Genotypic and Allelic Distribution of the CD36 rs1761667 Polymorphism in High-Level Moroccan Athletes: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
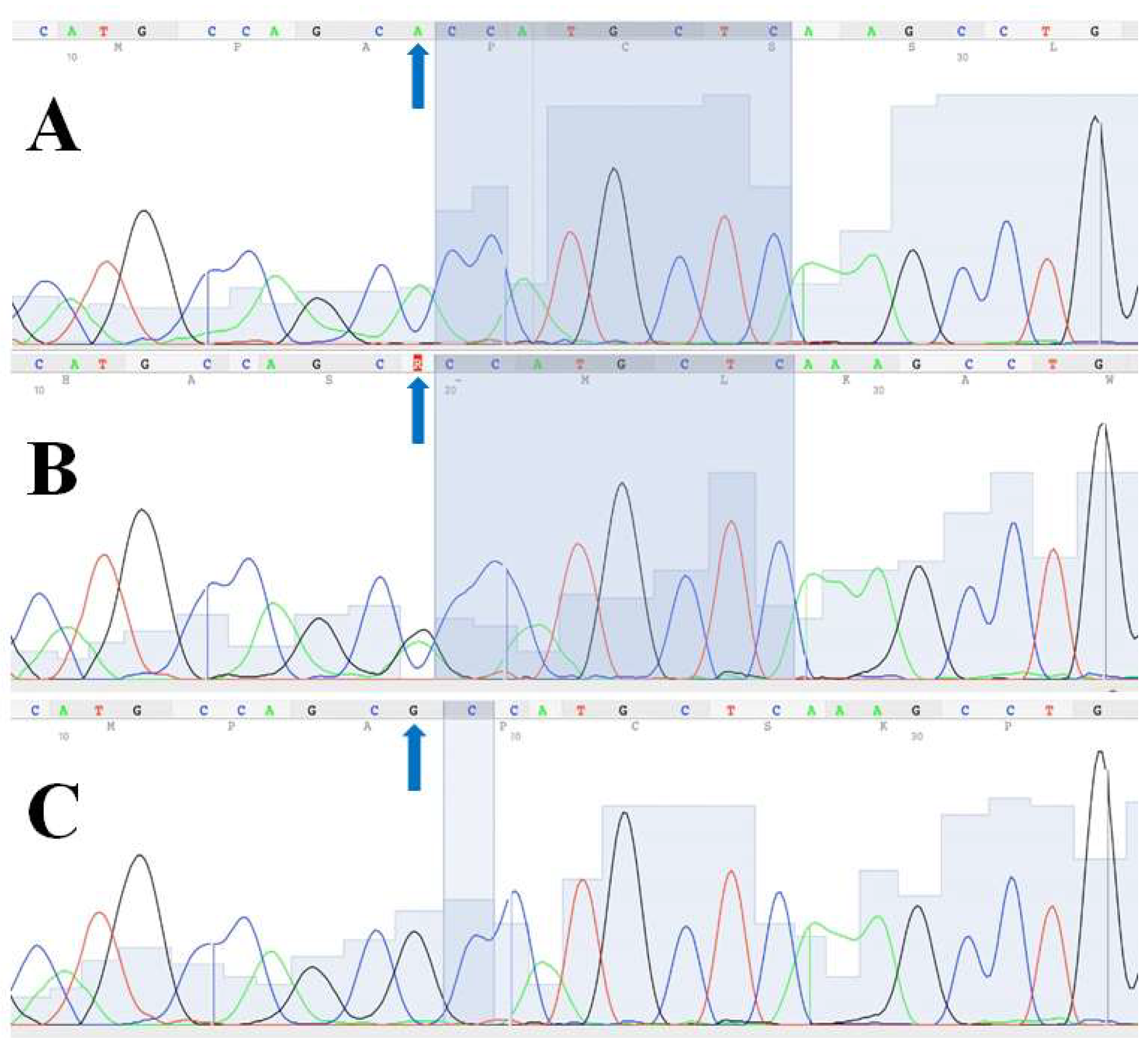
2.2. Genotyping
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nédélec, M.; Halson, S.; Delecroix, B.; Abaidia, A.-E.; Ahmaidi, S.; Dupont, G. Sleep Hygiene and Recovery Strategies in Elite Soccer Players. Sports Med. 2015, 45, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Kiely, J.; Grgic, J.; Lucia, A.; Del Coso, J. Can Genetic Testing Identify Talent for Sport? Genes 2019, 10, 972. [Google Scholar] [CrossRef]
- Yang, N.; MacArthur, D.G.; Gulbin, J.P.; Hahn, A.G.; Beggs, A.H.; Easteal, S.; North, K. ACTN3 genotype is associated with human elite athletic performance. Am. J. Hum. Genet. 2003, 73, 627–631. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Vinogradova, O.L.; Williams, A.G. Gene polymorphisms and fiber-type composition of human skeletal muscle. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 292–303. [Google Scholar] [CrossRef]
- Bray, M.S.; Hagberg, J.M.; Pérusse, L.; Rankinen, T.; Roth, S.M.; Wolfarth, B.; Bouchard, C. The human gene map for performance and health-related fitness phenotypes: The 2006–2007 update. Med. Sci. Sports Exerc. 2009, 41, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The Regulation of Fat Metabolism during Aerobic Exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Fediuc, S.; Hawke, T.J.; Riddell, M.C. Endurance exercise training increases adipose tissue glucocorticoid exposure: Adaptations that facilitate lipolysis. Metabolism 2009, 58, 651–660. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000, 72, 558S–563S. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [CrossRef]
- Dandanell, S.; Meinild-Lundby, A.-K.; Andersen, A.B.; Lang, P.F.; Oberholzer, L.; Keiser, S.; Robach, P.; Larsen, S.; Rønnestad, B.R.; Lundby, C. Determinants of maximal whole-body fat oxidation in elite cross-country skiers: Role of skeletal muscle mitochondria. Scand. J. Med. Sci. Sports 2018, 28, 2494–2504. [Google Scholar] [CrossRef]
- Hetlelid, K.J.; Plews, D.J.; Herold, E.; Laursen, P.B.; Seiler, S. Rethinking the role of fat oxidation: Substrate utilisation during high-intensity interval training in well-trained and recreationally trained runners. BMJ Open Sport Exerc. Med. 2015, 1, e000047. [Google Scholar] [CrossRef] [PubMed]
- Aslankeser, Z.; Balcı, Ş.S. Re-examination of the contribution of substrates to energy expenditure during high-intensity intermittent exercise in endurance athletes. PeerJ 2017, 5, e3769. [Google Scholar] [CrossRef] [PubMed]
- Harasim, E.; Kalinowska, A.; Chabowski, A.; Stepek, T. The role of fatty-acid transport proteins (FAT/CD36, FABPpm, FATP) in lipid metabolism in skeletal muscles. Postep. Hig. I Med. Dosw. Online 2008, 62, 433–441. [Google Scholar]
- Ibrahimi, A.; Bonen, A.; Blinn, W.D.; Hajri, T.; Li, X.; Zhong, K.; Cameron, R.; Abumrad, N.A. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J. Biol. Chem. 1999, 274, 26761–26766. [Google Scholar] [CrossRef]
- Bonen, A.; Han, X.-X.; Habets, D.D.J.; Febbraio, M.; Glatz, J.F.C.; Luiken, J.J.F.P. A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1740–E1749. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Watanabe, I.; Ishii, K.; Morimoto, M.; Fujiwara, H.; Yoshida, S.; Hui, S.-P.; Matsuno, K.; Chiba, H. Attenuated aerobic exercise capacity in CD36 deficiency. J. Med. Genet. 2007, 44, 445–447. [Google Scholar] [CrossRef]
- Karunakaran, U.; Elumalai, S.; Moon, J.-S.; Won, K.-C. CD36 Signal Transduction in Metabolic Diseases: Novel Insights and Therapeutic Targeting. Cells 2021, 10, 1833. [Google Scholar] [CrossRef]
- Su, X.; Abumrad, N.A. Cellular fatty acid uptake: A pathway under construction. Trends Endocrinol. Metab. 2009, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wu, F.; Chen, M.; Li, Y.; You, M.; Zhang, Y.; Yang, P.; Wei, L.; Ruan, X.Z.; Zhao, L.; et al. Inhibition of Fatty Acid Translocase (FAT/CD36) Palmitoylation Enhances Hepatic Fatty Acid β-Oxidation by Increasing Its Localization to Mitochondria and Interaction with Long-Chain Acyl-CoA Synthetase 1. Antioxid. Redox Signal. 2022, 36, 1081–1100. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Nabben, M.; Luiken, J.J.F.P. CD36 (SR-B2) as master regulator of cellular fatty acid homeostasis. Curr. Opin. Lipidol. 2022, 33, 103–111. [Google Scholar] [CrossRef]
- El Ouali, E.M.; Bosquet, L.; Elgharbaoui, B.; Laziri, F.; Laher, I.; Hackney, A.C.; Ibrahimi, A.; Taib, B.; El Harane, S.; Weiss, K.; et al. Association between “cluster of differentiation 36 (CD36)” and adipose tissue lipolysis during exercise training: A systematic review. Front. Physiol. 2023, 14, 1256440. [Google Scholar] [CrossRef]
- Ferreira, L.F. Mitochondrial basis for sex-differences in metabolism and exercise performance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R848–R849. [Google Scholar] [CrossRef] [PubMed]
- Bradley, N.S.; Snook, L.A.; Jain, S.S.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E183–E189. [Google Scholar] [CrossRef]
- Maunder, E.; Plews, D.J.; Wallis, G.A.; Brick, M.J.; Leigh, W.B.; Chang, W.-L.; Stewart, T.; Watkins, C.M.; Kilding, A.E. Peak fat oxidation is positively associated with vastus lateralis CD36 content, fed-state exercise fat oxidation, and endurance performance in trained males. Eur. J. Appl. Physiol. 2022, 122, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.L.; Hunter, G.R.; Gower, B.A.; Bamman, M.M.; Windham, S.T.; Moellering, D.R.; Fisher, G. Exercise Effects on Mitochondrial Function and Lipid Metabolism during Energy Balance. Med. Sci. Sports Exerc. 2020, 52, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.P.; Bezaire, V.; Heigenhauser, G.J.F.; Tandon, N.N.; Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A.; Spriet, L.L. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise: Human skeletal muscle mitochondrial fatty acid oxidation. J. Physiol. 2006, 571, 201–210. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef]
- Talanian, J.L.; Holloway, G.P.; Snook, L.A.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E180–E188. [Google Scholar] [CrossRef]
- Astorino, T.A.; Schubert, M.M.; Palumbo, E.; Stirling, D.; McMillan, D.W. Effect of two doses of interval training on maximal fat oxidation in sedentary women. Med. Sci. Sports Exerc. 2013, 45, 1878–1886. [Google Scholar] [CrossRef]
- Hames, K.C.; Vella, A.; Kemp, B.J.; Jensen, M.D. Free fatty acid uptake in humans with CD36 deficiency. Diabetes 2014, 63, 3606–3614. [Google Scholar] [CrossRef]
- Zhang, Y.; Zang, J.; Wang, B.; Li, B.; Yao, X.; Zhao, H.; Li, W. CD36 genotype associated with ischemic stroke in Chinese Han. Int. J. Clin. Exp. Med. 2015, 8, 16149–16157. [Google Scholar]
- Fernández-Ruiz, E.; Armesilla, A.L.; Sánchez-Madrid, F.; Vega, M.A. Gene Encoding the Collagen Type I and Thrombospondin Receptor CD36 Is Located on Chromosome 7q11.2. Genomics 1993, 17, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Rać, M.E.; Safranow, K.; Poncyljusz, W. Molecular basis of human CD36 gene mutations. Mol. Med. 2007, 13, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, X.; Zhu, F. CD36 gene variants and their clinical relevance: A narrative review. Ann. Blood 2021, 6. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakata, T.; Oka, T.; Ogawa, T.; Okamoto, F.; Kusaka, Y.; Sohmiya, K.; Shimamoto, K.; Itakura, K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 2001, 42, 751–759. [Google Scholar] [CrossRef]
- Tanaka, T.; Sohmiya, K.; Kawamura, K. Is CD36 deficiency an etiology of hereditary hypertrophic cardiomyopathy? J. Mol. Cell. Cardiol. 1997, 29, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, K.; Kuwasako, T.; Hirano, K.; Nozaki, S.; Yamashita, S.; Matsuzawa, Y. CD36 deficiency associated with insulin resistance. Lancet 2001, 357, 686–687. [Google Scholar] [CrossRef]
- Yamashita, S.; Hirano, K.-I.; Kuwasako, T.; Janabi, M.; Toyama, Y.; Ishigami, M.; Sakai, N. Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mol. Cell. Biochem. 2007, 299, 19–22. [Google Scholar] [CrossRef]
- Morii, T.; Ohno, Y.; Kato, N.; Hirose, H.; Kawabe, H.; Hirao, K.; Eguchi, T.; Maruyama, T.; Hayashi, M.; Saito, I.; et al. CD36 single nucleotide polymorphism is associated with variation in low-density lipoprotein-cholesterol in young Japanese men. Biomarkers 2009, 14, 207–212. [Google Scholar] [CrossRef]
- Ma, X.; Bacci, S.; Mlynarski, W.; Gottardo, L.; Soccio, T.; Menzaghi, C.; Iori, E.; Lager, R.A.; Shroff, A.R.; Gervino, E.V.; et al. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum. Mol. Genet. 2004, 13, 2197–2205. [Google Scholar] [CrossRef]
- Noel, S.E.; Lai, C.-Q.; Mattei, J.; Parnell, L.D.; Ordovas, J.M.; Tucker, K.L. Variants of the CD36 gene and metabolic syndrome in Boston Puerto Rican adults. Atherosclerosis 2010, 211, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, M.M.; Abdel-Hamed, A.R.; Abo-elmatty, D.M.; Mesbah, N.M.; Al-Sawaf, A.; Ezzat, O.; AL-Sawaf, H. A possible novel co-relation of locus 7q11 rs1761667 polymorphism with the severity of preeclampsia in Egyptian pregnant women. Meta Gene 2020, 24, 100650. [Google Scholar] [CrossRef]
- Mrizak, I.; Šerý, O.; Plesnik, J.; Arfa, A.; Fekih, M.; Bouslema, A.; Zaouali, M.; Tabka, Z.; Khan, N.A. The A allele of cluster of differentiation 36 (CD36) SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women. Br. J. Nutr. 2015, 113, 1330–1337. [Google Scholar] [CrossRef]
- Melis, M.; Carta, G.; Pintus, S.; Pintus, P.; Piras, C.A.; Murru, E.; Manca, C.; Di Marzo, V.; Banni, S.; Tomassini Barbarossa, I. Polymorphism rs1761667 in the CD36 Gene Is Associated to Changes in Fatty Acid Metabolism and Circulating Endocannabinoid Levels Distinctively in Normal Weight and Obese Subjects. Front. Physiol. 2017, 8, 1006. [Google Scholar] [CrossRef] [PubMed]
- Abbiss, C.R.; Menaspà, P.; Villerius, V.; Martin, D.T. Distribution of power output when establishing a breakaway in cycling. Int. J. Sports Physiol. Perform. 2013, 8, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, T.; Sanders, D. Demands of professional cycling races: Influence of race category and result. Eur. J. Sport Sci. 2021, 21, 666–677. [Google Scholar] [CrossRef]
- Kusnanik, N.W.; Rahayu, Y.S.; Rattray, B. Physiological Demands of Playing Field Hockey Game at Sub Elite Players. IOP Conf. Ser. Mater. Sci. Eng. 2018, 288, 012112. [Google Scholar] [CrossRef]
- Boyle, P.M.; Mahoney, C.A.; Wallace, W.F. The competitive demands of elite male field hockey. J. Sports Med. Phys. Fitness 1994, 34, 235–241. [Google Scholar] [PubMed]
- Tanisawa, K.; Wang, G.; Seto, J.; Verdouka, I.; Twycross-Lewis, R.; Karanikolou, A.; Tanaka, M.; Borjesson, M.; Di Luigi, L.; Dohi, M.; et al. Sport and exercise genomics: The FIMS 2019 consensus statement update. Br. J. Sports Med. 2020, 54, 969–975. [Google Scholar] [CrossRef]
- Houssaini, T.S.; Jaafour, S.; Ouldim, K.; Squali, F.-Z. CD36 Gene Polymorphism and Susceptibility to Nephropathies. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 9798–9804. [Google Scholar] [CrossRef]
- van Loon, L.J.C. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J. Appl. Physiol. 2004, 97, 1170–1187. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, M.V.; Madsen, K.; Simões, H.G.; Pereira, R.M.R.; Negrão, C.E.; Mendonça, R.Z.; Takayama, L.; Fukui, R.; da Silva, M.E.R. Effects of carbohydrate supplementation on competitive runners undergoing overload training followed by a session of intermittent exercise. Eur. J. Appl. Physiol. 2010, 109, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; DiMarco, N.M.; Langley, S.; American Dietetic Association; Dietitians of Canada; American College of Sports Medicine: Nutrition and Athletic Performance. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Am. Diet. Assoc. 2009, 109, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Pilegaard, H.; Ordway, G.A.; Saltin, B.; Neufer, P.D. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E806–E814. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.P.; Bonen, A.; Spriet, L.L. Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am. J. Clin. Nutr. 2009, 89, 455S–462S. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.-W.; Liao, M.-F.; Liu, L.; Xiong, X.-Y.; Zhang, Q.; Zhong, Q.; Zhou, K.; Yang, Y.-R.; Meng, Z.-Y.; Gong, C.-X.; et al. CD36 Gene Polymorphisms Are Associated with Intracerebral Hemorrhage Susceptibility in a Han Chinese Population. BioMed Res. Int. 2017, 2017, 5352071. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Anderson, M.J.; Carey, A.L.; Newman, D.G.; Bonen, A.; Kriketos, A.D.; Cooney, G.J.; Hawley, J.A. Muscle Oxidative Capacity Is a Better Predictor of Insulin Sensitivity than Lipid Status. J. Clin. Endocrinol. Metab. 2003, 88, 5444–5451. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.M.; Impey, S.G.; Currell, K.; Mitchell, N.; Shepherd, S.O.; Jeromson, S.; Hawley, J.A.; Close, G.L.; Hamilton, D.L.; Sharples, A.P. Postexercise high-fat feeding suppresses p70S6K1 activity in human skeletal muscle. Med. Sci. Sports Exerc. 2016, 48, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Li, L.; Hou, S.; Liu, P.; Kao, W.; Chiu, Y.; How, C. Expression of ABC transporter and scavenger receptor mRNAs in PBMCs in 100-km ultramarathon runners. Eur. J. Clin. Investig. 2021, 51, e13365. [Google Scholar] [CrossRef]
- Arkinstall, M.J.; Tunstall, R.J.; Cameron-Smith, D.; Hawley, J.A. Regulation of metabolic genes in human skeletal muscle by short-term exercise and diet manipulation. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E25–E31. [Google Scholar] [CrossRef]
- Fujii, R.; Hishida, A.; Suzuki, K.; Imaeda, N.; Goto, C.; Hamajima, N.; Wakai, K.; Kondo, T. Cluster of differentiation 36 gene polymorphism (rs1761667) is associated with dietary MUFA intake and hypertension in a Japanese population. Br. J. Nutr. 2019, 121, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Bajit, H.; Ait Si Mohammed, O.; Guennoun, Y.; Benaich, S.; Bouaiti, E.; Belghiti, H.; Mrabet, M.; Elfahime, E.M.; El Haloui, N.E.; Saeid, N.; et al. Single-nucleotide polymorphism rs1761667 in the CD36 gene is associated with orosensory perception of a fatty acid in obese and normal-weight Moroccan subjects. J. Nutr. Sci. 2020, 9, e24. [Google Scholar] [CrossRef] [PubMed]
- Hatmal, M.M.; Alshaer, W.; Mahmoud, I.S.; Al-Hatamleh, M.A.I.; Al-Ameer, H.J.; Abuyaman, O.; Zihlif, M.; Mohamud, R.; Darras, M.; Shhab, M.A.; et al. Investigating the association of CD36 gene polymorphisms (rs1761667 and rs1527483) with T2DM and dyslipidemia: Statistical analysis, machine learning based prediction, and meta-analysis. PLoS ONE 2021, 16, e0257857. [Google Scholar] [CrossRef] [PubMed]
- Momeni-Moghaddam, M.A.; Asadikaram, G.; Akbari, H.; Abolhassani, M.; Masoumi, M.; Nadimy, Z.; Khaksari, M. CD36 gene polymorphism rs1761667 (G > A) is associated with hypertension and coronary artery disease in an Iranian population. BMC Cardiovasc. Disord. 2019, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Yanagita, M. Targeting the fatty acid transport protein CD36, a class B scavenger receptor, in the treatment of renal disease. Kidney Int. 2016, 89, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.; Sahl, R.E.; Rømer, T.; Hansen, M.T.; Nielsen, A.B.; Lie-Olesen, M.M.; Rasmusen, H.K.; Søgaard, D.; Ingersen, A.; Rosenkilde, M.; et al. Extreme duration exercise affects old and younger men differently. Acta Physiol. 2022, 235, e13816. [Google Scholar] [CrossRef] [PubMed]
- Love-Gregory, L.; Sherva, R.; Schappe, T.; Qi, J.-S.; McCrea, J.; Klein, S.; Connelly, M.A.; Abumrad, N.A. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 2011, 20, 193–201. [Google Scholar] [CrossRef]
- Daoudi, H.; Plesník, J.; Sayed, A.; Šerý, O.; Rouabah, A.; Rouabah, L.; Khan, N.A. Oral Fat Sensing and CD36 Gene Polymorphism in Algerian Lean and Obese Teenagers. Nutrients 2015, 7, 9096–9104. [Google Scholar] [CrossRef]
| Cyclists | Field Hockey Players | All Athletes | Controls | p-Value | Controls vs. All Athletes | Cyclists vs. Field Hockey Players | |
|---|---|---|---|---|---|---|---|
| Age (year) | 23.58 ± 3.76 | 18.5 (18–20.75) | 20 (18–23) | 18.71 ± 0.76 | <0.0001 | 0.005 | <0.001 |
| Weight (kg) | 64.37 ± 4.90 | 64.25 ± 7.22 | 64.30 ± 6.23 | 72 (62–80.50) | 0.03 | 0.04 | >0.999 |
| Height (m) | 1.77 ± 0.05 | 1.76 ± 0.05 | 1.77 ± 0.05 | 1.76 ± 0.08 | 0.78 | 0.96 | 0.81 |
| BMI (kg/m2) | 20.50 ± 1.90 | 20.75 ± 2.00 | 20.64 ± 1.93 | 23.04 ± 3.43 | <0.001 | <0.001 | 0.98 |
| Variables | Cyclists | Field Hockey Players | All Athletes | Controls | |
|---|---|---|---|---|---|
| Sample size | 18 | 24 | 42 | 26 | |
| Genotype distribution, n (%) | AA | 0 (0.00%) | 10 (41.67%) | 10 (23.81%) | 5 (19.23%) |
| AG | 13 (72.22%) | 12 (50.00%) | 25 (59.52%) | 18 (69.23%) | |
| GG | 5 (27.78%) | 2 (8.33%) | 7 (16.67%) | 3 (11.54%) | |
| Allele distribution, n (%) | A | 13 (36.11%) | 32 (66.67%) | 45 (53.57%) | 28 (53.85%) |
| G | 23 (63.89%) | 16 (33.33%) | 39 (46.43%) | 24 (46.15%) | |
| HWE p-value | 0.06 | 0.83 | 0.44 | 0.13 |
| Groups | χ2 | df | p-Value |
|---|---|---|---|
| Controls vs. All athletes | 0.67 | 2 | 0.71 |
| Controls vs. Cyclists | 5.01 | 2 | 0.08 |
| Controls vs. Field hockey players | 2.99 | 2 | 0.22 |
| Cyclists vs. Field hockey players | 10.69 | 2 | 0.004 |
| Groups | χ2 | df | p-Value |
|---|---|---|---|
| Controls vs. All athletes | 0.00 | 1 | 0.97 |
| Controls vs. Cyclists | 2.69 | 1 | 0.10 |
| Controls vs. Field hockey players | 1.70 | 1 | 0.19 |
| Cyclists vs. Field hockey players | 7.72 | 1 | 0.005 |
| Groups | Dominant (AA+AG vs. GG) | p Value | Recessive (AA vs. AG+GG) | p-Value |
|---|---|---|---|---|
| Controls vs. All athletes | 0.65 [0.17–2.84] | 0.73 | 1.31 [0.38–3.93] | 0.76 |
| Controls vs. Cyclists | 0.33 [0.08–1.70] | 0.24 | 0.00 [0.00–0.95] | 0.06 |
| Controls vs. Field hockey players | 1.43 [0.26–8.62] | 0.99 | 3.00 [0.90–9.97] | 0.12 |
| Cyclists vs. Field hockey players | 0.23 [0.04–1.46] | 0.11 | 0.00 [0.00–0.35] | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Ouali, E.M.; Kartibou, J.; Del Coso, J.; El Makhzen, B.; Bouguenouch, L.; El Harane, S.; Taib, B.; Weiss, K.; Knechtle, B.; Mesfioui, A.; et al. Genotypic and Allelic Distribution of the CD36 rs1761667 Polymorphism in High-Level Moroccan Athletes: A Pilot Study. Genes 2024, 15, 419. https://doi.org/10.3390/genes15040419
El Ouali EM, Kartibou J, Del Coso J, El Makhzen B, Bouguenouch L, El Harane S, Taib B, Weiss K, Knechtle B, Mesfioui A, et al. Genotypic and Allelic Distribution of the CD36 rs1761667 Polymorphism in High-Level Moroccan Athletes: A Pilot Study. Genes. 2024; 15(4):419. https://doi.org/10.3390/genes15040419
Chicago/Turabian StyleEl Ouali, El Mokhtar, Jihan Kartibou, Juan Del Coso, Badreddine El Makhzen, Laila Bouguenouch, Sanae El Harane, Bouchra Taib, Katja Weiss, Beat Knechtle, Abdelhalem Mesfioui, and et al. 2024. "Genotypic and Allelic Distribution of the CD36 rs1761667 Polymorphism in High-Level Moroccan Athletes: A Pilot Study" Genes 15, no. 4: 419. https://doi.org/10.3390/genes15040419
APA StyleEl Ouali, E. M., Kartibou, J., Del Coso, J., El Makhzen, B., Bouguenouch, L., El Harane, S., Taib, B., Weiss, K., Knechtle, B., Mesfioui, A., & Zouhal, H. (2024). Genotypic and Allelic Distribution of the CD36 rs1761667 Polymorphism in High-Level Moroccan Athletes: A Pilot Study. Genes, 15(4), 419. https://doi.org/10.3390/genes15040419









