Comparison of Fecal MicroRNA Isolation Using Various Total RNA Isolation Kits
Abstract
1. Introduction
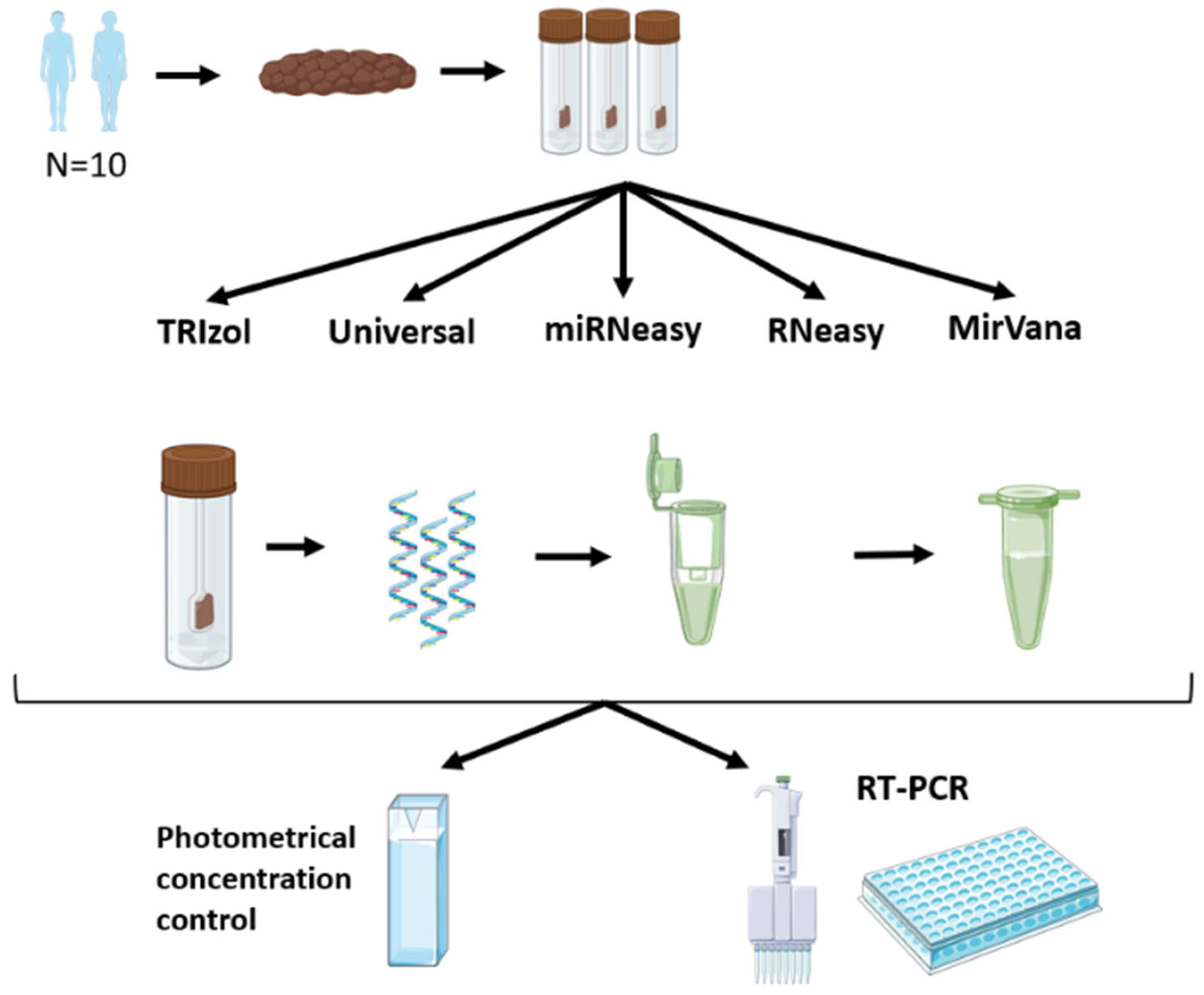
2. Materials and Methods
2.1. Stool Samples from Healthy Subjects
2.2. RNA Extraction Using the TRIzol Method
2.3. RNA Extraction Using Qiagen Kits
2.4. RNA Extraction Using mirVana Kit
2.5. MiRNA Quantification by Real-Time RT-PCR
2.6. Statistical Analysis
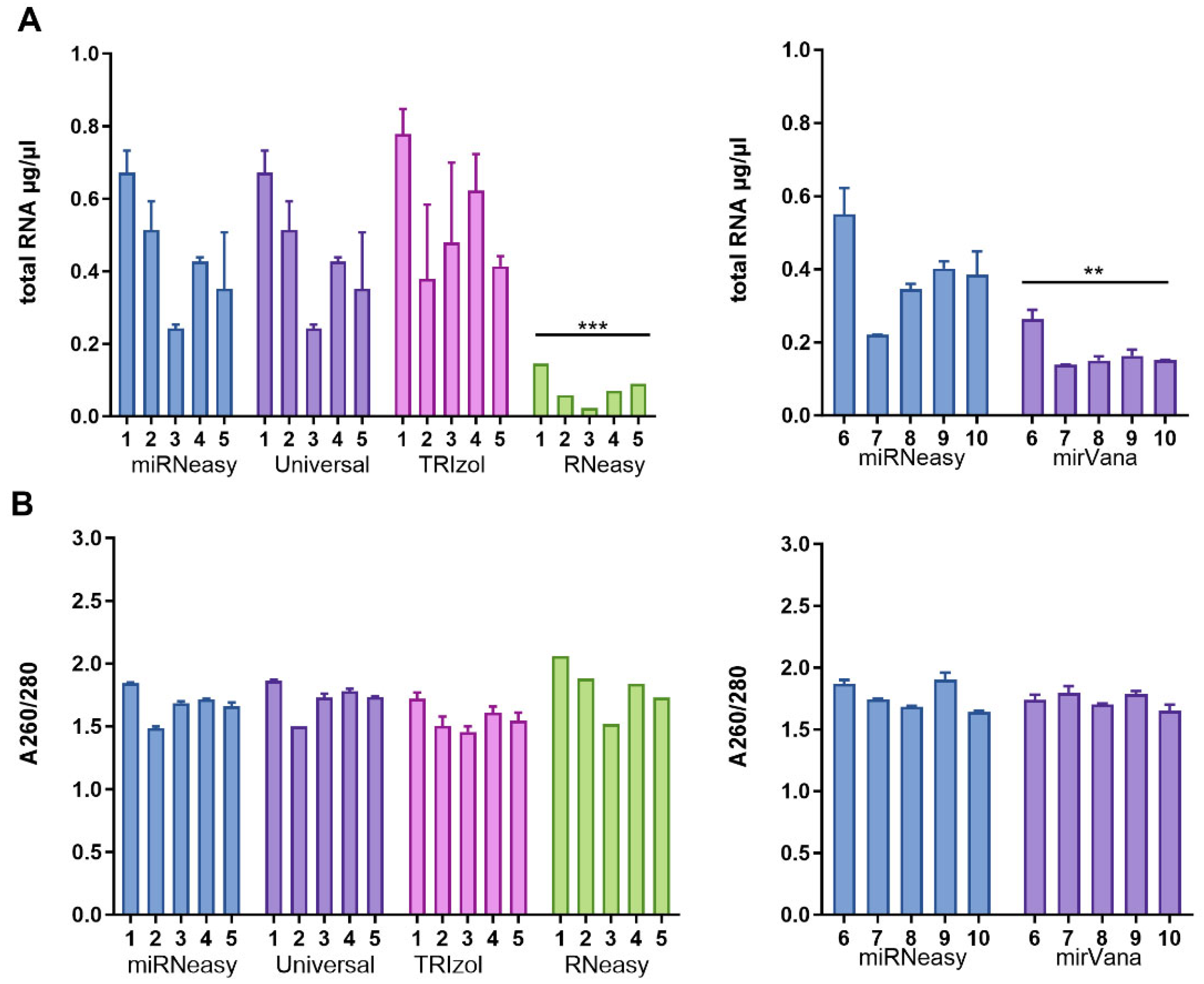
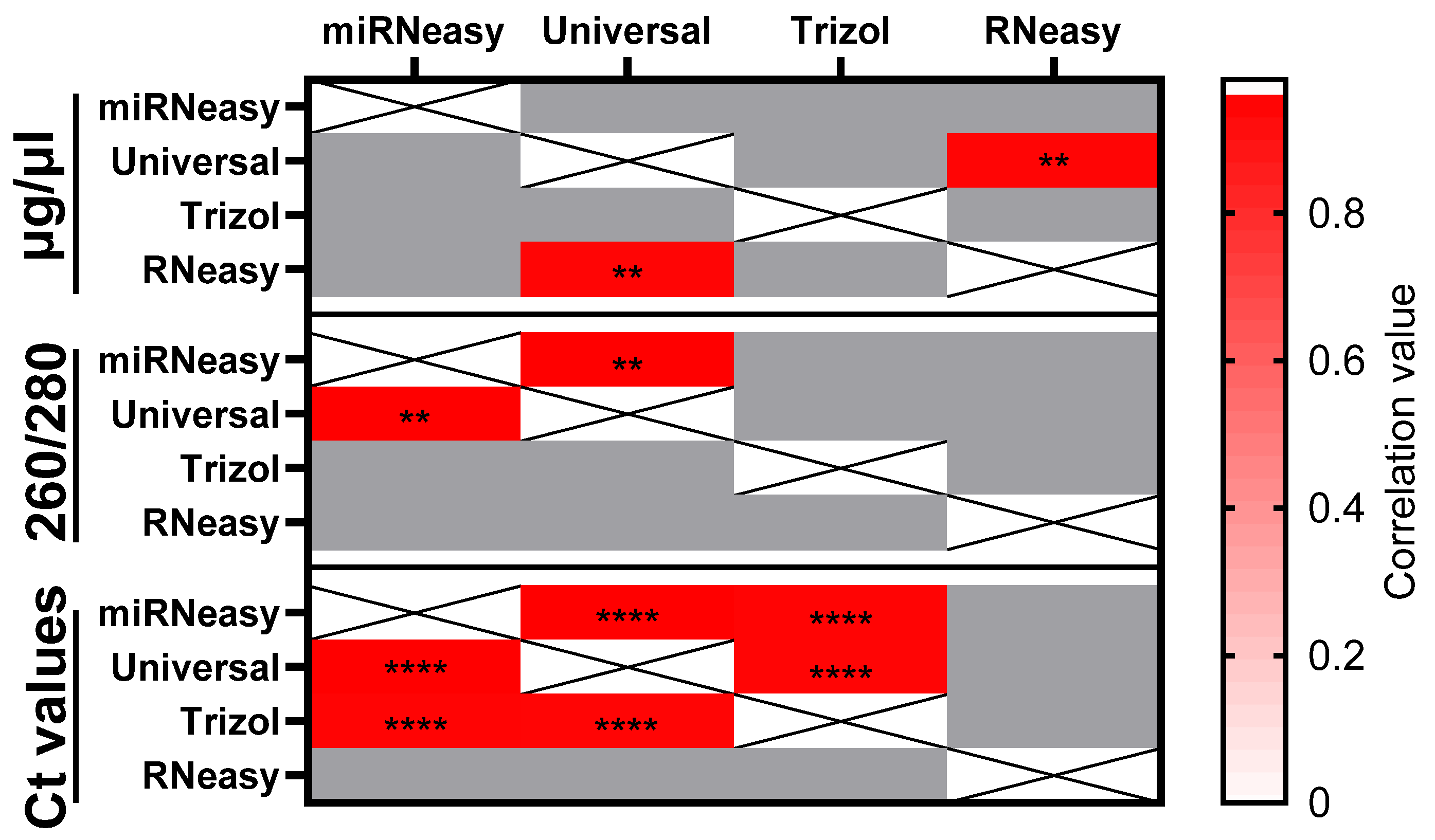
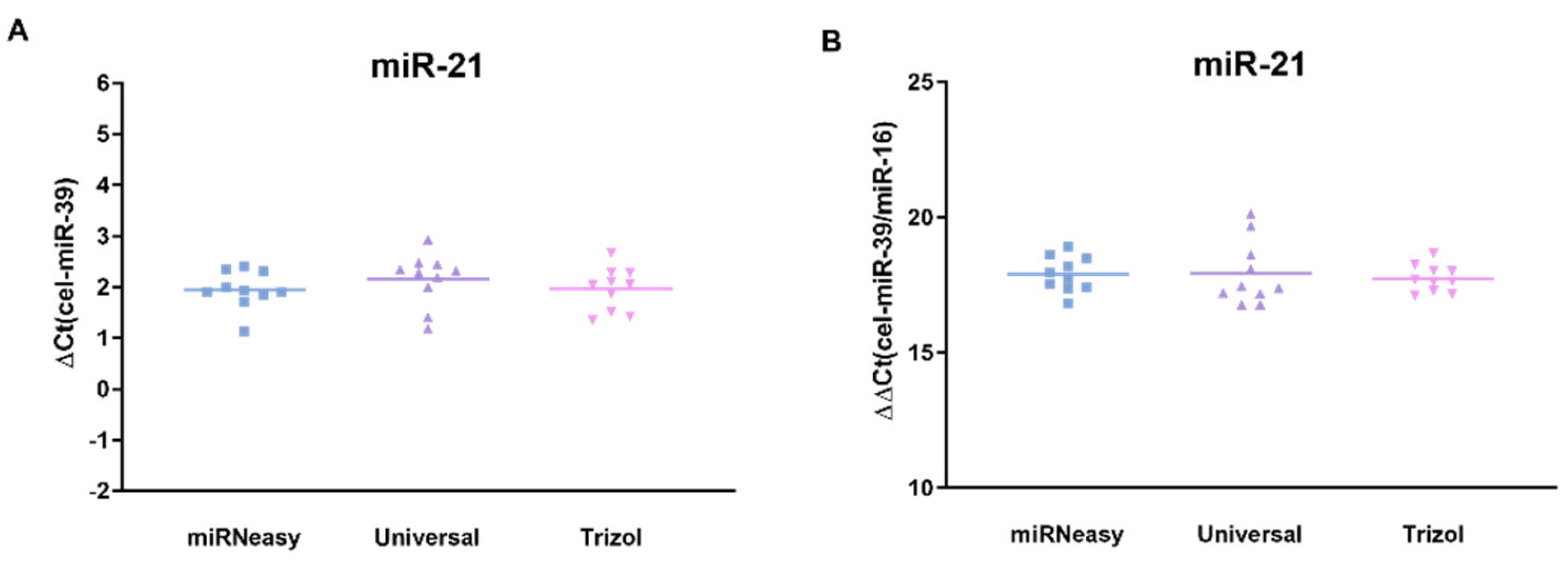
3. Results
3.1. Total RNA Purity
3.2. Comparison of miRNA Performance
3.3. Comparison of Normalized miR-21 Concentration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrow, D.A.; De Lemos, J.A. Benchmarks for the Assessment of Novel Cardiovascular Biomarkers. Circulation 2007, 115, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Saengow, U.; Chongsuwiwatvong, V.; Geater, A.; Birch, S. Preferences and Acceptance of Colorectal Cancer Screening in Thailand. Asian Pac. J. Cancer Prev. 2015, 16, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The Rising Tide of Early-Onset Colorectal Cancer: A Comprehensive Review of Epidemiology, Clinical Features, Biology, Risk Factors, Prevention, and Early Detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick-Lewis, D.; Ali, M.U.; Warren, R.; Kenny, M.; Sherifali, D.; Raina, P. Screening for Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin. Color. Cancer 2016, 15, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Schindler, P.; Kupcinskas, J.; Juzenas, S.; Skieceviciene, J.; Salteniene, V.; Schulz, C.; Weigt, J.; Malfertheiner, P.; Link, A. Expression of MicroRNAs in the Ascites of Patients with Peritoneal Carcinomatosis and Peritonitis. Cancer Cytopathol. 2018, 126, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Pauley, K.M.; Satoh, M.; Chan, A.L.; Bubb, M.R.; Reeves, W.H.; Chan, E.K. Upregulated MiR-146a Expression in Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Arthritis Res. Ther. 2008, 10, R101. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA Expression Profiles Classify Human Cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Piontek, K.B.; Kumbhari, V.; Ishida, M.; Selaru, F.M. Isolation and Profiling of MicroRNA-Containing Exosomes from Human Bile. J. Vis. Exp. 2016, 112, e54036. [Google Scholar] [CrossRef]
- Li, L.; Masica, D.; Ishida, M.; Tomuleasa, C.; Umegaki, S.; Kalloo, A.N.; Georgiades, C.; Singh, V.K.; Khashab, M.; Amateau, S.; et al. Human Bile Contains MicroRNA-Laden Extracellular Vesicles That Can Be Used for Cholangiocarcinoma Diagnosis. Hepatology 2014, 60, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Shen, Y.; Nagasaka, T.; Lozano, J.J.; Boland, C.R.; Goel, A. Fecal MicroRNAs as Novel Biomarkers for Colon Cancer Screening. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Link, J.; Thon, C.; Petkevicius, V.; Steponaitiene, R.; Malfertheiner, P.; Kupcinskas, J.; Link, A. The Translational Impact of Plant-Derived Xeno-MiRNA MiR-168 in Gastrointestinal Cancers and Preneoplastic Conditions. Diagnostics 2023, 13, 2701. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Vickers, K.; Xiong, Y.; Zhao, S.; Sheng, Q.; Zhang, P.; Zhou, W.; Flynn, C.R. Comprehensive Evaluation of Extracellular Small RNA Isolation Methods from Serum in High Throughput Sequencing. BMC Genom. 2017, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Schönauen, K.; Le, N.; von Arnim, U.; Schulz, C.; Malfertheiner, P.; Link, A. Circulating and Fecal MicroRNAs as Biomarkers for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2018, 24, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Qiu, P.; Wang, H.; Yang, H.; Yang, X.; Ye, M.; Wang, F.; Zhao, Q. Identification of MicroRNA-16-5p and MicroRNA-21-5p in Feces as Potential Noninvasive Biomarkers for Inflammatory Bowel Disease. Aging 2021, 13, 4634–4646. [Google Scholar] [CrossRef] [PubMed]
- Sorop, A.; Constantinescu, D.; Cojocaru, F.; Dinischiotu, A.; Cucu, D.; Dima, S.O. Exosomal MicroRNAs as Biomarkers and Therapeutic Targets for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 4997. [Google Scholar] [CrossRef] [PubMed]
- Chiorino, G.; Petracci, E.; Sehovic, E.; Gregnanin, I.; Camussi, E.; Mello-Grand, M.; Ostano, P.; Riggi, E.; Vergini, V.; Russo, A.; et al. Plasma MicroRNA Ratios Associated with Breast Cancer Detection in a Nested Case–Control Study from a Mammography Screening Cohort. Sci. Rep. 2023, 13, 12040. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, K.; Rong, Q. Diagnostic Value of Fecal MicroRNAs for Colorectal Cancer: A Meta-Analysis. Clin. Lab. 2015, 61, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Kupcinskas, J. MicroRNAs as Non-Invasive Diagnostic Biomarkers for Gastric Cancer: Current Insights and Future Perspectives. World J. Gastroenterol. 2018, 24, 3313–3329. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. The Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction: Twenty-Something Years On. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.P.; La Torre, J.; Kelley, D.E.; Greenberg, J.R. On the Lability of Poly(A) Sequences during Extraction of Messenger RNA from Polyribosomes. Biochim. Biophys. Acta BBA Nucleic Acids Protein Synth. 1972, 262, 220–226. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.M. A Solid-Phase Extraction Procedure for DNA Purification. Anal. Biochem. 1989, 181, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; Yiap, B.C. DNA, RNA, and Protein Extraction: The Past and The Present. J. Biomed. Biotechnol. 2009, 2009, 574398. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.; de Silva, K.; Purdie, A.C.; Plain, K.M. Comparison of Methods for MiRNA Isolation and Quantification from Ovine Plasma. Sci. Rep. 2020, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wang, C.; Lu, X.; Zhang, C.; Zhou, Z.; Chen, X.; Zhang, C.-Y.; Zen, K.; Zhang, C. Comparison of Commercial Exosome Isolation Kits for Circulating Exosomal MicroRNA Profiling. Anal. Bioanal. Chem. 2018, 410, 3805–3814. [Google Scholar] [CrossRef]
- Brunet-Vega, A.; Pericay, C.; Quílez, M.E.; Ramírez-Lázaro, M.J.; Calvet, X.; Lario, S. Variability in MicroRNA Recovery from Plasma: Comparison of Five Commercial Kits. Anal. Biochem. 2015, 488, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Urbizu, A.; Arnaldo, L.; Beyer, K. Obtaining MiRNA from Saliva—Comparison of Sampling and Purification Methods. Int. J. Mol. Sci. 2023, 24, 2386. [Google Scholar] [CrossRef] [PubMed]
- Bhati, A.; Varghese, A.; Rajan, G.; Sridhar, V.; Mohan, Y.; Pradeep, S.; Babu, S.; Kaikkolante, N.; Sarma, M.; Arun, S.; et al. An Effective Method for Saliva Stabilization and Magnetic Nanoparticles Based DNA Extraction for Genomic Applications. Anal. Biochem. 2021, 624, 114182. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA Profiling: Approaches and Considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Link, J.; Thon, C.; Schanze, D.; Steponaitiene, R.; Kupcinskas, J.; Zenker, M.; Canbay, A.; Malfertheiner, P.; Link, A. Food-Derived Xeno-MicroRNAs: Influence of Diet and Detectability in Gastrointestinal Tract—Proof-of-Principle Study. Mol. Nutr. Food Res. 2019, 63, e1800076. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lederer, T.; Hipler, N.M.; Thon, C.; Kupcinskas, J.; Link, A. Comparison of Fecal MicroRNA Isolation Using Various Total RNA Isolation Kits. Genes 2024, 15, 498. https://doi.org/10.3390/genes15040498
Lederer T, Hipler NM, Thon C, Kupcinskas J, Link A. Comparison of Fecal MicroRNA Isolation Using Various Total RNA Isolation Kits. Genes. 2024; 15(4):498. https://doi.org/10.3390/genes15040498
Chicago/Turabian StyleLederer, Theresa, Noam M. Hipler, Cosima Thon, Juozas Kupcinskas, and Alexander Link. 2024. "Comparison of Fecal MicroRNA Isolation Using Various Total RNA Isolation Kits" Genes 15, no. 4: 498. https://doi.org/10.3390/genes15040498
APA StyleLederer, T., Hipler, N. M., Thon, C., Kupcinskas, J., & Link, A. (2024). Comparison of Fecal MicroRNA Isolation Using Various Total RNA Isolation Kits. Genes, 15(4), 498. https://doi.org/10.3390/genes15040498





