Genetic Variants Underlying Plasticity in Natural Populations of Spadefoot Toads: Environmental Assessment versus Phenotypic Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study System
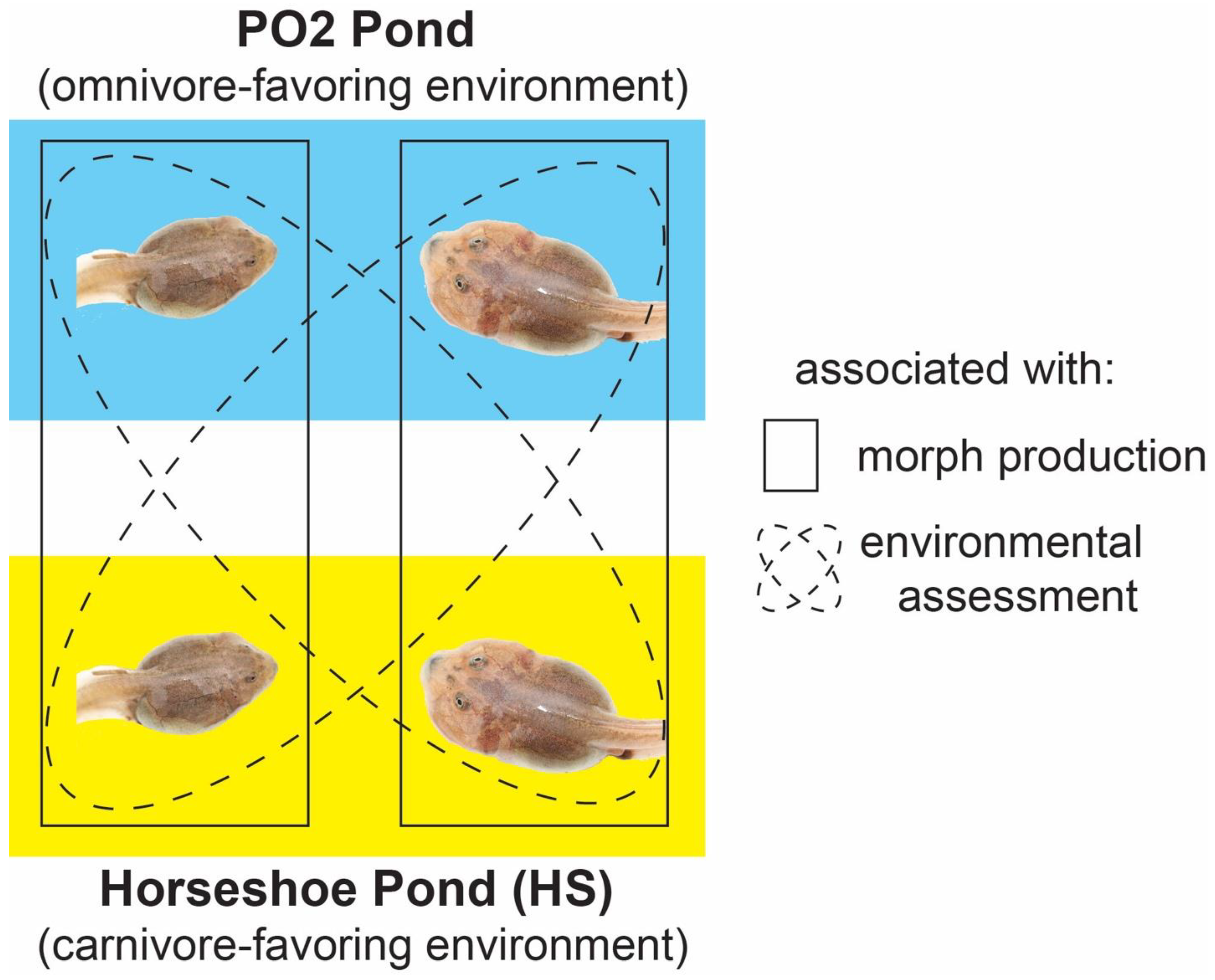
2.2. Natural Experiment
2.3. Sample Collection
2.4. Hi-C Library Preparation, Pseudogenome Assembly, and Annotation Transfer
2.5. DNA Extraction and ddRAD-seq Library Construction
2.6. Quality Control and SNP Detection
2.7. Genome-Wide Association Scans
2.8. Statistical Analysis of Putative QTLs
2.9. Analysis of Nearby Genes
3. Results
3.1. Improved Genome Assembly Properties
3.2. SNP Detection
3.3. Genome-Wide Association Scans
3.4. Nearby Gene Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nijhout, H.F. Development and evolution of adaptive polyphenisms. Evol. Dev. 2003, 5, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, D.W. (Ed.) Phenotypic Plasticity and Evolution: Causes, Consequences, Controversies; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Sultan, S.E. Phenotypic plasticity as an intrinsic property of organisms. In Phenotypic Plasticity and Evolution: Causes, Consequences, Controversies; Pfennig, D.W., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 3–24. [Google Scholar]
- Turcotte, M.M.; Levine, J.M. Phenotypic plasticity and species coexistence. Trends Ecol. Evol. 2016, 31, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. Phenotypic plasticity in the interactions and evolution of species. Science 2001, 294, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.P. Key questions on the role of phenotypic plasticity in eco-evolutionary dynamics. J. Hered. 2016, 107, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.; Levine, J.M.; Turcotte, M.M.; Hart, S.P. Phenotypic plasticity promotes species coexistence. Nat. Ecol. Evol. 2022, 6, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Foxx, A.J. Induced plasticity alters responses to conspecific interactions in seedlings of a perennial grass. Sci. Rep. 2021, 11, 14581. [Google Scholar] [CrossRef]
- Moczek, A.P.; Sultan, S.; Foster, S.; Ledon-Rettig, C.; Dworkin, I.; Nijhout, H.F.; Abouheif, E.; Pfennig, D.W. The role of developmental plasticity in evolutionary innovation. Proc. Biol. Sci. 2011, 278, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Levis, N.A.; Pfennig, D.W. Innovation and diversification via plasticity-led evolution. In Phenotypic Plasticity and Evolution: Causes, Consequences, Controversies; Pfennig, D.W., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 211–240. [Google Scholar]
- Moczek, A.P. When the end modifies its means: The origins of novelty and the evolution of innovation. Biol. J. Linn. Soc. 2023, 139, 433–440. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 1989, 20, 249–278. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Wund, M.A.; Baker, J.A.; Clancy, B.; Golub, J.L.; Foster, S.A. A test of the “flexible stem” model of evolution: Ancestral plasticity, genetic accommodation, and morphological divergence in the threespine stickleback radiation. Am. Nat. 2008, 172, 449–462. [Google Scholar] [CrossRef]
- Pfennig, D.W.; Wund, M.A.; Snell-Rood, E.C.; Cruickshank, T.; Schlichting, C.D.; Moczek, A.P. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol. Evol. 2010, 25, 459–467. [Google Scholar] [CrossRef]
- Schneider, R.F.; Meyer, A. How plasticity, genetic assimilation and cryptic genetic variation may contribute to adaptive radiations. Mol. Ecol. 2017, 26, 330–350. [Google Scholar] [CrossRef] [PubMed]
- De Lisle, S.P.; Mäenpää, M.I.; Svensson, E.I. Phenotypic plasticity is aligned with phenological adaptation on both micro-and macroevolutionary timescales. Ecol. Lett. 2022, 25, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Davison, D.R.; Michod, R.E. Phenotypic plasticity and evolutionary transitions in individuality. In Phenotypic Plasticity and Evolution: Causes, Consequences, Controversies; Pfennig, D.W., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 241–266. [Google Scholar]
- Levis, N.A.; Pfennig, D.W. Evaluating ‘plasticity-first’ evolution in nature: Key criteria and empirical approaches. Trends Ecol. Evol. 2016, 31, 563–574. [Google Scholar] [CrossRef]
- Lafuente, E.; Beldade, P. Genomics of developmental plasticity in animals. Front. Genet. 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.J. Phenotypic plasticity: From theory and genetics to current and future challenges. Genetics 2020, 215, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Ehrenreich, I.M. Genetic variation in phenotypic plasticity. In Phenotypic Plasticity and Evolution: Causes, Consequences, Controversies; Pfennig, D.W., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 91–111. [Google Scholar]
- Lafuente, E.; Duneau, D.; Beldade, P. Genetic basis of thermal plasticity variation in Drosophila melanogaster body size. PLoS Genet. 2018, 14, e1007686. [Google Scholar] [CrossRef] [PubMed]
- Ørsted, M.; Rohde, P.D.; Hoffmann, A.A.; Sørensen, P.; Kristensen, T.N. Environmental variation partitioned into separate heritable components. Evolution 2018, 72, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.M.; Lyman, R.F. The genetics of phenotypic plasticity. II. Response to selection. J. Evol. Biol. 1991, 4, 23–50. [Google Scholar] [CrossRef]
- Maulana, M.I.; Riksen, J.A.G.; Snoek, B.L.; Kammenga, J.E.; Sterken, M.G. The genetic architecture underlying body-size traits plasticity over different temperatures and developmental stages in Caenorhabditis elegans. Heredity 2022, 128, 313–324. [Google Scholar] [CrossRef]
- Windig, J.J.; De Kovel, C.G.F.; De Jong, G. Genetics and mechanics of plasticity. In Phenotypic Plasticity; DeWitt, T.J., Scheiner, S.M., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 31–49. [Google Scholar]
- Diouf, I.; Derivot, L.; Koussevitzky, S.; Carretero, Y.; Bitton, F.; Moreau, L.; Causse, M. Genetic basis of phenotypic plasticity and genotype x environment interactions in a multi-parental tomato population. J. Exp. Bot. 2020, 71, 5365–5376. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Organisms and Environment: Ecological Development, Niche Construction, and Adaptation; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Levis, N.A.; Reed, E.M.X.; Pfennig, D.W.; Buford Reiskind, M.O. Identification of candidate loci for adaptive phenotypic plasticity in natural populations of spadefoot toads. Ecol. Evol. 2020, 10, 8976–8988. [Google Scholar] [CrossRef]
- Scherer, A.E.; Lunt, J.; Draper, A.M.; Smee, D.L. Phenotypic plasticity in oysters (Crassostrea virginica) mediated by chemical signals from predators and injured prey. Invertebr. Biol. 2016, 135, 97–107. [Google Scholar] [CrossRef]
- Weiss, L.C. Sensory ecology of predator-induced phenotypic plasticity. Front. Behav. Neurosci. 2019, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Prasannakumar, I.; Kodandaramaiah, U. Adaptive phenotypic plasticity of mandibles with respect to host plants. Arthropod-Plant Interact. 2024, 18, 77–88. [Google Scholar] [CrossRef]
- Simpson, S.J.; Despland, E.; Hägele, B.F.; Dodgson, T. Gregarious behavior in desert locusts is evoked by touching their back legs. Proc. Natl. Acad. Sci. USA 2001, 98, 3895–3897. [Google Scholar] [CrossRef] [PubMed]
- Heyland, A.; Hodin, J. Heterochronic developmental shift caused by thyroid hormone in larval sand dollars and its implications for phenotypic plasticity and the evolution of nonfeeding development. Evolution 2004, 58, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Fogg, L.G.; Cortesi, F.; Gache, C.; Lecchini, D.; Marshall, N.J.; de Busserolles, F. Developing and adult reef fish show rapid light-induced plasticity in their visual system. Mol. Ecol. 2023, 32, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Aphalo, P.J.; Ballaré, C.L.; Scopel, A.L. Plant-plant signalling, the shade-avoidance response and competition. J. Exp. Bot. 1999, 50, 1629–1634. [Google Scholar] [CrossRef]
- Cortesi, F.; Feeney, W.E.; Ferrari, M.C.O.; Waldie, P.A.; Phillips, G.A.C.; McClure, E.C.; Sköld, H.N.; Salzburger, W.; Marshall, N.J.; Cheney, K.L. Phenotypic Plasticity Confers Multiple Fitness Benefits to a Mimic. Curr. Biol. 2015, 25, 949–954. [Google Scholar] [CrossRef]
- Noble, D.W.A.; Stenhouse, V.; Schwanz, L.E. Developmental temperatures and phenotypic plasticity in reptiles: A systematic review and meta-analysis. Biol. Rev. 2018, 93, 72–97. [Google Scholar] [CrossRef]
- Mallard, F.; Nolte, V.; Schlötterer, C. The Evolution of Phenotypic Plasticity in Response to Temperature Stress. Genome Biol. Evol. 2020, 12, 2429–2440. [Google Scholar] [CrossRef]
- Angert, A.L.; Horst, J.L.; Huxman, T.E.; Venable, D.L. Phenotypic plasticity and precipitation response in Sonoran Desert winter annuals. Am. J. Bot. 2010, 97, 405–411. [Google Scholar] [CrossRef]
- Zhang, C.-X.; Brisson, J.A.; Xu, H.-J. Molecular Mechanisms of Wing Polymorphism in Insects. Annu. Rev. Entomol. 2019, 64, 297–314. [Google Scholar] [CrossRef]
- Relyea, R.A. Fine-Tuned Phenotypes: Tadpole Plasticity under 16 Combinations of Predators and Competitors. Ecology 2004, 85, 172–179. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Wallin, W.G.; Hitchcock, L.J.; Fox, C.W. Phenotypic plasticity in a complex world: Interactive effects of food and temperature on fitness components of a seed beetle. Oecologia 2007, 153, 309–321. [Google Scholar] [CrossRef]
- Yoon, K.J.; Cunningham, C.B.; Bretman, A.; Duncan, E.J. One genome, multiple phenotypes: Decoding the evolution and mechanisms of environmentally induced developmental plasticity in insects. Biochem. Soc. Trans. 2023, 51, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Levis, N.A.; Ragsdale, E.J. Linking Molecular Mechanisms and Evolutionary Consequences of Resource Polyphenism. Front. Integr. Neurosci. 2022, 16, 805061. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.W.; Kern, A.D. Comparative Genomics of Centrality and Essentiality in Three Eukaryotic Protein-Interaction Networks. Mol. Biol. Evol. 2005, 22, 803–806. [Google Scholar] [CrossRef] [PubMed]
- de la Serna Buzon, S.M.; Martin, R.A.; Pfennig, D.W. Carryover effects and the evolution of polyphenism. Biol. J. Linn. Soc. 2020, 131, 622–631. [Google Scholar] [CrossRef]
- Michener, C.D. Social polymorphism in Hymenoptera. Symp. R. Entomol. Soc. Lond. 1961, 1, 43–56. [Google Scholar]
- Pfennig, D.W. Polyphenism in spadefoot toad tadpoles as a locally adjusted evolutionarily stable strategy. Evolution 1992, 46, 1408–1420. [Google Scholar] [CrossRef]
- Pfennig, D.W. The adaptive significance of an environmentally-cued developmental switch in an anuran tadpole. Oecologia 1990, 85, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Paull, J.S.; Martin, R.A.; Pfennig, D.W. Increased competition as a cost of specialization during the evolution of resource polymorphism. Biol. J. Linn. Soc. 2012, 107, 845–853. [Google Scholar] [CrossRef]
- Pfennig, D.W.; Mabry, A.; Orange, D. Environmental causes of correlations between age and size at metamorphosis in Scaphiopus multiplicatus. Ecology 1991, 72, 2240–2248. [Google Scholar] [CrossRef]
- Pomeroy, L.V. Developmental Polymorphism in the Tadpoles of the Spadefoot Toad Scaphiopus multiplicatus. Doctoral Dissertation, University of California Riverside, Riverside, CA, USA, 1981. [Google Scholar]
- Pfennig, D.W. Cannibalistic tadpoles that pose the greatest threat to kin are most likely to discriminate kin. Proc. R. Soc. Lond. Ser. B 1999, 266, 57–81. [Google Scholar] [CrossRef]
- Martin, R.A.; Pfennig, D.W. Evaluating the targets of selection during character displacement. Evolution 2011, 65, 2946–2958. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, D.W.; Murphy, P.J. Character displacement in polyphenic tadpoles. Evolution 2000, 54, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.W.; Pfennig, D.W.; de la Serna Buzón, S.; Pfennig, K.S. Male sexual signals predict phenotypic plasticity in offspring: Implications for the evolution of plasticity and local adaptation. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180179. [Google Scholar] [CrossRef]
- Martin, R.A.; Pfennig, D.W. Disruptive selection in natural populations: The roles of ecological specialization and resource competition. Am. Nat. 2009, 174, 268–281. [Google Scholar] [CrossRef]
- Levis, N.A.; Martin, R.A.; O’Donnell, K.A.; Pfennig, D.W. Intraspecific adaptive radiation: Competition, ecological opportunity, and phenotypic diversification within species. Evolution 2017, 71, 2496–2509. [Google Scholar] [CrossRef]
- Pfennig, D.W.; Rice, A.M.; Martin, R.A. Field and experimental evidence for competition’s role in phenotypic divergence. Evolution 2007, 61, 257–271. [Google Scholar] [CrossRef]
- Pfennig, D.W. Proximate and functional causes of polyphenism in an anuran tadpole. Funct. Ecol. 1992, 6, 167–174. [Google Scholar] [CrossRef]
- Seidl, F.; Levis, N.A.; Schell, R.; Pfennig, D.W.; Pfennig, K.S.; Ehrenreich, I.M. Genome of Spea multiplicata, a rapidly developing, phenotypically plastic, and desert-adapted spadefoot toad. G3 Genes Genomes Genet. 2019, 9, 3909–3919. [Google Scholar] [CrossRef]
- Durand, N.C.; Shamim, M.S.; Machol, I.; Rao, S.S.P.; Huntley, M.H.; Lander, E.S.; Aiden, E.L. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst. 2016, 3, 95–98. [Google Scholar] [CrossRef]
- Dudchenko, O.; Batra, S.S.; Omer, A.D.; Nyquist, S.K.; Hoeger, M.; Durand, N.C.; Shamim, M.S.; Machol, I.; Lander, E.S.; Aiden, A.P.; et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 2017, 356, 92–95. [Google Scholar] [CrossRef]
- Durand, N.C.; Robinson, J.T.; Shamim, M.S.; Machol, I.; Mesirov, J.P.; Lander, E.S.; Aiden, E.L. Juicebox provides a visualization system for Hi-C contact maps with unlimited zoom. Cell Syst. 2016, 3, 99–101. [Google Scholar] [CrossRef]
- Rhie, A.; McCarthy, S.A.; Fedrigo, O.; Damas, J.; Formenti, G.; Koren, S.; Uliano-Silva, M.; Chow, W.; Fungtammasan, A.; Kim, J.; et al. Towards complete and error-free genome assemblies of all vertebrate species. Nature 2021, 592, 737–746. [Google Scholar] [CrossRef]
- Alonge, M.; Lebeigle, L.; Kirsche, M.; Jenike, K.; Ou, S.; Aganezov, S.; Wang, X.; Lippman, Z.B.; Schatz, M.C.; Soyk, S. Automated assembly scaffolding using RagTag elevates a new tomato system for high-throughput genome editing. Genome Biol. 2022, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, A.O. Chromosomal studies of the Pelobatidae (Salientia) and some instances of ploidy. Southwest. Nat. 1970, 15, 239–248. [Google Scholar] [CrossRef]
- Pracana, R.; Priyam, A.; Levantis, I.; Nichols, R.A.; Wurm, Y. The fire ant social chromosome supergene variant Sb shows low diversity but high divergence from SB. Mol. Ecol. 2017, 26, 2864–2879. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.M.; Haussler, D.; Kent, W.J. The UCSC genome browser and associated tools. Brief. Bioinform. 2012, 14, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Isdaner, A.J.; Levis, N.A.; Pfennig, D.W. Comparative transcriptomics reveals that a novel form of phenotypic plasticity evolved via lineage-specific changes in gene expression. Ecol. Evol. 2023, 13, e10646. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Watanabe, C.K. GMAP: A genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 2005, 21, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Burford Reiskind, M.O.; Labadie, P.; Bargielowski, I.; Lounibos, L.P.; Reiskind, M.H. Rapid evolution and the genomic consequences of selection against interspecific mating. Mol. Ecol. 2018, 27, 3641–3654. [Google Scholar] [CrossRef] [PubMed]
- Burford Reiskind, M.O.; Coyle, K.; Daniels, H.V.; Labadie, P.; Reiskind, M.H.; Roberts, N.B.; Roberts, R.B.; Schaff, J.; Vargo, E.L. Development of a universal double-digest RAD sequencing approach for a group of nonmodel, ecologically and economically important insect and fish taxa. Mol. Ecol. Resour. 2016, 16, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Krueger, F. Trim Galore! 2015. Available online: http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 10 May 2024).
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high-confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Lippert, C.; Listgarten, J.; Liu, Y.; Kadie, C.M.; Davidson, R.I.; Heckerman, D. FaST linear mixed models for genome-wide association studies. Nat. Methods 2011, 8, 833–835. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Zeileis, A.; Hothorn, T. Diagnostic checking in regression relationships. R News 2002, 2, 7–10. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.43.17. 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 10 May 2024).
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Levis, N.A.; McKay, D.J.; Pfennig, D.W. Disentangling the developmental origins of a novel phenotype: Enhancement versus reversal of environmentally induced gene expression. Proc. R. Soc. B 2022, 289, 20221764. [Google Scholar] [CrossRef]
- Levis, N.A.; Kelly, P.W.; Harmon, E.A.; Ehrenreich, I.M.; McKay, D.J.; Pfennig, D.W. Transcriptomic bases of a polyphenism. J. Exp. Zool. Part B Mol. Dev. Evol. 2021, 336, 482–485. [Google Scholar] [CrossRef]
- Zhou, S.; Han, L.; Weng, M.; Zhu, H.; Heng, Y.; Wang, G.; Shen, Z.; Chen, X.; Fu, X.; Zhang, M.; et al. Paxbp1 controls a key checkpoint for cell growth and survival during early activation of quiescent muscle satellite cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2021093118. [Google Scholar] [CrossRef]
- Diao, Y.; Guo, X.; Li, Y.; Sun, K.; Lu, L.; Jiang, L.; Fu, X.; Zhu, H.; Sun, H.; Wang, H.; et al. Pax3/7BP Is a Pax7- and Pax3-Binding Protein that Regulates the Proliferation of Muscle Precursor Cells by an Epigenetic Mechanism. Cell Stem Cell 2012, 11, 231–241. [Google Scholar] [CrossRef]
- Deryabin, A.; Moraleva, A.; Dobrochaeva, K.; Kovaleva, D.; Rubtsova, M.; Dontsova, O.; Rubtsov, Y. Human RPF1 and ESF1 in Pre-rRNA Processing and the Assembly of Pre-Ribosomal Particles: A Functional Study. Cells 2024, 13, 326. [Google Scholar] [CrossRef]
- Schlichting, C.D.; Pigliucci, M. Control of phenotypic plasticity via regulatory genes. Am. Nat. 1993, 142, 366–370. [Google Scholar] [CrossRef]
- Genuth, N.R.; Barna, M. The Discovery of Ribosome Heterogeneity and Its Implications for Gene Regulation and Organismal Life. Mol. Cell 2018, 71, 364–374. [Google Scholar] [CrossRef]



| Scan Type | Chr | Position | p-Value | Percentage Point Change in Proportion between Major Allele Homozygote and Heterozygote | Percentage Point Change in Proportion between Heterozygote and Minor Allele Homozygote |
|---|---|---|---|---|---|
| Environmental Assessment | 2 | 77,068,752 | 8.32 × 10−8 | 34.9 | 30.6 |
| 2 | 139,184,079 | 1.81 × 10−7 | 62.0 | 0.6 | |
| 3 | 19,873,503 | 1.27 × 10−7 | 14.5 | 54.1 | |
| 5 | 7,752,008 | 1.04 × 10−7 | 54.2 | 1.7 | |
| 5 | 7,752,029 | 1.04 × 10−7 | 54.2 | 1.7 | |
| 5 | 90,293,461 | 1.40 × 10−9 | 30.7 | 45.5 | |
| 7 | 30,349,593 | 1.32 × 10−7 | 19.2 | 49.3 | |
| 8 | 31,495,326 | 4.19 × 10−8 | 66.5 | −7.4 | |
| 11 | 16,020,207 | 1.84 × 10−11 | 60.4 | n/a | |
| 13 | 22,448,116 | 2.56 × 10−7 | 17.1 | 50.3 | |
| Morph Production (HS) | 2 | 9,988,823 | 2.21 × 10−7 | 22.9 | 46.2 |
| 3 | 19,873,503 | 1.45 × 10−8 | 24.5 | 50.0 | |
| 5 | 90,293,461 | 1.11 × 10−7 | 38.9 | 39.3 | |
| 6 | 52,066,113 | 1.01 × 10−7 | −68.5 | n/a | |
| 6 | 52,066,114 | 1.01 × 10−7 | −68.5 | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isdaner, A.J.; Levis, N.A.; Ehrenreich, I.M.; Pfennig, D.W. Genetic Variants Underlying Plasticity in Natural Populations of Spadefoot Toads: Environmental Assessment versus Phenotypic Response. Genes 2024, 15, 611. https://doi.org/10.3390/genes15050611
Isdaner AJ, Levis NA, Ehrenreich IM, Pfennig DW. Genetic Variants Underlying Plasticity in Natural Populations of Spadefoot Toads: Environmental Assessment versus Phenotypic Response. Genes. 2024; 15(5):611. https://doi.org/10.3390/genes15050611
Chicago/Turabian StyleIsdaner, Andrew J., Nicholas A. Levis, Ian M. Ehrenreich, and David W. Pfennig. 2024. "Genetic Variants Underlying Plasticity in Natural Populations of Spadefoot Toads: Environmental Assessment versus Phenotypic Response" Genes 15, no. 5: 611. https://doi.org/10.3390/genes15050611
APA StyleIsdaner, A. J., Levis, N. A., Ehrenreich, I. M., & Pfennig, D. W. (2024). Genetic Variants Underlying Plasticity in Natural Populations of Spadefoot Toads: Environmental Assessment versus Phenotypic Response. Genes, 15(5), 611. https://doi.org/10.3390/genes15050611





