Computational Tools to Assist in Analyzing Effects of the SERPINA1 Gene Variation on Alpha-1 Antitrypsin (AAT)
Abstract
:1. Introduction
2. Molecular Variation: SNVs, CNVs, and Other
3. Molecular Variability of SERPINA1
4. Clinical Consequences of Pathogenic Variants in SERPINA1
5. Variant Interpretation in the Genomics Era—Future Perspective for AATD Diagnostics
6. Computational Tools for Predicting the Outcome of SNVs
6.1. Sequence-Based Computational Analysis
6.2. In Silico Protein Stability Analysis
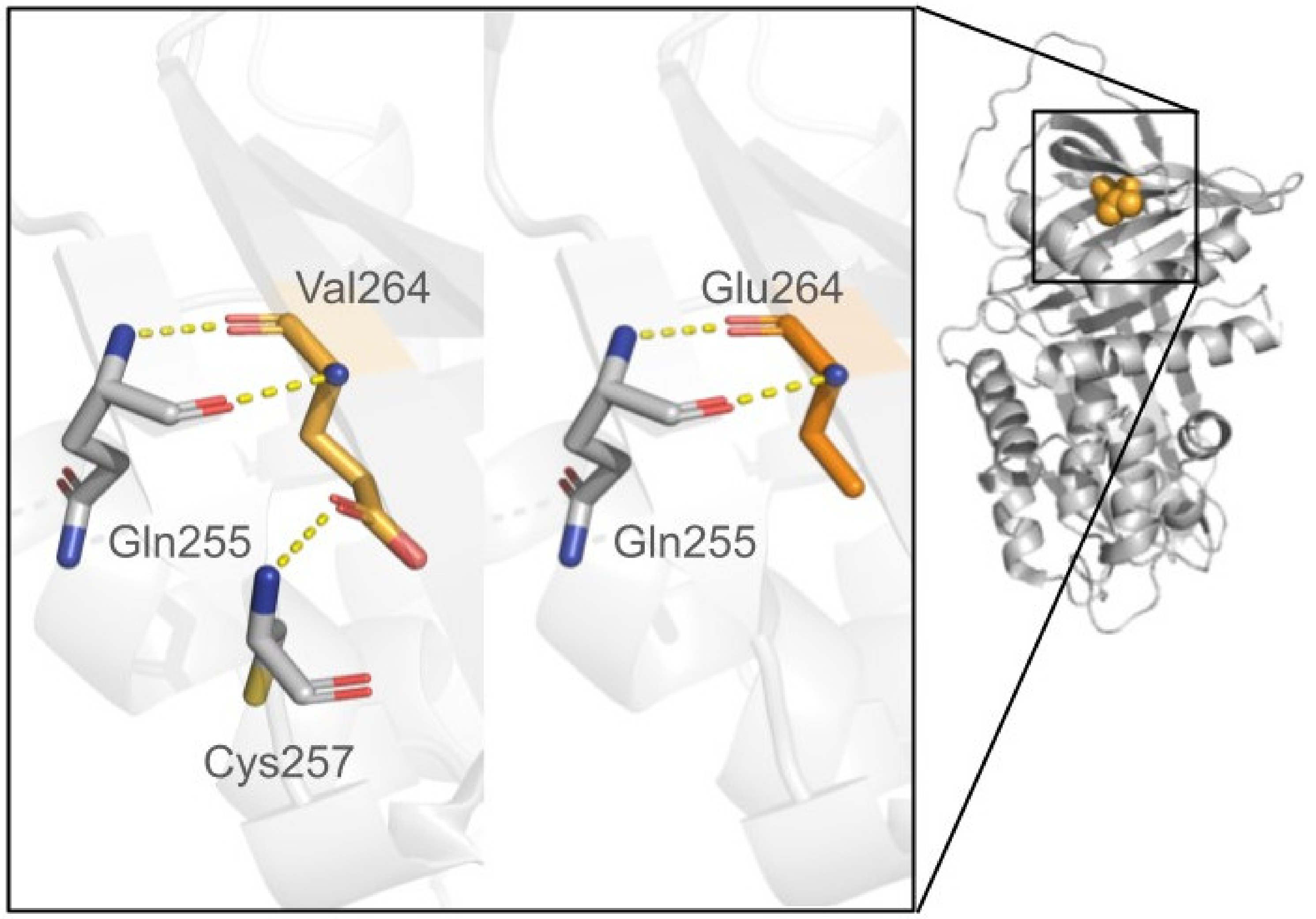
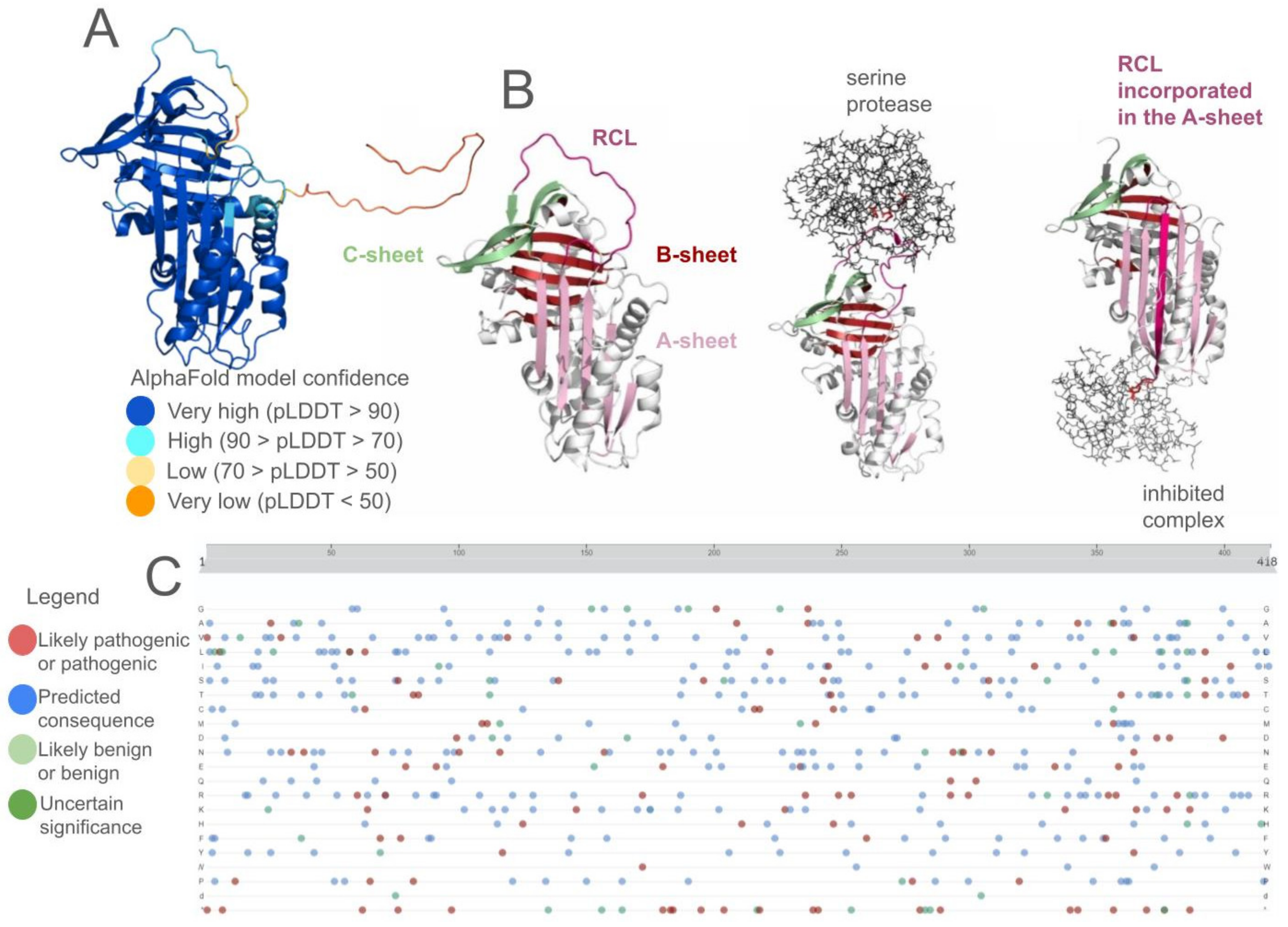
6.3. Homology Modeling and Structure Predictions
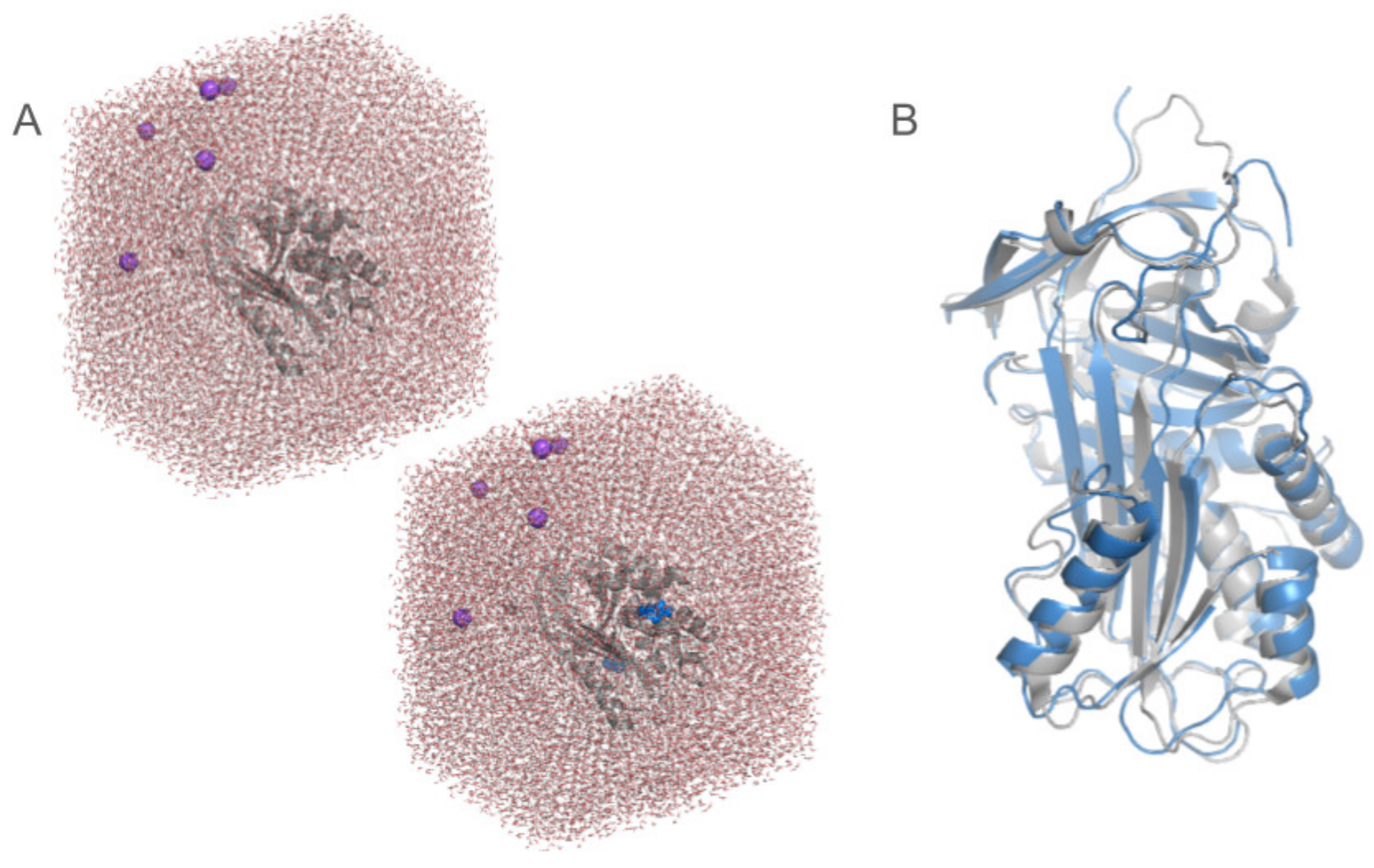
6.4. Molecular Dynamics Simulations
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusa, K.; Rashid, S.T.; Strick-Marchand, H.; Varela, I.; Liu, P.-Q.; Paschon, D.E.; Miranda, E.; Ordóñez, A.; Hannan, N.R.F.; Rouhani, F.J.; et al. Targeted Gene Correction of α1-Antitrypsin Deficiency in Induced Pluripotent Stem Cells. Nature 2011, 478, 391–394. [Google Scholar] [CrossRef]
- Foil, K.E. Variants of SERPINA1 and the Increasing Complexity of Testing for Alpha-1 Antitrypsin Deficiency. Ther. Adv. Chronic Dis. 2021, 12, 20406223211015954. [Google Scholar] [CrossRef]
- Seixas, S.; Marques, P.I. Known Mutations at the Cause of Alpha-1 Antitrypsin Deficiency an Updated Overview of SERPINA1 Variation Spectrum. Appl. Clin. Genet. 2021, 14, 173–194. [Google Scholar] [CrossRef]
- Tuteja, S.; Kadri, S.; Yap, K.L. A Performance Evaluation Study: Variant Annotation Tools—The Enigma of Clinical next Generation Sequencing (NGS) Based Genetic Testing. J. Pathol. Inform. 2022, 13, 100130. [Google Scholar] [CrossRef]
- Snustad, D.P.; Simmons, M.J. Principles of Genetics, 7th ed.; Wiley: Hoboken, NJ, USA, 2015; Available online: https://www.wiley.com/en-us/Principles+of+Genetics%2C+7th+Edition-p-9781119142287 (accessed on 10 February 2024).
- Biesecker, L.G.; Spinner, N.B. A Genomic View of Mosaicism and Human Disease. Nat. Rev. Genet. 2013, 14, 307–320. [Google Scholar] [CrossRef]
- GnomAD. Available online: https://gnomad.broadinstitute.org/about (accessed on 23 November 2023).
- den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.-F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E.M. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Taschner, P.E.M.; den Dunnen, J.T. Describing Structural Changes by Extending HGVS Sequence Variation Nomenclature. Hum. Mutat. 2011, 32, 507–511. [Google Scholar] [CrossRef]
- Goldstein, D.B.; Allen, A.; Keebler, J.; Margulies, E.H.; Petrou, S.; Petrovski, S.; Sunyaev, S. Sequencing Studies in Human Genetics: Design and Interpretation. Nat. Rev. Genet. 2013, 14, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.M.; Bals, R.; Koczulla, R.; Vogelmeier, C.; Köhnlein, T.; Welte, T. The Discovery of α1-Antitrypsin and Its Role in Health and Disease. Respir. Med. 2011, 105, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Serpina1[Gene]—ClinVar—NCBI. Available online: https://www.ncbi.nlm.nih.gov/clinvar/?term=serpina1%5Bgene%5D&redir=gene (accessed on 20 November 2023).
- HGMD® Home Page. Available online: https://www.hgmd.cf.ac.uk/ac/index.php (accessed on 20 November 2023).
- Stoller, J.K.; Hupertz, V.; Aboussouan, L.S. Alpha-1 Antitrypsin Deficiency. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 1993. [Google Scholar]
- Tan, L.; Dickens, J.A.; Demeo, D.L.; Miranda, E.; Perez, J.; Rashid, S.T.; Day, J.; Ordoñez, A.; Marciniak, S.J.; Haq, I.; et al. Circulating Polymers in A1-Antitrypsin Deficiency. Eur. Respir. J. 2014, 43, 1501–1504. [Google Scholar] [CrossRef]
- Kueppers, F.; Andrake, M.D.; Xu, Q.; Dunbrack, R.L.; Kim, J.; Sanders, C.L. Protein Modeling to Assess the Pathogenicity of Rare Variants of SERPINA1 in Patients Suspected of Having Alpha 1 Antitrypsin Deficiency. BMC Med. Genet. 2019, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.P.; Dina, M.A.; Howe, S.C.; Butz, M.L.; Willkomm, K.S.; Murray, D.L.; Snyder, M.R.; Rumilla, K.M.; Halling, K.C.; Highsmith, W.E. SERPINA1 Full-Gene Sequencing Identifies Rare Mutations Not Detected in Targeted Mutation Analysis. J. Mol. Diagn. 2015, 17, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotti, I.; Carroll, T.P.; Ottaviani, S.; Fra, A.M.; O’Brien, G.; Molloy, K.; Corda, L.; Medicina, D.; Curran, D.R.; McElvaney, N.G.; et al. Identification and Characterisation of Eight Novel SERPINA1 Null Mutations. Orphanet J. Rare Dis. 2014, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Matamala, N.; Lara, B.; Gomez-Mariano, G.; Martínez, S.; Retana, D.; Fernandez, T.; Silvestre, R.A.; Belmonte, I.; Rodriguez-Frias, F.; Vilar, M.; et al. Characterization of Novel Missense Variants of SERPINA1 Gene Causing Alpha-1 Antitrypsin Deficiency. Am. J. Respir. Cell Mol. Biol. 2018, 58, 706–716. [Google Scholar] [CrossRef]
- Stefl, S.; Nishi, H.; Petukh, M.; Panchenko, A.R.; Alexov, E. Molecular Mechanisms of Disease-Causing Missense Mutations. J. Mol. Biol. 2013, 425, 3919–3936. [Google Scholar] [CrossRef]
- Gonzalez, A.; Belmonte, I.; Nuñez, A.; Farago, G.; Barrecheguren, M.; Pons, M.; Orriols, G.; Gabriel-Medina, P.; Rodríguez-Frías, F.; Miravitlles, M.; et al. New Variants of Alpha-1-Antitrypsin: Structural Simulations and Clinical Expression. Respir. Res. 2022, 23, 339. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, P.; Sun, S.; Wang, X.; Balch, W.E. Profiling Genetic Diversity Reveals the Molecular Basis for Balancing Function with Misfolding in Alpha-1 Antitrypsin. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kalsheker, N. Alpha1-Antitrypsin: Structure, Function and Molecular Biology of the Gene. Biosci. Rep. 1989, 9, 129–138. [Google Scholar] [CrossRef]
- Lomas, D.A.; Evans, D.L.; Finch, J.T.; Carrell, R.W. The Mechanism of Z Alpha 1-Antitrypsin Accumulation in the Liver. Nature 1992, 357, 605–607. [Google Scholar] [CrossRef]
- Coonrod, E.M.; Durtschi, J.D.; Margraf, R.L.; Voelkerding, K.V. Developing Genome and Exome Sequencing for Candidate Gene Identification in Inherited Disorders: An Integrated Technical and Bioinformatics Approach. Arch. Pathol. Lab. Med. 2013, 137, 415–433. [Google Scholar] [CrossRef]
- Neveling, K.; Feenstra, I.; Gilissen, C.; Hoefsloot, L.H.; Kamsteeg, E.-J.; Mensenkamp, A.R.; Rodenburg, R.J.T.; Yntema, H.G.; Spruijt, L.; Vermeer, S.; et al. A Post-Hoc Comparison of the Utility of Sanger Sequencing and Exome Sequencing for the Diagnosis of Heterogeneous Diseases. Hum. Mutat. 2013, 34, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, F.; Wu, J.; Schubert, J.; Li, M.M. Application of Next Generation Sequencing in Laboratory Medicine. Ann. Lab. Med. 2021, 41, 25–43. [Google Scholar] [CrossRef] [PubMed]
- MRC Holland. Available online: https://www.mrcholland.com/product/P459/4115 (accessed on 23 November 2023).
- Stuppia, L.; Antonucci, I.; Palka, G.; Gatta, V. Use of the MLPA Assay in the Molecular Diagnosis of Gene Copy Number Alterations in Human Genetic Diseases. Int. J. Mol. Sci. 2012, 13, 3245–3276. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha1-Antitrypsin Deficiency. N. Engl. J. Med. 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- Orphanet: Alpha 1 Antitrypsin Deficiency. Available online: https://www.orpha.net/consor/www/cgi-bin/OC_Exp.php?lng=EN&Expert=60 (accessed on 20 November 2023).
- Miravitlles, M. Alpha1-Antitrypsin Deficiency: Epidemiology and Prevalence. Respir. Med. 2000, 94 (Suppl. C), S12–S15. [Google Scholar] [CrossRef]
- Blanco, I.; Bueno, P.; Diego, I.; Pérez-Holanda, S.; Casas-Maldonado, F.; Esquinas, C.; Miravitlles, M. Alpha-1 Antitrypsin Pi*Z Gene Frequency and Pi*ZZ Genotype Numbers Worldwide: An Update. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 561–569. [Google Scholar] [CrossRef]
- de Serres, F.J. Alpha-1 Antitrypsin Deficiency Is Not a Rare Disease but a Disease That Is Rarely Diagnosed. Environ. Health Perspect. 2003, 111, 1851–1854. [Google Scholar] [CrossRef]
- Stoller, J.K.; Brantly, M. The Challenge of Detecting Alpha-1 Antitrypsin Deficiency. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10 (Suppl. S1), 26–34. [Google Scholar] [CrossRef]
- Mahadeva, R.; Atkinson, C.; Li, Z.; Stewart, S.; Janciauskiene, S.; Kelley, D.G.; Parmar, J.; Pitman, R.; Shapiro, S.D.; Lomas, D.A. Polymers of Z Alpha1-Antitrypsin Co-Localize with Neutrophils in Emphysematous Alveoli and Are Chemotactic in Vivo. Am. J. Pathol. 2005, 166, 377–386. [Google Scholar] [CrossRef]
- Lomas, D.A.; Parfrey, H. Alpha1-Antitrypsin Deficiency. 4: Molecular Pathophysiology. Thorax 2004, 59, 529–535. [Google Scholar] [CrossRef]
- Mahadeva, R.; Chang, W.S.; Dafforn, T.R.; Oakley, D.J.; Foreman, R.C.; Calvin, J.; Wight, D.G.; Lomas, D.A. Heteropolymerization of S, I, and Z Alpha1-Antitrypsin and Liver Cirrhosis. J. Clin. Investig. 1999, 103, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Catarino, S.; Alonso, L.; Landeta, M.; Vivanco, A.; Artieda, M.; Larruskain, A.; Lopez, M. Development of a next Generation Sequencing Assay for Detection of A1AT Deficiency. Eur. Respir. J. 2023, 62, PA5212. [Google Scholar] [CrossRef]
- Kalia, S.S.; Adelman, K.; Bale, S.J.; Chung, W.K.; Eng, C.; Evans, J.P.; Herman, G.E.; Hufnagel, S.B.; Klein, T.E.; Korf, B.R.; et al. Recommendations for Reporting of Secondary Findings in Clinical Exome and Genome Sequencing, 2016 Update (ACMG SF v2.0): A Policy Statement of the American College of Medical Genetics and Genomics. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Saelaert, M.; Mertes, H.; Moerenhout, T.; De Baere, E.; Devisch, I. Criteria for Reporting Incidental Findings in Clinical Exome Sequencing—A Focus Group Study on Professional Practices and Perspectives in Belgian Genetic Centres. BMC Med. Genom. 2019, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.M.; Biesecker, L.G.; Rehm, H.L. Overview of Specifications to the ACMG/AMP Variant Interpretation Guidelines. Curr. Protoc. Hum. Genet. 2019, 103, e93. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Oliveira, J.; Sousa, M. Bioinformatics and Computational Tools for Next-Generation Sequencing Analysis in Clinical Genetics. J. Clin. Med. 2020, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.Org: Online Mendelian Inheritance in Man (OMIM®), an Online Catalog of Human Genes and Genetic Disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef]
- Home—OMIM. Available online: https://www.omim.org/ (accessed on 23 November 2023).
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public Archive of Relationships among Sequence Variation and Human Phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef]
- ClinVar. Available online: https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 23 November 2023).
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Orphanet. Available online: http://www.orpha.net/consor/www/cgi-bin/index.php?lng=EN (accessed on 23 November 2023).
- Zia, A.; Moses, A.M. Ranking Insertion, Deletion and Nonsense Mutations Based on Their Effect on Genetic Information. BMC Bioinformatics 2011, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Shaik, N.A.; Saud Al-Saud, N.B.; Abdulhamid Aljuhani, T.; Jamil, K.; Alnuman, H.; Aljeaid, D.; Sultana, N.; El-Harouni, A.A.; Awan, Z.A.; Elango, R.; et al. Structural Characterization and Conformational Dynamics of Alpha-1 Antitrypsin Pathogenic Variants Causing Alpha-1-Antitrypsin Deficiency. Front. Mol. Biosci. 2022, 9, 1051511. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Arapoglou, T.; Li, X.; Li, Z.; Zheng, X.; Moore, J.; Asok, A.; Kumar, S.; Blue, E.E.; Buyske, S.; et al. FAVOR: Functional Annotation of Variants Online Resource and Annotator for Variation across the Human Genome. Nucleic Acids Res. 2023, 51, D1300–D1311. [Google Scholar] [CrossRef]
- Genomics in the Cloud [Book]. Available online: https://www.oreilly.com/library/view/genomics-in-the/9781491975183/ (accessed on 10 February 2024).
- Liu, X.; Jian, X.; Boerwinkle, E. dbNSFP: A Lightweight Database of Human Nonsynonymous SNPs and Their Functional Predictions. Hum. Mutat. 2011, 32, 894–899. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Gunasekaran, P.; Leung, K.; Bindal, N.; Boutselakis, H.; Ding, M.; Bamford, S.; Cole, C.; Ward, S.; et al. COSMIC: Exploring the World’s Knowledge of Somatic Mutations in Human Cancer. Nucleic Acids Res. 2015, 43, D805–D811. [Google Scholar] [CrossRef]
- Funcotator Information and Tutorial. Available online: https://gatk.broadinstitute.org/hc/en-us/articles/360035889931-Funcotator-Information-and-Tutorial (accessed on 10 February 2024).
- Ip, E.; Chapman, G.; Winlaw, D.; Dunwoodie, S.L.; Giannoulatou, E. VPOT: A Customizable Variant Prioritization Ordering Tool for Annotated Variants. Genom. Proteom. Bioinform. 2019, 17, 540–545. [Google Scholar] [CrossRef]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef]
- Giacopuzzi, E.; Laffranchi, M.; Berardelli, R.; Ravasio, V.; Ferrarotti, I.; Gooptu, B.; Borsani, G.; Fra, A. Real-World Clinical Applicability of Pathogenicity Predictors Assessed on SERPINA1 Mutations in Alpha-1-Antitrypsin Deficiency. Hum. Mutat. 2018, 39, 1203–1213. [Google Scholar] [CrossRef]
- Feng, B.-J. PERCH: A Unified Framework for Disease Gene Prioritization. Hum. Mutat. 2017, 38, 243–251. [Google Scholar] [CrossRef]
- Ball, M.P.; Thakuria, J.V.; Zaranek, A.W.; Clegg, T.; Rosenbaum, A.M.; Wu, X.; Angrist, M.; Bhak, J.; Bobe, J.; Callow, M.J.; et al. A Public Resource Facilitating Clinical Use of Genomes. Proc. Natl. Acad. Sci. USA 2012, 109, 11920–11927. [Google Scholar] [CrossRef] [PubMed]
- Licata, L.; Via, A.; Turina, P.; Babbi, G.; Benevenuta, S.; Carta, C.; Casadio, R.; Cicconardi, A.; Facchiano, A.; Fariselli, P.; et al. Resources and Tools for Rare Disease Variant Interpretation. Front. Mol. Biosci. 2023, 10, 1169109. [Google Scholar] [CrossRef] [PubMed]
- Katsonis, P.; Wilhelm, K.; Williams, A.; Lichtarge, O. Genome Interpretation Using in Silico Predictors of Variant Impact. Hum. Genet. 2022, 141, 1549–1577. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Oak, N.; Plon, S.E. Evaluation of in Silico Algorithms for Use with ACMG/AMP Clinical Variant Interpretation Guidelines. Genome Biol. 2017, 18, 225. [Google Scholar] [CrossRef]
- Jain, S.; Bakolitsa, C.; Brenner, S.E.; Radivojac, P.; Moult, J.; Repo, S.; Hoskins, R.A.; Andreoletti, G.; Barsky, D.; Chellapan, A.; et al. CAGI, the Critical Assessment of Genome Interpretation, Establishes Progress and Prospects for Computational Genetic Variant Interpretation Methods. Genome Biol. 2024, 25, 53. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. SIFT: Predicting Amino Acid Changes That Affect Protein Function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting Functional Effect of Human Missense Mutations Using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 76, 7–20. [Google Scholar] [CrossRef]
- Jagadeesh, K.A.; Wenger, A.M.; Berger, M.J.; Guturu, H.; Stenson, P.D.; Cooper, D.N.; Bernstein, J.A.; Bejerano, G. M-CAP Eliminates a Majority of Variants of Uncertain Significance in Clinical Exomes at High Sensitivity. Nat. Genet. 2016, 48, 1581–1586. [Google Scholar] [CrossRef]
- Rogers, M.F.; Shihab, H.A.; Mort, M.; Cooper, D.N.; Gaunt, T.R.; Campbell, C. FATHMM-XF: Accurate Prediction of Pathogenic Point Mutations via Extended Features. Bioinformatics 2018, 34, 511–513. [Google Scholar] [CrossRef]
- Rigobello, C.; Baraldo, S.; Tinè, M.; Ferrarotti, I.; Corsico, A.G.; Bazzan, E.; Turato, G.; Balestro, E.; Biondini, D.; Valle, G.; et al. Exome Sequencing Reveals Immune Genes as Susceptibility Modifiers in Individuals with A1-Antitrypsin Deficiency. Sci. Rep. 2019, 9, 13088. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, L.; Forcato, C.; Vitulo, N.; Birolo, G.; De Pascale, F.; Feltrin, E.; Schiavon, R.; Anglani, F.; Negrisolo, S.; Zanetti, A.; et al. QueryOR: A Comprehensive Web Platform for Genetic Variant Analysis and Prioritization. BMC Bioinform. 2017, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Krishnan, V.G.; Mort, M.E.; Xin, F.; Kamati, K.K.; Cooper, D.N.; Mooney, S.D.; Radivojac, P. Automated Inference of Molecular Mechanisms of Disease from Amino Acid Substitutions. Bioinformatics 2009, 25, 2744–2750. [Google Scholar] [CrossRef]
- Bao, L.; Zhou, M.; Cui, Y. nsSNPAnalyzer: Identifying Disease-Associated Nonsynonymous Single Nucleotide Polymorphisms. Nucleic Acids Res. 2005, 33, W480–W482. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Bromberg, Y.; Rost, B. SNAP: Predict Effect of Non-Synonymous Polymorphisms on Function. Nucleic Acids Res. 2007, 35, 3823–3835. [Google Scholar] [CrossRef]
- Stone, E.A.; Sidow, A. Physicochemical Constraint Violation by Missense Substitutions Mediates Impairment of Protein Function and Disease Severity. Genome Res. 2005, 15, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.D.; Kejariwal, A. Coding Single-Nucleotide Polymorphisms Associated with Complex vs. Mendelian Disease: Evolutionary Evidence for Differences in Molecular Effects. Proc. Natl. Acad. Sci. USA 2004, 101, 15398–15403. [Google Scholar] [CrossRef]
- Capriotti, E.; Calabrese, R.; Casadio, R. Predicting the Insurgence of Human Genetic Diseases Associated to Single Point Protein Mutations with Support Vector Machines and Evolutionary Information. Bioinformatics 2006, 22, 2729–2734. [Google Scholar] [CrossRef]
- Calabrese, R.; Capriotti, E.; Fariselli, P.; Martelli, P.L.; Casadio, R. Functional Annotations Improve the Predictive Score of Human Disease-Related Mutations in Proteins. Hum. Mutat. 2009, 30, 1237–1244. [Google Scholar] [CrossRef]
- Castaldi, P.J.; Dahabreh, I.J.; Ioannidis, J.P.A. An Empirical Assessment of Validation Practices for Molecular Classifiers. Brief. Bioinform. 2011, 12, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Bendl, J.; Stourac, J.; Salanda, O.; Pavelka, A.; Wieben, E.D.; Zendulka, J.; Brezovsky, J.; Damborsky, J. PredictSNP: Robust and Accurate Consensus Classifier for Prediction of Disease-Related Mutations. PLoS Comput. Biol. 2014, 10, e1003440. [Google Scholar] [CrossRef] [PubMed]
- Ramensky, V.; Bork, P.; Sunyaev, S. Human Non-Synonymous SNPs: Server and Survey. Nucleic Acids Res. 2002, 30, 3894–3900. [Google Scholar] [CrossRef] [PubMed]
- Denden, S.; Leban, N.; Hayek, D.; Knani, J.; Chibani, J.B.; Khelil, A.H. In Silico Analysis of Alpha1-Antitrypsin Variants: The Effects of a Novel Mutation. Genet. Mol. Biol. 2010, 33, 633–636. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Patschull, A.O.M.; Segu, L.; Nyon, M.P.; Lomas, D.A.; Nobeli, I.; Barrett, T.E.; Gooptu, B. Therapeutic Target-Site Variability in A1-Antitrypsin Characterized at High Resolution. Acta Crystallograph. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Huntington, J.A.; Read, R.J.; Carrell, R.W. Structure of a Serpin–Protease Complex Shows Inhibition by Deformation. Nature 2000, 407, 923–926. [Google Scholar] [CrossRef]
- Yamasaki, M.; Li, W.; Johnson, D.J.D.; Huntington, J.A. Crystal Structure of a Stable Dimer Reveals the Molecular Basis of Serpin Polymerization. Nature 2008, 455, 1255–1258. [Google Scholar] [CrossRef]
- Yamasaki, M.; Sendall, T.J.; Pearce, M.C.; Whisstock, J.C.; Huntington, J.A. Molecular Basis of α1-antitrypsin Deficiency Revealed by the Structure of a Domain-swapped Trimer. EMBO Rep. 2011, 12, 1011–1017. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, Y.; Zhang, F.; Wei, Z.; Wang, Y.; Carrell, R.W.; Read, R.J.; Chen, G.-Q.; Zhou, A. Molecular Mechanism of Z α1-Antitrypsin Deficiency. J. Biol. Chem. 2016, 291, 15674–15686. [Google Scholar] [CrossRef]
- Pancotti, C.; Benevenuta, S.; Birolo, G.; Alberini, V.; Repetto, V.; Sanavia, T.; Capriotti, E.; Fariselli, P. Predicting Protein Stability Changes upon Single-Point Mutation: A Thorough Comparison of the Available Tools on a New Dataset. Brief. Bioinform. 2022, 23, bbab555. [Google Scholar] [CrossRef]
- Iqbal, S.; Li, F.; Akutsu, T.; Ascher, D.B.; Webb, G.I.; Song, J. Assessing the Performance of Computational Predictors for Estimating Protein Stability Changes upon Missense Mutations. Brief. Bioinform. 2021, 22, bbab184. [Google Scholar] [CrossRef]
- Hassan, M.S.; Shaalan, A.A.; Dessouky, M.I.; Abdelnaiem, A.E.; ElHefnawi, M. A Review Study: Computational Techniques for Expecting the Impact of Non-Synonymous Single Nucleotide Variants in Human Diseases. Gene 2019, 680, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Novati, G.; Pan, J.; Bycroft, C.; Žemgulytė, A.; Applebaum, T.; Pritzel, A.; Wong, L.H.; Zielinski, M.; Sargeant, T.; et al. Accurate Proteome-Wide Missense Variant Effect Prediction with AlphaMissense. Science 2023, 381, eadg7492. [Google Scholar] [CrossRef] [PubMed]
- Buß, O.; Rudat, J.; Ochsenreither, K. FoldX as Protein Engineering Tool: Better Than Random Based Approaches? Comput. Struct. Biotechnol. J. 2018, 16, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, Q.; Dunbrack, R.L. Prediction of Phenotypes of Missense Mutations in Human Proteins from Biological Assemblies. Proteins 2013, 81, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Schwede, T.; Sali, A.; Honig, B.; Levitt, M.; Berman, H.M.; Jones, D.; Brenner, S.E.; Burley, S.K.; Das, R.; Dokholyan, N.V.; et al. Outcome of a Workshop on Applications of Protein Models in Biomedical Research. Structure 2009, 17, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Mitusińska, K.; Góra, A.; Bogdańska, A.; Rożdżyńska-Świątkowska, A.; Tylki-Szymańska, A.; Jezela-Stanek, A. Structural Analysis of the Effect of Asn107Ser Mutation on Alg13 Activity and Alg13-Alg14 Complex Formation and Expanding the Phenotypic Variability of ALG13-CDG. Biomolecules 2022, 12, 398. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling Protein Tertiary and Quaternary Structure Using Evolutionary Information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Sali, A. Comparative Protein Modeling by Satisfaction of Spatial Restraints. Mol. Med. Today 1995, 1, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate Prediction of Protein Structures and Interactions Using a Three-Track Neural Network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.M.; Polev, K.; Abdirasulov, A.; Reinharz, V.; Grzybowski, B.A.; Tlusty, T. AlphaFold2 Can Predict Single-Mutation Effects. Phys. Rev. Lett. 2023, 131, 218401. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Rodríguez, P.; Carmena-Bargueño, M.; de la Morena-Barrio, M.E.; Bravo-Pérez, C.; de la Morena-Barrio, B.; Cifuentes-Riquelme, R.; Lozano, M.L.; Pérez-Sánchez, H.; Corral, J. Analysis of AlphaFold and Molecular Dynamics Structure Predictions of Mutations in Serpins. bioRxiv 2023. [Google Scholar] [CrossRef]
- Sinha, S.; Tam, B.; Wang, S.M. Applications of Molecular Dynamics Simulation in Protein Study. Membranes 2022, 12, 844. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A Message-Passing Parallel Molecular Dynamics Implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber Biomolecular Simulation Programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Nelson, M.T.; Humphrey, W.; Gursoy, A.; Dalke, A.; Kalé, L.V.; Skeel, R.D.; Schulten, K. NAMD: A Parallel, Object-Oriented Molecular Dynamics Program. Int. J. Supercomput. Appl. High Perform. Comput. 1996, 10, 251–268. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Kumar, A.; Rajendran, V.; Sethumadhavan, R.; Purohit, R. Evidence of Colorectal Cancer-Associated Mutation in MCAK: A Computational Report. Cell Biochem. Biophys. 2013, 67, 837–851. [Google Scholar] [CrossRef]
- Acosta-Tapia, N.; Galindo, J.F.; Baldiris, R. Insights into the Effect of Lowe Syndrome-Causing Mutation p.Asn591Lys of OCRL-1 through Protein–Protein Interaction Networks and Molecular Dynamics Simulations. J. Chem. Inf. Model. 2020, 60, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Hazari, Y.; Pal, D.; Maity, D.; Bashir, S.; Singh, L.R.; Shah, N.N.; Fazili, K.M. Aggregation of M3 (E376D) Variant of Alpha1- Antitrypsin. Sci. Rep. 2020, 10, 8290. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Góra, A.; Spruijt, R.; Mitusińska, K.; Suarez-Diez, M.; Martins dos Santos, V.; Schaap, P.J. Modulating D-Amino Acid Oxidase (DAAO) Substrate Specificity through Facilitated Solvent Access. PLoS ONE 2018, 13, e0198990. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.J.; Risør, M.W.; Poulsen, E.C.; Nielsen, N.C.; Miao, Y.; Enghild, J.J.; Schiøtt, B. Reactive Center Loop Insertion in α-1-Antitrypsin Captured by Accelerated Molecular Dynamics Simulation. Biochemistry 2017, 56, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Kass, I.; Knaupp, A.S.; Bottomley, S.P.; Buckle, A.M. Conformational Properties of the Disease-Causing Z Variant of α1-Antitrypsin Revealed by Theory and Experiment. Biophys. J. 2012, 102, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ehtesham, M.S.; Ali, R.; Idrees, M. Effects of Normal and Aberrant Glycosylation on the Stability of α1-Anti Trypsin through Molecular Dynamic Simulation. Pak. J. Med. Health Sci. 2021, 15, 3066–3069. [Google Scholar] [CrossRef]
- Cooper, G.M.; Shendure, J. Needles in Stacks of Needles: Finding Disease-Causal Variants in a Wealth of Genomic Data. Nat. Rev. Genet. 2011, 12, 628–640. [Google Scholar] [CrossRef]
- Hekkelman, M.L.; de Vries, I.; Joosten, R.P.; Perrakis, A. AlphaFill: Enriching AlphaFold Models with Ligands and Cofactors. Nat. Methods 2023, 20, 205–213. [Google Scholar] [CrossRef]
- Wayment-Steele, H.K.; Ojoawo, A.; Otten, R.; Apitz, J.M.; Pitsawong, W.; Hömberger, M.; Ovchinnikov, S.; Colwell, L.; Kern, D. Predicting Multiple Conformations via Sequence Clustering and AlphaFold2. Nature 2024, 625, 832–839. [Google Scholar] [CrossRef]
- Banerjee, J.; Taroni, J.N.; Allaway, R.J.; Prasad, D.V.; Guinney, J.; Greene, C. Machine Learning in Rare Disease. Nat. Methods 2023, 20, 803–814. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mróz, J.; Pelc, M.; Mitusińska, K.; Chorostowska-Wynimko, J.; Jezela-Stanek, A. Computational Tools to Assist in Analyzing Effects of the SERPINA1 Gene Variation on Alpha-1 Antitrypsin (AAT). Genes 2024, 15, 340. https://doi.org/10.3390/genes15030340
Mróz J, Pelc M, Mitusińska K, Chorostowska-Wynimko J, Jezela-Stanek A. Computational Tools to Assist in Analyzing Effects of the SERPINA1 Gene Variation on Alpha-1 Antitrypsin (AAT). Genes. 2024; 15(3):340. https://doi.org/10.3390/genes15030340
Chicago/Turabian StyleMróz, Jakub, Magdalena Pelc, Karolina Mitusińska, Joanna Chorostowska-Wynimko, and Aleksandra Jezela-Stanek. 2024. "Computational Tools to Assist in Analyzing Effects of the SERPINA1 Gene Variation on Alpha-1 Antitrypsin (AAT)" Genes 15, no. 3: 340. https://doi.org/10.3390/genes15030340
APA StyleMróz, J., Pelc, M., Mitusińska, K., Chorostowska-Wynimko, J., & Jezela-Stanek, A. (2024). Computational Tools to Assist in Analyzing Effects of the SERPINA1 Gene Variation on Alpha-1 Antitrypsin (AAT). Genes, 15(3), 340. https://doi.org/10.3390/genes15030340






