Resistome, Virulome, and Clonal Variation in Methicillin-Resistant Staphylococcus aureus (MRSA) in Healthy Swine Populations: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
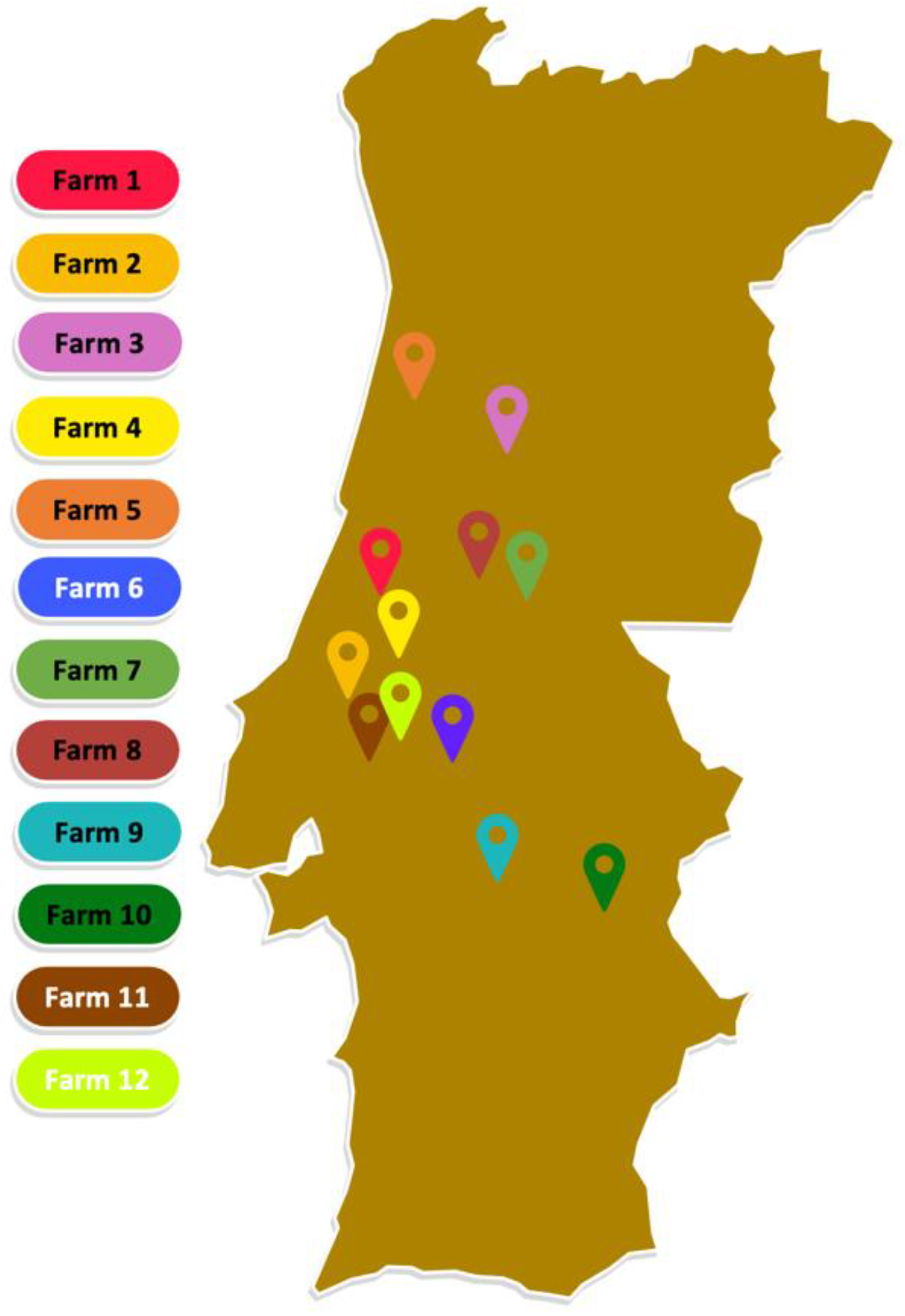
2.1. Sample Collection
2.2. MRSA Isolation
2.3. Antimicrobial Susceptibility Testing
2.4. Pulsed-Field Gel Electrophoresis (PFGE)
2.5. Whole Genome Sequencing (WGS)
3. Results
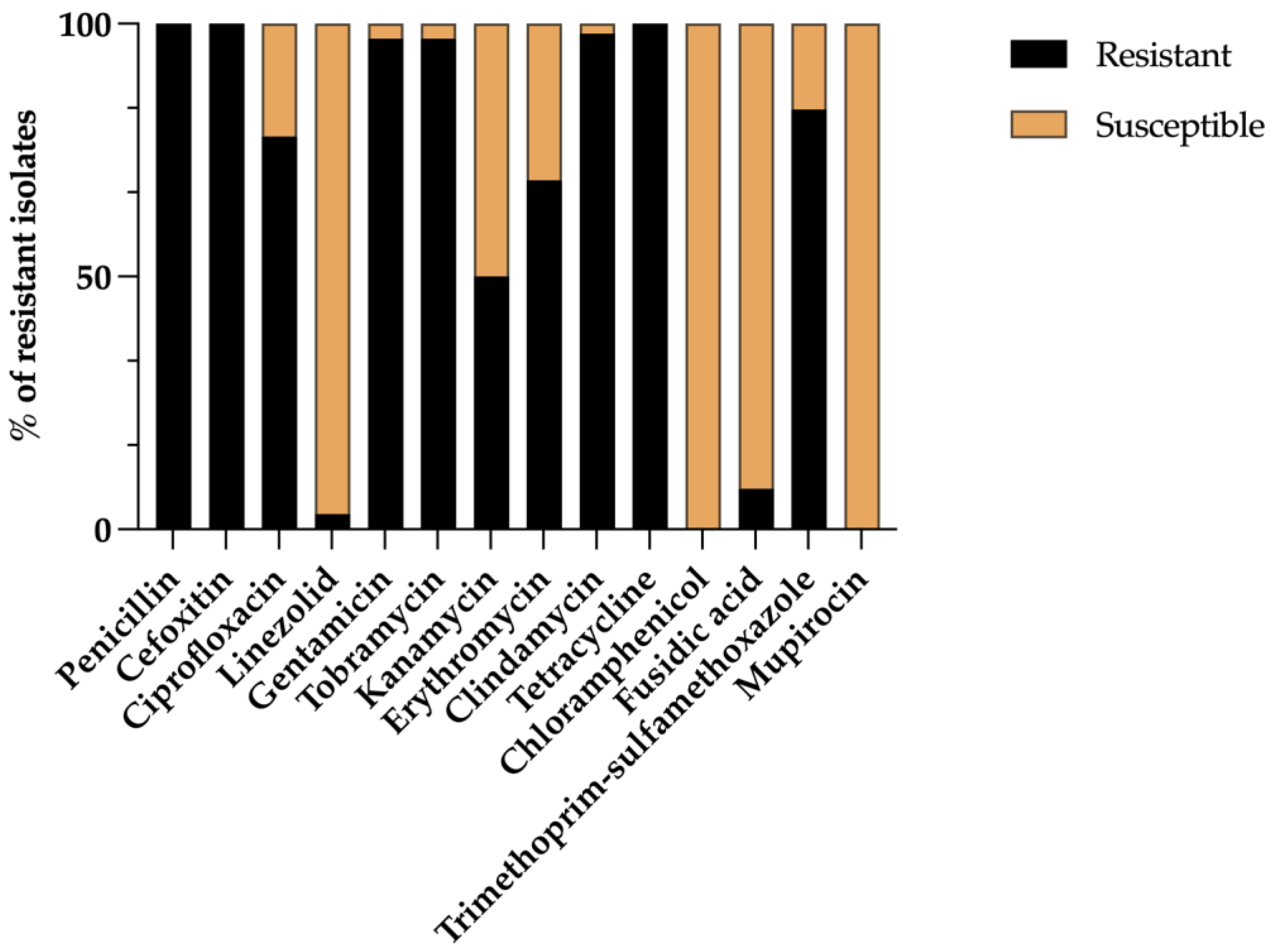
3.1. Prevalence and Phenotypic Antimicrobial Resistance
3.2. Whole Genome Sequencing
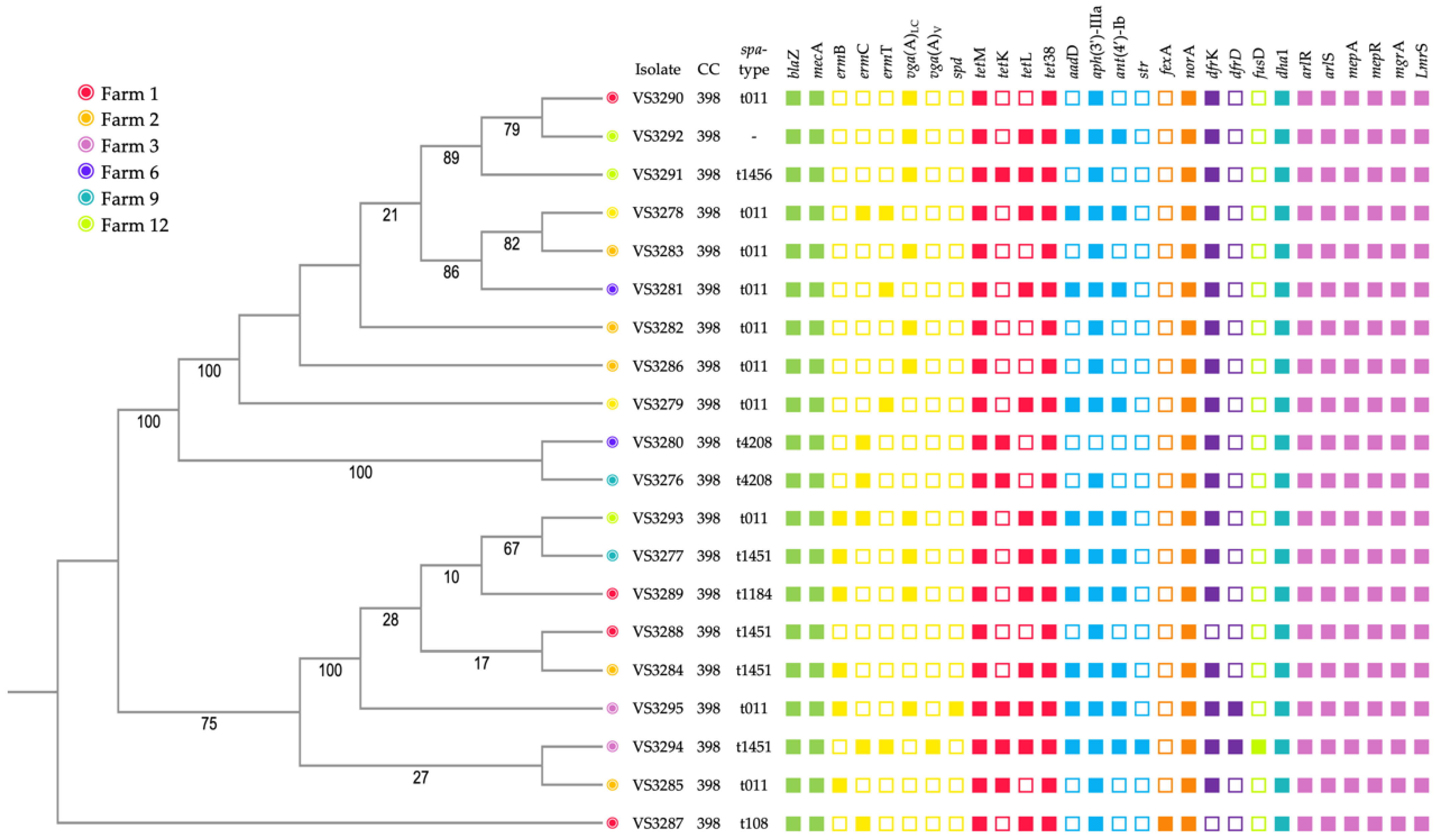
3.3. Antimicrobial Resistance and Virulence Genes
3.4. Molecular Typing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crespo-Piazuelo, D.; Lawlor, P.G. Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) prevalence in humans in close contact with animals and measures to reduce on-farm colonization. Ir. Vet. J. 2021, 74, 21. [Google Scholar] [CrossRef] [PubMed]
- Cuny, C.; Wieler, L.; Witte, W. Livestock-Associated MRSA: The Impact on Humans. Antibiotics 2015, 4, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Pantosti, A. Methicillin-resistant Staphylococcus aureus associated with animals and its relevance to human health. Front. Microbiol. 2012, 3, 127. [Google Scholar] [CrossRef] [PubMed]
- Kayano, T.; Pulford, J.; Thomas, L.F. Identifying Pig- and Pork-Associated Zoonotic and Foodborne Hazards in Eastern and Southern Africa: A Systematized Review. Zoonotic Dis. 2023, 3, 120–133. [Google Scholar] [CrossRef]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host adaptation and emergence of methicillin resistance in livestock. MBio 2012, 3, e00305-11. [Google Scholar] [CrossRef] [PubMed]
- van Wamel, W.J.B.; Rooijakkers, S.H.M.; Ruyken, M.; van Kessel, K.P.M.; van Strijp, J.A.G. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on β-hemolysin-converting bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. World Agriculture: Towards 2015/2030; Food and Agriculture Organization: Rome, Italy, 2022. [Google Scholar]
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2021–2030; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- Bidaisee, S.; Macpherson, C.N.L. Zoonoses and one health: A review of the literature. J. Parasitol. Res. 2014, 2014, 874345. [Google Scholar] [CrossRef] [PubMed]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Bellini, S. 7. The pig sector in the European Union. In Understanding and Combatting African Swine Fever; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; pp. 183–195. ISBN 978-90-8686-357-0. [Google Scholar]
- Rauw, W.M.; Rydhmer, L.; Kyriazakis, I.; Øverland, M.; Gilbert, H.; Dekkers, J.C.M.; Hermesch, S.; Bouquet, A.; Gómez Izquierdo, E.; Louveau, I.; et al. Prospects for sustainability of pig production in relation to climate change and novel feed resources. J. Sci. Food Agric. 2020, 100, 3575–3586. [Google Scholar] [CrossRef] [PubMed]
- Pirolo, M.; Visaggio, D.; Gioffrè, A.; Artuso, I.; Gherardi, M.; Pavia, G.; Samele, P.; Ciambrone, L.; Di Natale, R.; Spatari, G. Unidirectional animal-to-human transmission of methicillin-resistant Staphylococcus aureus ST398 in pig farming; evidence from a surveillance study in southern Italy. Antimicrob. Resist. Infect. Control 2019, 8, 187. [Google Scholar] [CrossRef]
- Golob, M.; Pate, M.; Kušar, D.; Zajc, U.; Papić, B.; Ocepek, M.; Zdovc, I.; Avberšek, J. Antimicrobial Resistance and Molecular Characterization of Methicillin-Resistant Staphylococcus aureus from Two Pig Farms: Longitudinal Study of LA-MRSA. Antibiotics 2022, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Moodley, A.; Nielsen, S.S.; Guardabassi, L. Effects of tetracycline and zinc on selection of methicillin-resistant Staphylococcus aureus (MRSA) sequence type 398 in pigs. Vet. Microbiol. 2011, 152, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Mesa Varona, O.; Chaintarli, K.; Muller-Pebody, B.; Anjum, M.F.; Eckmanns, T.; Norström, M.; Boone, I.; Tenhagen, B.-A. Monitoring antimicrobial resistance and drug usage in the human and livestock sector and foodborne antimicrobial resistance in six European countries. Infect. Drug Resist. 2020, 13, 957–993. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar]
- Conceição, T.; De Lencastre, H.; Aires-De-Sousa, M. Frequent isolation of methicillin resistant Staphylococcus aureus (MRSA) ST398 among healthy pigs in Portugal. PLoS ONE 2017, 12, e0175340. [Google Scholar] [CrossRef] [PubMed]
- Leão, C.; Clemente, L.; Cara d’Anjo, M.; Albuquerque, T.; Amaro, A. Emergence of Cfr-Mediated Linezolid Resistance among Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) from Healthy Pigs in Portugal. Antibiotics 2022, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Yamanaka, M.; Nara, K.; Tanaka, S.; Uema, M.; Asai, T.; Tamura, Y. Isolation of ST398 methicillin-resistant Staphylococcus aureus from pigs at abattoirs in Tohoku region, Japan. J. Vet. Med. Sci. 2020, 82, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Silva, N.; Manageiro, V.; Ramos, S.; Coelho, A.; Gonçalves, D.; Caniça, M.; Torres, C.; Igrejas, G.; Poeta, P. First report on MRSA CC398 recovered from wild boars in the north of Portugal. Are we facing a problem? Sci. Total Environ. 2017, 596–597, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Porrero, M.C.; Mentaberre, G.; Sánchez, S.; Fernández-Llario, P.; Casas-Díaz, E.; Mateos, A.; Vidal, D.; Lavín, S.; Fernández-Garayzábal, J.-F.; Domínguez, L. Carriage of Staphylococcus aureus by Free-Living Wild Animals in Spain. Appl. Environ. Microbiol. 2014, 80, 4865–4870. [Google Scholar] [CrossRef] [PubMed]
- Quero, S.; Serras-Pujol, M.; Párraga-Niño, N.; Torres, C.; Navarro, M.; Vilamala, A.; Puigoriol, E.; de los Ríos, J.D.; Arqué, E.; Serra-Pladevall, J.; et al. Methicillin-resistant and methicillin-sensitive Staphylococcus aureus in pork industry workers, Catalonia, Spain. One Health 2023, 16, 100538. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.; Gomes, A.; Ruiz-Ripa, L.; Mama, O.M.; Sabença, C.; Sousa, M.; Silva, V.; Sousa, T.; Vieira-Pinto, M.; Igrejas, G.; et al. Methicillin-Resistant Staphylococcus aureus CC398 in Purulent Lesions of Piglets and Fattening Pigs in Portugal. Microb. Drug Resist. 2020, 26, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Kinross, P.; Petersen, A.; Skov, R.; Van Hauwermeiren, E.; Pantosti, A.; Laurent, F.; Voss, A.; Kluytmans, J.; Struelens, M.J.; Heuer, O. Livestock-associated meticillin-resistant Staphylococcus aureus (MRSA) among human MRSA isolates, European Union/European Economic Area countries, 2013. Eurosurveillance 2017, 22, 16–696. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Araújo, S.; Monteiro, A.; Eira, J.; Pereira, J.E.; Maltez, L.; Igrejas, G.; Lemsaddek, T.S.; Poeta, P. Staphylococcus aureus and MRSA in Livestock: Antimicrobial Resistance and Genetic Lineages. Microorganisms 2023, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Wu, J.; Chen, S.; Jin, Y.; Long, J.; Duan, G.; Yang, H. Transmission of livestock-associated methicillin-resistant Staphylococcus aureus between animals, environment, and humans in the farm. Environ. Sci. Pollut. Res. 2023, 30, 86521–86539. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Sipos, R.; Perreten, V.; Tóth, Á.; Ungvári, E.; Papp, M.; Dán, Á.; Biksi, I. High Prevalence of Livestock-Associated Methicillin-Resistant Staphylococcus aureus in Hungarian Pig Farms and Genomic Evidence for the Spillover of the Pathogen to Humans. Transbound. Emerg. Dis. 2023, 2023, 5540019. [Google Scholar] [CrossRef]
- van der Mee-Marquet, N.; Corvaglia, A.-R.; Valentin, A.-S.; Hernandez, D.; Bertrand, X.; Girard, M.; Kluytmans, J.; Donnio, P.-Y.; Quentin, R.; François, P. Analysis of prophages harbored by the human-adapted subpopulation of Staphylococcus aureus CC398. Infect. Genet. Evol. 2013, 18, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Cuny, C.; Abdelbary, M.; Layer, F.; Werner, G.; Witte, W. Prevalence of the immune evasion gene cluster in Staphylococcus aureus CC398. Vet. Microbiol. 2015, 177, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Stegger, M.; Liu, C.M.; Larsen, J.; Soldanova, K.; Aziz, M.; Contente-Cuomo, T.; Petersen, A.; Vandendriessche, S.; Jiménez, J.N.; Mammina, C.; et al. Rapid Differentiation between Livestock-Associated and Livestock-Independent Staphylococcus aureus CC398 Clades. PLoS ONE 2013, 8, e79645. [Google Scholar] [CrossRef] [PubMed]
- Porrero, M.C.; Mentaberre, G.; Sánchez, S.; Fernández-Llario, P.; Gómez-Barrero, S.; Navarro-Gonzalez, N.; Serrano, E.; Casas-Díaz, E.; Marco, I.; Fernández-Garayzabal, J.F.; et al. Methicillin resistant Staphylococcus aureus (MRSA) carriage in different free-living wild animal species in Spain. Vet. J. 2013, 198, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Pantosti, A.; Sanchini, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Futur. Microbiol. 2007, 2, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Truong-Bolduc, Q.C.; Bolduc, G.R.; Medeiros, H.; Vyas, J.M.; Wang, Y.; Hooper, D.C. Role of the Tet38 efflux pump in Staphylococcus aureus internalization and survival in epithelial cells. Infect. Immun. 2015, 83, 4362–4372. [Google Scholar] [CrossRef] [PubMed]
- Truong-Bolduc, Q.C.; Dunman, P.M.; Strahilevitz, J.; Projan, S.J.; Hooper, D.C. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J. Bacteriol. 2005, 187, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Surveillance of Veterinary Antimicrobial Consumption, 2021. ‘Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020’ EMA/58183/2021; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Abreu, R.; Rodríguez-Álvarez, C.; Lecuona, M.; Castro, B.; González, J.C.; Aguirre-Jaime, A.; Arias, Á. Increased antimicrobial resistance of MRSA strains isolated from pigs in Spain between 2009 and 2018. Vet. Sci. 2019, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cai, C.; Chen, J.; Cheng, C.; Cheng, G.; Hu, X.; Liu, C. Inducible Expression of both ermB and ermT Conferred High Macrolide Resistance in Streptococcus gallolyticus subsp. pasteurianus Isolates in China. Int. J. Mol. Sci. 2016, 17, 1599. [Google Scholar] [CrossRef] [PubMed]
- Tkadlec, J.; Vařeková, E.; Pantůček, R.; Doškař, J.; Růžičková, V.; Botka, T.; Fila, L.; Melter, O. Characterization of Staphylococcus aureus strains isolated from Czech cystic fibrosis patients: High rate of ribosomal mutation conferring resistance to MLSB antibiotics as a result of long-term and low-dose azithromycin treatment. Microb. Drug Resist. 2015, 21, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, I.N.; Lozano, C.; Zarazaga, M.; Simón, C.; Höfle, U.; Sieber, R.N.; Latorre-Fernández, J.; Stegger, M.; Torres, C. Comparative genomics of Staphylococcus aureus strains from wild birds and pig farms elucidates levels of mobilomes, antibiotic pressure and host adaptation. J. Glob. Antimicrob. Resist. 2024, 36, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Mama, O.M.; Morales, L.; Ruiz-Ripa, L.; Zarazaga, M.; Torres, C. High prevalence of multidrug resistant S. aureus-CC398 and frequent detection of enterotoxin genes among non-CC398 S. aureus from pig-derived food in Spain. Int. J. Food Microbiol. 2020, 320, 108510. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, I.N.; Lozano, C.; Simon, C.; Latorre-Fernandez, J.; Zarazaga, M.; Torres, C. Nasal staphylococci community of healthy pigs and pig-farmers in Aragon (Spain). Predominance and within-host resistome diversity in MRSA-CC398 and MSSA-CC9 lineages. One Health 2023, 16, 100505. [Google Scholar] [CrossRef] [PubMed]
- Feßler, A.; Kadlec, K.; Wang, Y.; Zhang, W.-J.; Wu, C.; Shen, J.; Schwarz, S. Small antimicrobial resistance plasmids in livestock-associated methicillin-resistant Staphylococcus aureus CC398. Front. Microbiol. 2018, 9, 2063. [Google Scholar] [CrossRef] [PubMed]
- Feßler, A.T.; Wang, Y.; Wu, C.; Schwarz, S. Mobile lincosamide resistance genes in staphylococci. Plasmid 2018, 99, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Wang, H.; Liao, Y.; Li, J.; Feßler, A.T.; Michael, G.B.; Schwarz, S.; Wang, Y. Detection and genetic environment of pleuromutilin-lincosamide-streptogramin A resistance genes in staphylococci isolated from pets. Front. Microbiol. 2017, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Henrike, K.-H.; Xing, J.; Dennis, H.; Stefan, F.; Feßler, A.T.; Nansong, J.; Heike, K.; Yang, W.; Congming, W.; Stefan, S. Genomic Diversity of Methicillin-Resistant Staphylococcus aureus CC398 Isolates Collected from Diseased Swine in the German National Resistance Monitoring Program GERM-Vet from 2007 to 2019. Microbiol. Spectr. 2023, 11, e00770-23. [Google Scholar]
- Soliman, E.S.; Moawed, S.A.; Ziaan, A.M.G. Assessing cleaning and disinfection regime in a slaughterhouse against carcasses contamination. Adv. Anim. Vet. Sci 2016, 4, 449–457. [Google Scholar] [CrossRef]
- Asanin, J.; Misic, D.; Aksentijevic, K.; Tambur, Z.; Rakonjac, B.; Kovacevic, I.; Spergser, J.; Loncaric, I. Genetic Profiling and Comparison of Human and Animal Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Serbia. Antibiotics 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Vieira-Pinto, M.; Saraiva, C.; Manageiro, V.; Reis, L.; Ferreira, E.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Prevalence and Characteristics of Multidrug-Resistant Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) CC398 Isolated from Quails (Coturnix Coturnix Japonica) Slaughtered for Human Consumption. Animals 2021, 11, 2038. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Tenhagen, B.A.; Fetsch, A.; Sachsenröder, J.; Käsbohrer, A.; Schroeter, A.; Hammer, J.A.; Hertwig, S.; Helmuth, R.; Bräunig, J.; et al. Virulence and Resistance Determinants of German Staphylococcus aureus ST398 Isolates from Nonhuman Sources. Appl. Environ. Microbiol. 2011, 77, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Weidenmaier, C.; Lee, J.C. Structure and function of surface polysaccharides of Staphylococcus aureus. In Staphylococcus aureus Microbiology, Pathology, Immunology, Therapy and Prophylaxis; Springer International Publishing: Cham, Switzerland, 2017; Volume 409, pp. 57–93. [Google Scholar]
- Keinhoerster, D.; George, S.E.; Weidenmaier, C.; Wolz, C. Function and regulation of Staphylococcus aureus wall teichoic acids and capsular polysaccharides. Int. J. Med. Microbiol. 2019, 309, 151333. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.; Lee, J.C. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Löffler, B.; Buzzola, F.R.; Sordelli, D.O. Staphylococcus aureus adaptation to the host and persistence: Role of loss of capsular polysaccharide expression. Future Microbiol. 2010, 5, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ko, Y.-P.; Liang, X.; Ross, C.L.; Liu, Q.; Murray, B.E.; Höök, M. Collagen-binding microbial surface components recognizing adhesive matrix molecule (MSCRAMM) of Gram-positive bacteria inhibit complement activation via the classical pathway. J. Biol. Chem. 2013, 288, 20520–20531. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. The MSCRAMM Family of Cell-Wall-Anchored Surface Proteins of Gram-Positive Cocci. Trends Microbiol. 2019, 27, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Capelo, J.L.; Igrejas, G.; Poeta, P. Molecular Mechanisms of Antimicrobial Resistance in Staphylococcus aureus Biofilms BT—Emerging Modalities in Mitigation of Antimicrobial Resistance; Akhtar, N., Singh, K.S., Prerna, Goyal, D., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 291–314. ISBN 978-3-030-84126-3. [Google Scholar]
- Miao, J.; Lin, S.; Soteyome, T.; Peters, B.M.; Li, Y.; Chen, H.; Su, J.; Li, L.; Li, B.; Xu, Z.; et al. Biofilm Formation of Staphylococcus aureus under Food Heat Processing Conditions: First Report on CML Production within Biofilm. Sci. Rep. 2019, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.; Palmer, T. The type VII secretion system of Staphylococcus. Annu. Rev. Microbiol. 2021, 75, 471–494. [Google Scholar] [CrossRef] [PubMed]
- Zapotoczna, M.; Heilbronner, S.; Speziale, P.; Foster, T.J. Iron-regulated surface determinant (Isd) proteins of Staphylococcus lugdunensis. J. Bacteriol. 2012, 194, 6453–6467. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, G.; Pellegrini, A.; Alfeo, M.J.; Marchese, L.; Foster, T.J.; Speziale, P. The iron-regulated surface determinant B (IsdB) protein from Staphylococcus aureus acts as a receptor for the host protein vitronectin. J. Biol. Chem. 2020, 295, 10008–10022. [Google Scholar] [CrossRef] [PubMed]
- Pineda, A.P.A.; Cueva, C.L.R.; Chacón, R.D.; Ramírez, M.; De Almeida, O.G.G.; De Oliveira, D.P.; Franco, B.D.G.M.; Lacorte, G.; Landgraf, M.; Silva, N.C.C. Genomic characterization of Staphylococcus aureus from Canastra Minas Artisanal Cheeses. Braz. J. Microbiol. 2023, 54, 2103–2116. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Silva, A.; Barbero, R.; Romero, M.; del Campo, R.; Caniça, M.; Cordeiro, R.; Igrejas, G.; Poeta, P. Resistome, Virulome, and Clonal Variation in Methicillin-Resistant Staphylococcus aureus (MRSA) in Healthy Swine Populations: A Cross-Sectional Study. Genes 2024, 15, 532. https://doi.org/10.3390/genes15050532
Silva V, Silva A, Barbero R, Romero M, del Campo R, Caniça M, Cordeiro R, Igrejas G, Poeta P. Resistome, Virulome, and Clonal Variation in Methicillin-Resistant Staphylococcus aureus (MRSA) in Healthy Swine Populations: A Cross-Sectional Study. Genes. 2024; 15(5):532. https://doi.org/10.3390/genes15050532
Chicago/Turabian StyleSilva, Vanessa, Adriana Silva, Raquel Barbero, Mario Romero, Rosa del Campo, Manuela Caniça, Rui Cordeiro, Gilberto Igrejas, and Patricia Poeta. 2024. "Resistome, Virulome, and Clonal Variation in Methicillin-Resistant Staphylococcus aureus (MRSA) in Healthy Swine Populations: A Cross-Sectional Study" Genes 15, no. 5: 532. https://doi.org/10.3390/genes15050532
APA StyleSilva, V., Silva, A., Barbero, R., Romero, M., del Campo, R., Caniça, M., Cordeiro, R., Igrejas, G., & Poeta, P. (2024). Resistome, Virulome, and Clonal Variation in Methicillin-Resistant Staphylococcus aureus (MRSA) in Healthy Swine Populations: A Cross-Sectional Study. Genes, 15(5), 532. https://doi.org/10.3390/genes15050532








