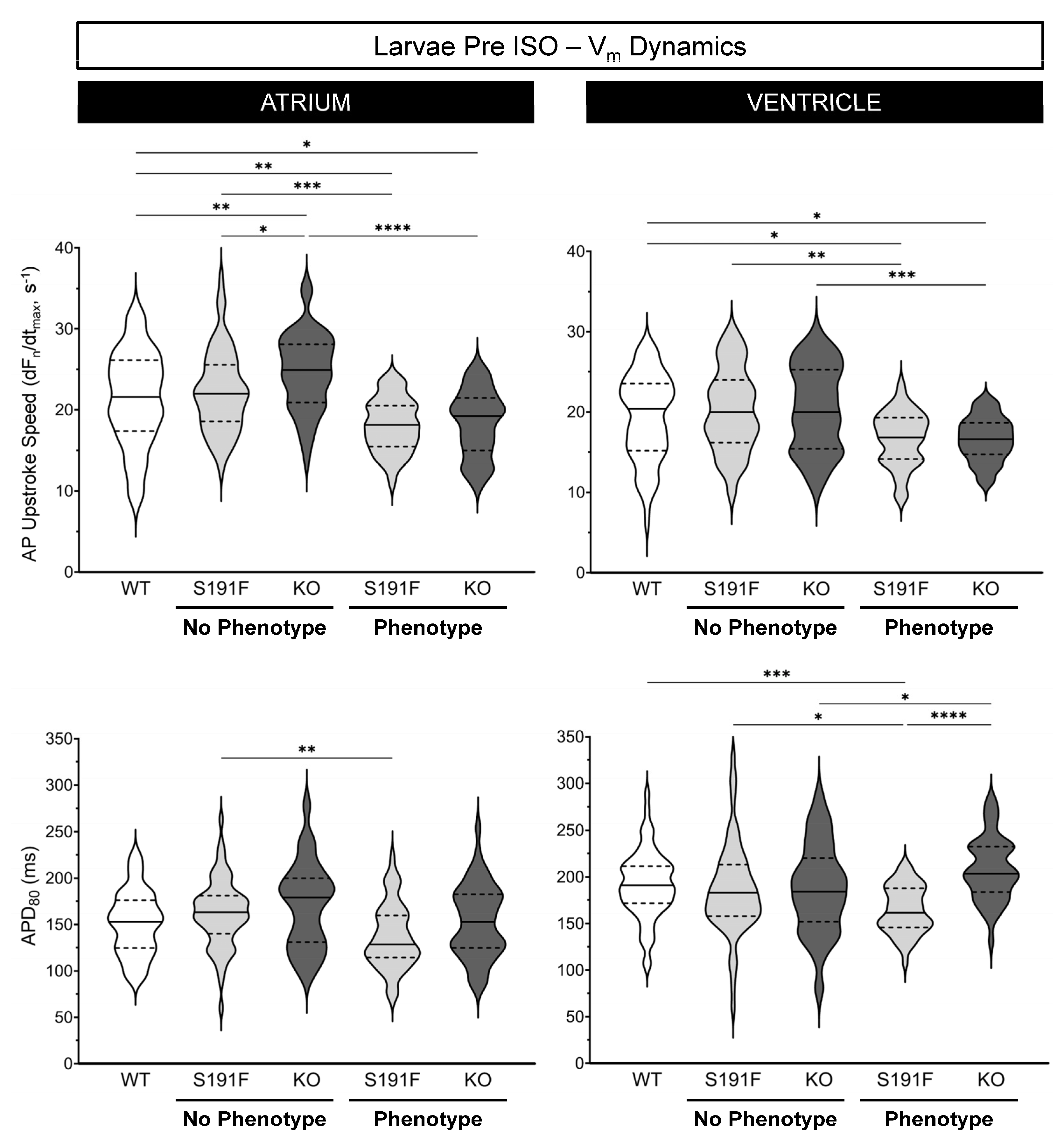
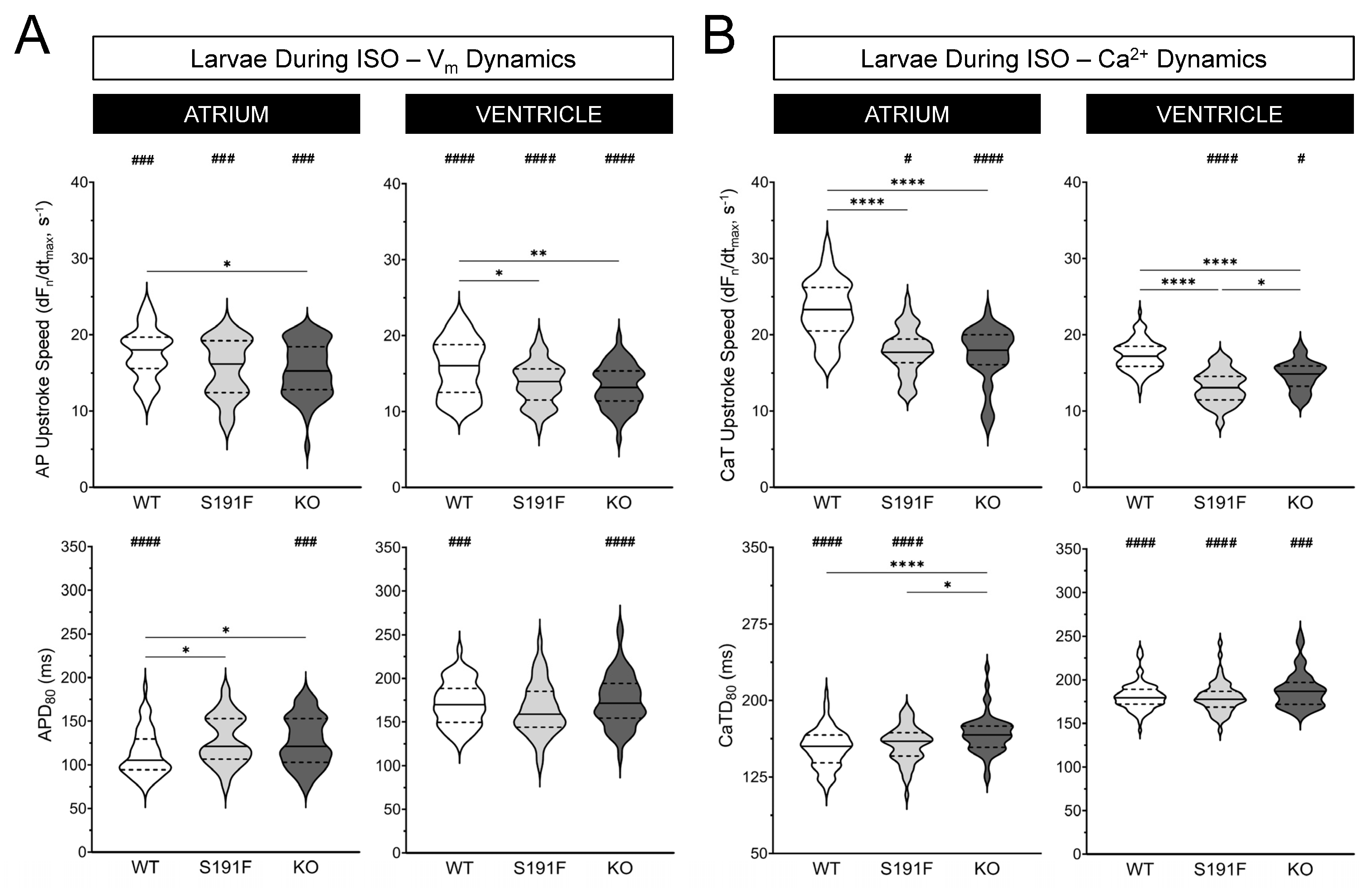
3.3. Effects of popdc1 Dysfunction on Vm Dynamics in Larval Zebrafish
3.4. Effects of popdc1 Dysfunction on Ca2+ Dynamics in Larval Zebrafish
3.5. Cardiac Effects of SNS Stress in Larval Zebrafish with popdc1 Dysfunction
3.6. Cardiac Effects of popdc1 Dysfunction and SNS Stress in Adult Isolated Hearts
4. Discussion
4.1. Summary of Results
4.2. Implications of Current Findings
4.3. Study Limitations
4.4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartos, D.C.; Grandi, E.; Ripplinger, C.M. Ion Channels in the Heart. Compr. Physiol. 2015, 5, 1423–1464. [Google Scholar] [CrossRef] [PubMed]
- Varró, A.; Tomek, J.; Nagy, N.; Virág, L.; Passini, E.; Rodriguez, B.; Baczkó, I. Cardiac Transmembrane Ion Channels and Action Potentials: Cellular Physiology and Arrhythmogenic Behavior. Physiol. Rev. 2021, 101, 1083–1176. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, E.A.; Rose, R.A.; Quinn, T.A. Neurohumoral Control of Sinoatrial Node Activity and Heart Rate: Insight from Experimental Models and Findings from Humans. Front. Physiol. 2020, 11, 170. [Google Scholar] [CrossRef]
- Gruscheski, L.; Brand, T. The Role of POPDC Proteins in Cardiac Pacemaking and Conduction. J. Cardiovasc. Dev. Dis. 2021, 8, 160. [Google Scholar] [CrossRef]
- Schindler, R.F.R.; Brand, T. The Popeye Domain Containing Protein Family—A Novel Class of cAMP Effectors with Important Functions in Multiple Tissues. Prog. Biophys. Mol. Biol. 2016, 120, 28–36. [Google Scholar] [CrossRef]
- Swan, A.H.; Gruscheski, L.; Boland, L.A.; Brand, T. The Popeye Domain Containing Gene Family Encoding a Family of cAMP-Effector Proteins with Important Functions in Striated Muscle and Beyond. J. Musc. Res. Cell Motil. 2019, 40, 169–183. [Google Scholar] [CrossRef]
- Tibbo, A.J.; Mika, D.; Dobi, S.; Ling, J.; McFall, A.; Tejeda, G.S.; Blair, C.; MacLeod, R.; MacQuaide, N.; Gök, C.; et al. Phosphodiesterase Type 4 Anchoring Regulates cAMP Signaling to Popeye Domain-Containing Proteins. J. Mol. Cell. Cardiol. 2022, 165, 86–102. [Google Scholar] [CrossRef]
- Froese, A.; Breher, S.S.; Waldeyer, C.; Schindler, R.F.R.; Nikolaev, V.O.; Rinné, S.; Wischmeyer, E.; Schlueter, J.; Becher, J.; Simrick, S.; et al. Popeye Domain Containing Proteins Are Essential for Stress-Mediated Modulation of Cardiac Pacemaking in Mice. J. Clin. Investig. 2012, 122, 1119–1130. [Google Scholar] [CrossRef]
- Holt, I.; Fuller, H.R.; Schindler, R.F.R.; Shirran, S.L.; Brand, T.; Morris, G.E. An Interaction of Heart Disease-Associated Proteins POPDC1/2 with XIRP1 in Transverse Tubules and Intercalated Discs. BMC Mol. Cell Biol. 2020, 21, 88. [Google Scholar] [CrossRef]
- Soni, S.; Raaijmakers, A.J.A.; Raaijmakers, L.M.; Damen, J.M.A.; van Stuijvenberg, L.; Vos, M.A.; Heck, A.J.R.; van Veen, T.A.B.; Scholten, A. A Proteomics Approach to Identify New Putative Cardiac Intercalated Disk Proteins. PLoS ONE 2016, 11, e0152231. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Chung, M.K.; Smith, J.D.; Hsu, J.; Serre, D.; Newton, D.W.; Castel, L.; Soltesz, E.; Pettersson, G.; Gillinov, A.M.; et al. Weighted Gene Coexpression Network Analysis of Human Left Atrial Tissue Identifies Gene Modules Associated with Atrial Fibrillation. Circ. Cardiovasc. Genet. 2013, 6, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.F.R.; Scotton, C.; Zhang, J.; Passarelli, C.; Ortiz-Bonnin, B.; Simrick, S.; Schwerte, T.; Poon, K.-L.; Fang, M.; Rinné, S.; et al. POPDC1S201F Causes Muscular Dystrophy and Arrhythmia by Affecting Protein Trafficking. J. Clin. Investig. 2015, 126, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tucker, N.R.; Rizki, G.; Mills, R.; Krijger, P.H.; de Wit, E.; Subramanian, V.; Bartell, E.; Nguyen, X.-X.; Ye, J.; et al. Discovery and Validation of Sub-Threshold Genome-Wide Association Study Loci Using Epigenomic Signatures. eLife 2016, 5, e10557. [Google Scholar] [CrossRef] [PubMed]
- Swan, A.H.; Schindler, R.F.R.; Savarese, M.; Mayer, I.; Rinné, S.; Bleser, F.; Schänzer, A.; Hahn, A.; Sabatelli, M.; Perna, F.; et al. Differential Effects of Mutations of POPDC Proteins on Heteromeric Interaction and Membrane Trafficking. Acta Neuropathol. Commun. 2023, 11, 4. [Google Scholar] [CrossRef]
- Mahmood, A.; Samad, A.; Shah, A.A.; Wadood, A.; Alkathiri, A.; Alshehri, M.A.; Alam, M.Z.; Hussain, T.; He, P.; Umair, M. A Novel Biallelic Variant in the Popeye Domain-containing Protein 1 (POPDC1) Underlies Limb Girdle Muscle Dystrophy Type 25. Clin. Genet. 2023, 103, 219–225. [Google Scholar] [CrossRef]
- Gangfuß, A.; Hentschel, A.; Heil, L.; Gonzalez, M.; Schönecker, A.; Depienne, C.; Nishimura, A.; Zengeler, D.; Kohlschmidt, N.; Sickmann, A.; et al. Proteomic and Morphological Insights and Clinical Presentation of Two Young Patients with Novel Mutations of BVES (POPDC1). Mol. Genet. Metab. 2022, 136, 226–237. [Google Scholar] [CrossRef]
- Beecher, G.; Tang, C.; Liewluck, T. Severe Adolescent-Onset Limb-Girdle Muscular Dystrophy Due to a Novel Homozygous Nonsense BVES Variant. J. Neurol. Sci. 2021, 420, 117259. [Google Scholar] [CrossRef]
- Indrawati, L.A.; Iida, A.; Tanaka, Y.; Honma, Y.; Mizoguchi, K.; Yamaguchi, T.; Ikawa, M.; Hayashi, S.; Noguchi, S.; Nishino, I. Two Japanese LGMDR25 Patients with a Biallelic Recurrent Nonsense Variant of BVES. Neuromuscul. Disord. 2020, 30, 674–679. [Google Scholar] [CrossRef]
- Meinke, P.; Kerr, A.R.W.; Czapiewski, R.; De Las Heras, J.I.; Dixon, C.R.; Harris, E.; Kölbel, H.; Muntoni, F.; Schara, U.; Straub, V.; et al. A Multistage Sequencing Strategy Pinpoints Novel Candidate Alleles for Emery-Dreifuss Muscular Dystrophy and Supports Gene Misregulation as Its Pathomechanism. EBioMedicine 2020, 51, 102587. [Google Scholar] [CrossRef]
- De Ridder, W.; Nelson, I.; Asselbergh, B.; De Paepe, B.; Beuvin, M.; Ben Yaou, R.; Masson, C.; Boland, A.; Deleuze, J.-F.; Maisonobe, T.; et al. Muscular Dystrophy with Arrhythmia Caused by Loss-of-Function Mutations in BVES. Neurol. Genet. 2019, 5, e321. [Google Scholar] [CrossRef]
- Stoyek, M.R.; MacDonald, E.A.; Mantifel, M.; Baillie, J.S.; Selig, B.M.; Croll, R.P.; Smith, F.M.; Quinn, T.A. Drivers of Sinoatrial Node Automaticity in Zebrafish: Comparison With Mechanisms of Mammalian Pacemaker Function. Front. Physiol. 2022, 13, 818122. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Quinn, T.A.; Croll, R.P.; Smith, F.M. Zebrafish Heart as a Model to Study the Integrative Autonomic Control of Pacemaker Function. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H676–H688. [Google Scholar] [CrossRef]
- MacDonald, E.A.; Stoyek, M.R.; Rose, R.A.; Quinn, T.A. Intrinsic Regulation of Sinoatrial Node Function and the Zebrafish as a Model of Stretch Effects on Pacemaking. Prog. Biophys. Mol. Biol. 2017, 130, 198–211. [Google Scholar] [CrossRef]
- Ravens, U. Ionic Basis of Cardiac Electrophysiology in Zebrafish Compared to Human Hearts. Prog. Biophys. Mol. Biol. 2018, 138, 38–44. [Google Scholar] [CrossRef] [PubMed]
- van Opbergen, C.J.M.; van der Voorn, S.M.; Vos, M.A.; de Boer, T.P.; van Veen, T.A.B. Cardiac Ca2+ Signalling in Zebrafish: Translation of Findings to Man. Prog. Biophys. Mol. Biol. 2018, 138, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Echeazarra, L.; Hortigón-Vinagre, M.P.; Casis, O.; Gallego, M. Adult and Developing Zebrafish as Suitable Models for Cardiac Electrophysiology and Pathology in Research and Industry. Front. Physiol. 2021, 11, 607860. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef] [PubMed]
- Stoyek, M.R.; Quinn, T.A. One Fish, Two Fish, Red Fish, Blue Fish*: Zebrafish as a Model for Cardiac Research. Prog. Biophys. Mol. Biol. 2018, 138, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, S.A.; Quinn, T.A. A Beginner’s Guide to Understanding and Implementing the Genetic Modification of Zebrafish. Prog. Biophys. Mol. Biol. 2018, 138, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kithcart, A.P.; MacRae, C.A. Zebrafish Assay Development for Cardiovascular Disease Mechanism and Drug Discovery. Prog. Biophys. Mol. Biol. 2018, 138, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Baillie, J.S.; Stoyek, M.R.; Quinn, T.A. Seeing the Light: The Use of Zebrafish for Optogenetic Studies of the Heart. Front. Physiol. 2021, 12, 748570. [Google Scholar] [CrossRef] [PubMed]
- van Opbergen, C.J.M.; Koopman, C.D.; Kok, B.J.M.; Knöpfel, T.; Renninger, S.L.; Orger, M.B.; Vos, M.A.; van Veen, T.A.B.; Bakkers, J.; de Boer, T.P. Optogenetic Sensors in the Zebrafish Heart: A Novel In Vivo Electrophysiological Tool to Study Cardiac Arrhythmogenesis. Theranostics 2018, 8, 4750–4764. [Google Scholar] [CrossRef] [PubMed]
- Stoyek, M.R.; Rog-Zielinska, E.A.; Quinn, T.A. Age-Associated Changes in Electrical Function of the Zebrafish Heart. Prog. Biophys. Mol. Biol. 2018, 138, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book; University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Tsutsui, H.; Karasawa, S.; Okamura, Y.; Miyawaki, A. Improving Membrane Voltage Measurements Using FRET with New Fluorescent Proteins. Nat. Methods 2008, 5, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Hires, S.A.; Mao, T.; Huber, D.; Chiappe, M.E.; Chalasani, S.H.; Petreanu, L.; Akerboom, J.; McKinney, S.A.; Schreiter, E.R.; et al. Imaging Neural Activity in Worms, Flies and Mice with Improved GCaMP Calcium Indicators. Nat. Methods 2009, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Bedell, V.M.; Wang, Y.; Campbell, J.M.; Poshusta, T.L.; Starker, C.G.; Krug Ii, R.G.; Tan, W.; Penheiter, S.G.; Ma, A.C.; Leung, A.Y.H.; et al. In Vivo Genome Editing Using a High-Efficiency TALEN System. Nature 2012, 491, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Verhasselt, S.; Roman, B.I.; De Wever, O.; Van Hecke, K.; Van Deun, R.; Bracke, M.E.; Stevens, C.V. Discovery of (S)-3′-Hydroxyblebbistatin and (S)-3′-Aminoblebbistatin: Polar Myosin II Inhibitors with Superior Research Tool Properties. Org. Biomol. Chem. 2017, 15, 2104–2118. [Google Scholar] [CrossRef]
- Baillie, J.S.; Gendernalik, A.; Garrity, D.M.; Bark, D.; Quinn, T.A. The In Vivo Study of Cardiac Mechano-Electric and Mechano-Mechanical Coupling during Heart Development in Zebrafish. Front. Physiol. 2023, 14, 1086050. [Google Scholar] [CrossRef]
- Abu Nahia, K.; Migdał, M.; Quinn, T.A.; Poon, K.-L.; Łapiński, M.; Sulej, A.; Liu, J.; Mondal, S.S.; Pawlak, M.; Bugajski, Ł.; et al. Genomic and Physiological Analyses of the Zebrafish Atrioventricular Canal Reveal Molecular Building Blocks of the Secondary Pacemaker Region. Cell. Mol. Life Sci. 2021, 78, 6669–6687. [Google Scholar] [CrossRef]
- Lin, E.; Ribeiro, A.; Ding, W.; Hove-Madsen, L.; Sarunic, M.V.; Beg, M.F.; Tibbits, G.F. Optical Mapping of the Electrical Activity of Isolated Adult Zebrafish Hearts: Acute Effects of Temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R823–R836. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yost, H.J.; Clark, E.B. Cardiac Morphology and Blood Pressure in the Adult Zebrafish. Anat. Rec. 2001, 264, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baxter, W.T.; Mironov, S.F.; Zaitsev, A.V.; Jalife, J.; Pertsov, A.M. Visualizing Excitation Waves inside Cardiac Muscle Using Transillumination. Biophys. J. 2001, 80, 516–530. [Google Scholar] [CrossRef]
- Bishop, M.J.; Rodriguez, B.; Qu, F.; Efimov, I.R.; Gavaghan, D.J.; Trayanova, N.A. The Role of Photon Scattering in Optical Signal Distortion during Arrhythmia and Defibrillation. Biophys. J. 2007, 93, 3714–3726. [Google Scholar] [CrossRef]
- Joyce, W.; Perry, S.F. Hif-1α Is Not Required for the Development of Cardiac Adrenergic Control in Zebrafish (Danio rerio). J. Exp. Zool. Part A 2021, 335, 623–631. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Croll, R.P.; Smith, F.M. Intrinsic and Extrinsic Innervation of the Heart in Zebrafish (Danio rerio): Zebrafish Cardiac Innervation. J. Comp. Neurol. 2015, 523, 1683–1700. [Google Scholar] [CrossRef]
- Méndez-Giráldez, R.; Gogarten, S.M.; Below, J.E.; Yao, J.; Seyerle, A.A.; Highland, H.M.; Kooperberg, C.; Soliman, E.Z.; Rotter, J.I.; Kerr, K.F.; et al. GWAS of the Electrocardiographic QT Interval in Hispanics/Latinos Generalizes Previously Identified Loci and Identifies Population-Specific Signals. Sci. Rep. 2017, 7, 17075. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, Y.; Hochhauser, E.; Kliminski, V.; Dick, J.; Zahalka, M.A.; Parnes, D.; Schlesinger, H.; Abassi, Z.; Shainberg, A.; Schindler, R.F.R.; et al. Popeye Domain Containing 1 (Popdc1/Bves) Is a Caveolae-Associated Protein Involved in Ischemia Tolerance. PLoS ONE 2013, 8, e71100. [Google Scholar] [CrossRef]
- Martin, K.E.; Waxman, J.S. Atrial and Sinoatrial Node Development in the Zebrafish Heart. J. Cardiovasc. Dev. Dis. 2021, 8, 15. [Google Scholar] [CrossRef]
- Hou, J.H.; Kralj, J.M.; Douglass, A.D.; Engert, F.; Cohen, A.E. Simultaneous Mapping of Membrane Voltage and Calcium in Zebrafish Heart In Vivo Reveals Chamber-Specific Developmental Transitions in Ionic Currents. Front. Physiol. 2014, 5, 344. [Google Scholar] [CrossRef]
- Steele, S.L.; Yang, X.; Debiais-Thibaud, M.; Schwerte, T.; Pelster, B.; Ekker, M.; Tiberi, M.; Perry, S.F. In Vivo and in Vitro Assessment of Cardiac β-Adrenergic Receptors in Larval Zebrafish (Danio rerio). J. Exp. Biol. 2011, 214, 1445–1457. [Google Scholar] [CrossRef]
- Steele, S.L.; Lo, K.H.A.; Li, V.W.T.; Cheng, S.H.; Ekker, M.; Perry, S.F. Loss of M2 Muscarinic Receptor Function Inhibits Development of Hypoxic Bradycardia and Alters Cardiac β-Adrenergic Sensitivity in Larval Zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R412–R420. [Google Scholar] [CrossRef]
- Schwerte, T.; Prem, C.; Mairösl, A.; Pelster, B. Development of the Sympatho-Vagal Balance in the Cardiovascular System in Zebrafish (Danio rerio) Characterized by Power Spectrum and Classical Signal Analysis. J. Exp. Biol. 2006, 209, 1093–1100. [Google Scholar] [CrossRef]
- Stoyek, M.R.; Hortells, L.; Quinn, T.A. From Mice to Mainframes: Experimental Models for Investigation of the Intracardiac Nervous System. J. Cardiovasc. Dev. Dis. 2021, 8, 149. [Google Scholar] [CrossRef]
- Brette, F.; Luxan, G.; Cros, C.; Dixey, H.; Wilson, C.; Shiels, H.A. Characterization of Isolated Ventricular Myocytes from Adult Zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2008, 374, 143–146. [Google Scholar] [CrossRef]
- Poon, K.-L.; Liebling, M.; Kondrychyn, I.; Brand, T.; Korzh, V. Development of the Cardiac Conduction System in Zebrafish. Gene Expr. Patterns 2016, 21, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Doris, D.; Karche, S.; Petkova, M.; Borbas, B.; Logantha, S.J.R.J.; Fedorenko, O.; Maczewski, M.; Mackiewcz, U.; Zhang, Y.; Chahal, A.; et al. A Sexy Approach to Pacemaking: Differences in Function and Molecular Make up of the Sinoatrial Node. Histol. Histopathol. 2019, 34, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Shetty, M.S.; Ris, L.; Schindler, R.F.R.; Mizuno, K.; Fedele, L.; Giese, K.P.; Brand, T.; Abel, T. Mice Lacking the cAMP Effector Protein POPDC1 Show Enhanced Hippocampal Synaptic Plasticity. Cereb. Cortex 2022, 32, 3457–3471. [Google Scholar] [CrossRef] [PubMed]



| Larvae Pre ISO | |||||
|---|---|---|---|---|---|
| No Phenotype | Phenotype | ||||
| WT | S191F | KO | S191F | KO | |
| HR (bpm) | 244 ± 2 | 252 ± 2 | 228 ± 2 | 227 ± 4 | 231 ± 3 |
| AV Delay (ms) | 70.0 ± 1.5 | 61.8 ± 1.3 | 52.2 ± 1.2 | 54.3 ± 2.3 | 54.4 ± 1.2 |
| AP Upstroke Speed (dFn/dtmax, s−1) | |||||
| Atrium | 21.4 ± 0.7 | 22.3 ± 0.7 | 24.7 ± 0.6 | 18.1 ± 0.5 | 18.4 ± 0.5 |
| Ventricle | 19.3 ± 0.7 | 20.2 ± 0.6 | 20.4 ± 0.7 | 16.6 ± 0.5 | 16.6 ± 0.4 |
| APD80 (ms) | |||||
| Atrium | 151 ± 4 | 162 ± 4 | 171 ± 6 | 137 ± 5 | 153 ± 5 |
| Ventricle | 192 ± 4 | 186 ± 6 | 187 ± 6 | 165 ± 4 | 209 ± 5 |
| CaTD Upstroke Speed (dFn/dtmax, s−1) | |||||
| Atrium | 23.3 ± 0.3 | 24.3 ± 0.3 | 24.6 ± 0.3 | 19.2 ± 0.3 | 20.7 ± 0.4 |
| Ventricle | 17.4 ± 0.2 | 17.8 ± 0.2 | 17.6 ± 0.2 | 15.3 ± 0.3 | 15.6 ± 0.3 |
| CaTD80 (ms) | |||||
| Atrium | 169 ± 2 | 169 ± 2 | 173 ± 3 | 183 ± 3 | 170 ± 3 |
| Ventricle | 193 ± 1 | 187 ± 2 | 207 ± 2 | 206 ± 4 | 203 ± 3 |
| Larvae during ISO | |||
|---|---|---|---|
| WT | S191F | KO | |
| HR (bpm) | 262 ± 2 | 251 ± 3 | 243 ± 3 |
| AV Delay (ms) | 73.5 ± 1.0 | 64.8 ± 1.3 | 60.8 ± 1.4 |
| AP Upstroke Speed (dFn/dtmax, s−1) | |||
| Atrium | 17.6 ± 0.5 | 15.6 ± 0.5 | 15.4 ± 0.5 |
| Ventricle | 15.8 ± 0.5 | 13.7 ± 0.4 | 13.3 ± 0.4 |
| APD80 (ms) | |||
| Atrium | 113 ± 4 | 128 ± 4 | 127 ± 4 |
| Ventricle | 172 ± 3 | 165 ± 4 | 175 ± 4 |
| CaTD Upstroke Speed (dFn/dtmax, s−1) | |||
| Atrium | 23.3 ± 0.4 | 17.8 ± 0.4 | 17.4 ± 0.5 |
| Ventricle | 17.3 ± 0.2 | 13.1 ± 0.3 | 14.7 ± 0.3 |
| CaTD80 (ms) | |||
| Atrium | 153 ± 2 | 157 ± 2 | 167 ± 3 |
| Ventricle | 181 ± 2 | 180 ± 2 | 188 ± 3 |
| Adult | ||||
|---|---|---|---|---|
| WT | S191F | |||
| AV Delay (ms) | 50.1 ± 3.0 | 62.7 ± 3.5 | 51.6 ± 4.7 | 87.1 ± 6.6 |
| AP Upstroke Speed (dFn/dtmax, s−1) | ||||
| Atrium | 148.1 ± 4.4 | 144.9 ± 6.0 | 134.7 ± 4.4 | 136.1 ± 3.0 |
| Ventricle | 132.7 ± 5.7 | 132.1 ± 6.7 | 106.0 ± 5.0 | 101.8 ± 5.9 |
| APD80 (ms) | ||||
| Atrium | 67 ± 3 | 74 ± 4 | 71 ± 7 | 73 ± 7 |
| Ventricle | 170 ± 5 | 164 ± 5 | 196 ± 8 | 187 ± 6 |
| CaTD Upstroke Speed (dFn/dtmax, s−1) | ||||
| Atrium | 83.4 ± 4.7 | 81.8 ± 5.8 | 94.3 ± 2.8 | 94.2 ± 2.4 |
| Ventricle | 90.9 ± 5.3 | 91.1 ± 5.5 | 89.5 ± 2.5 | 79.8 ± 5.6 |
| CaTD80 (ms) | ||||
| Atrium | 133 ± 8 | 153 ± 47 | 127 ± 4 | 121 ± 4 |
| Ventricle | 261 ± 16 | 241 ± 15 | 253 ± 12 | 228 ± 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyek, M.R.; Doane, S.E.; Dallaire, S.E.; Long, Z.D.; Ramia, J.M.; Cassidy-Nolan, D.L.; Poon, K.-L.; Brand, T.; Quinn, T.A. POPDC1 Variants Cause Atrioventricular Node Dysfunction and Arrhythmogenic Changes in Cardiac Electrophysiology and Intracellular Calcium Handling in Zebrafish. Genes 2024, 15, 280. https://doi.org/10.3390/genes15030280
Stoyek MR, Doane SE, Dallaire SE, Long ZD, Ramia JM, Cassidy-Nolan DL, Poon K-L, Brand T, Quinn TA. POPDC1 Variants Cause Atrioventricular Node Dysfunction and Arrhythmogenic Changes in Cardiac Electrophysiology and Intracellular Calcium Handling in Zebrafish. Genes. 2024; 15(3):280. https://doi.org/10.3390/genes15030280
Chicago/Turabian StyleStoyek, Matthew R., Sarah E. Doane, Shannon E. Dallaire, Zachary D. Long, Jessica M. Ramia, Donovan L. Cassidy-Nolan, Kar-Lai Poon, Thomas Brand, and T. Alexander Quinn. 2024. "POPDC1 Variants Cause Atrioventricular Node Dysfunction and Arrhythmogenic Changes in Cardiac Electrophysiology and Intracellular Calcium Handling in Zebrafish" Genes 15, no. 3: 280. https://doi.org/10.3390/genes15030280
APA StyleStoyek, M. R., Doane, S. E., Dallaire, S. E., Long, Z. D., Ramia, J. M., Cassidy-Nolan, D. L., Poon, K.-L., Brand, T., & Quinn, T. A. (2024). POPDC1 Variants Cause Atrioventricular Node Dysfunction and Arrhythmogenic Changes in Cardiac Electrophysiology and Intracellular Calcium Handling in Zebrafish. Genes, 15(3), 280. https://doi.org/10.3390/genes15030280







