Expression of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Candidate Genes EDA2R, PCDH9, and TRAF7 in Normal Human Kidney Development and CAKUT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procurement and Processing of Tissues
2.2. Immunofluorescence
2.3. Data Acquisition
2.4. Image Analysis of Area Percentage
2.5. Statistical Analysis of Area Percentage
3. Results
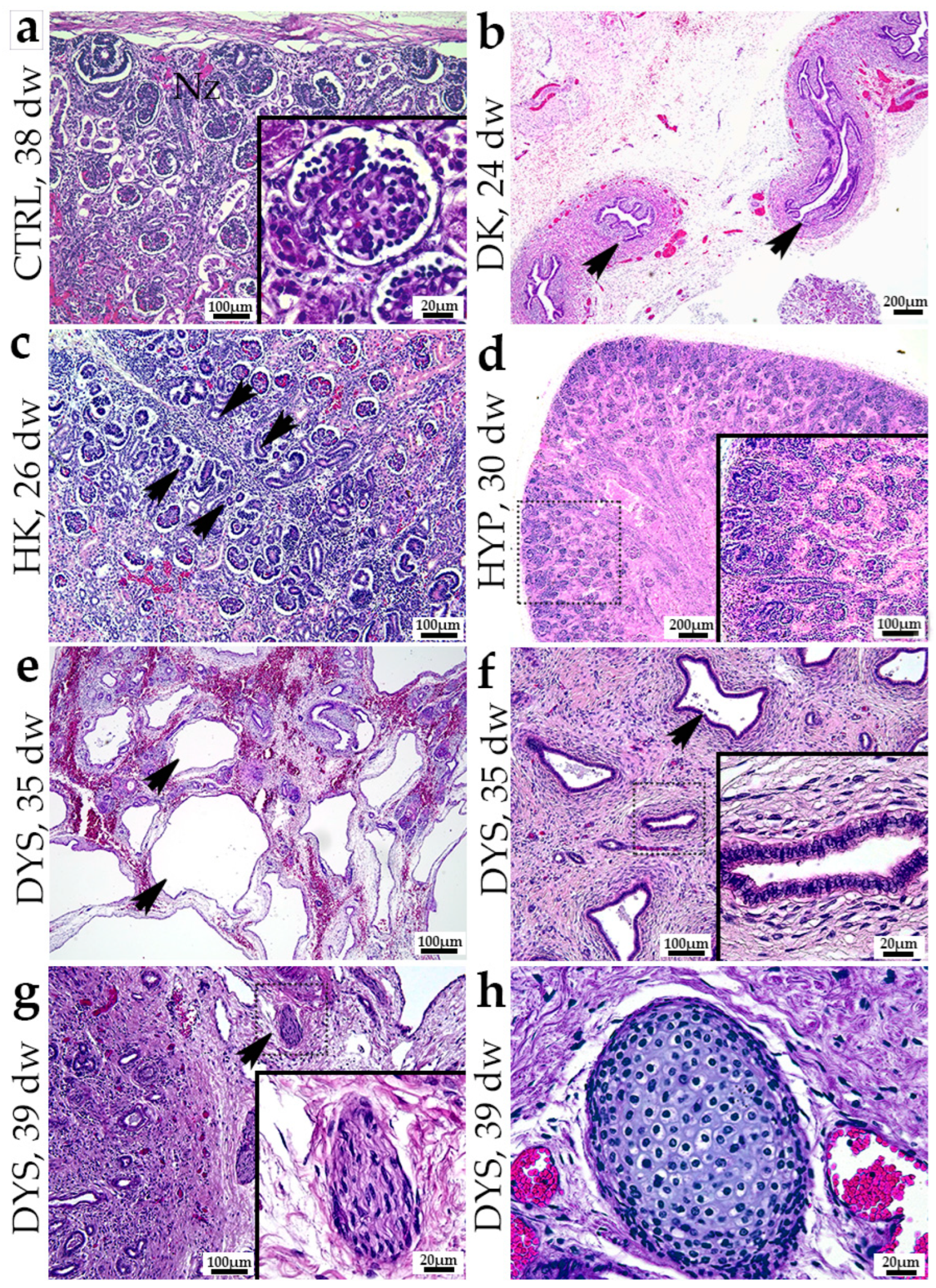
3.1. Hematoxylin and Eosin (H&E) of Normal Human Fetal Kidneys and Kidneys with Congenital Anomalies of the Kidney and Urinary Tract (CAKUT)
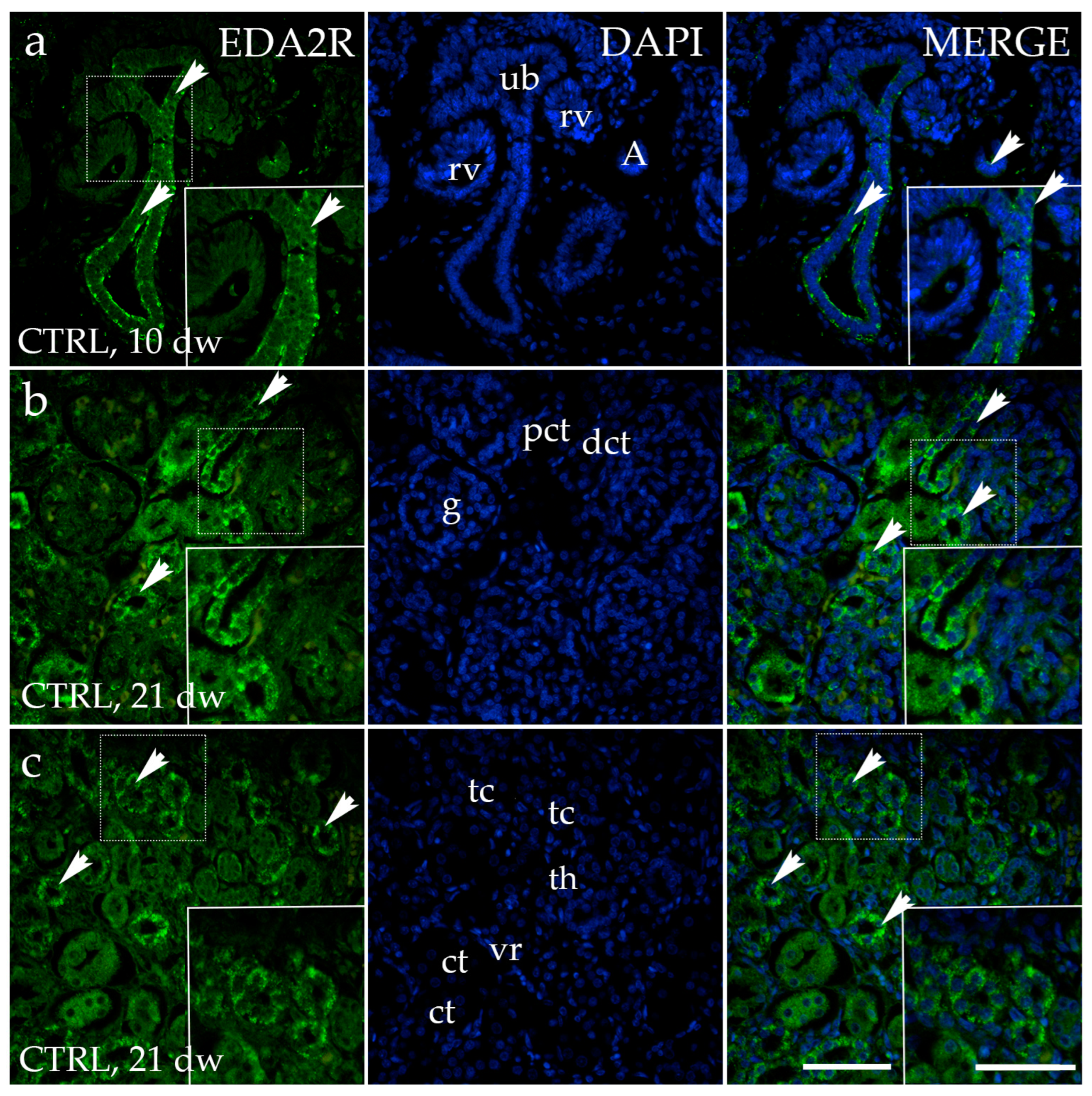
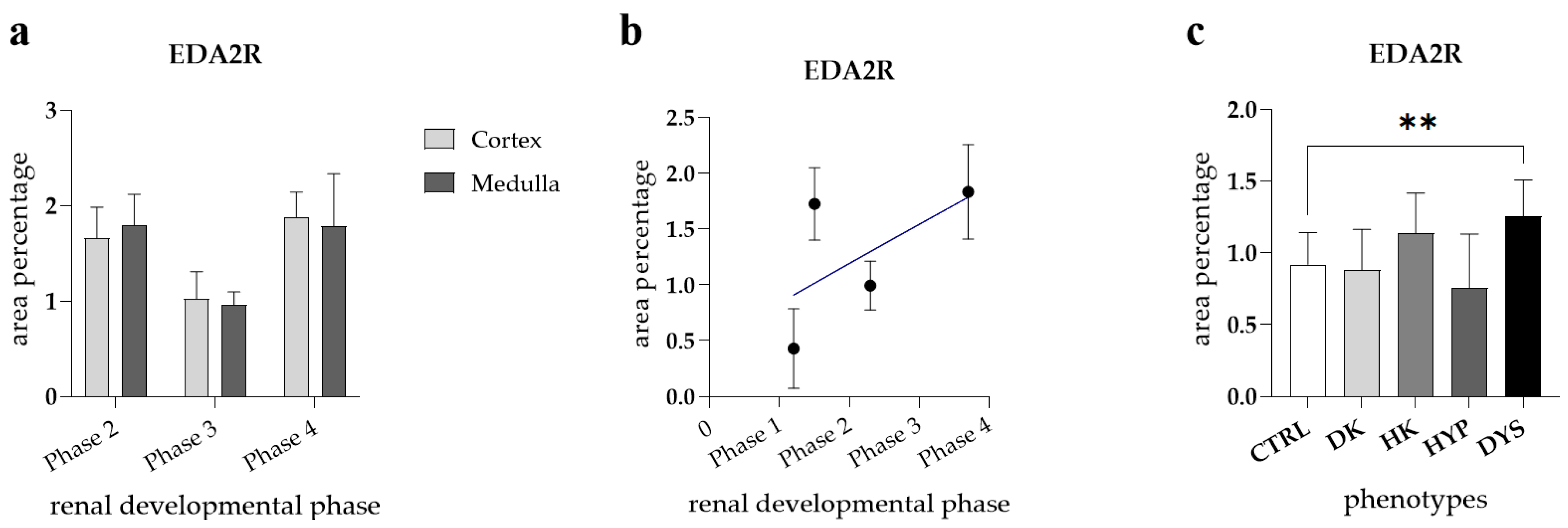
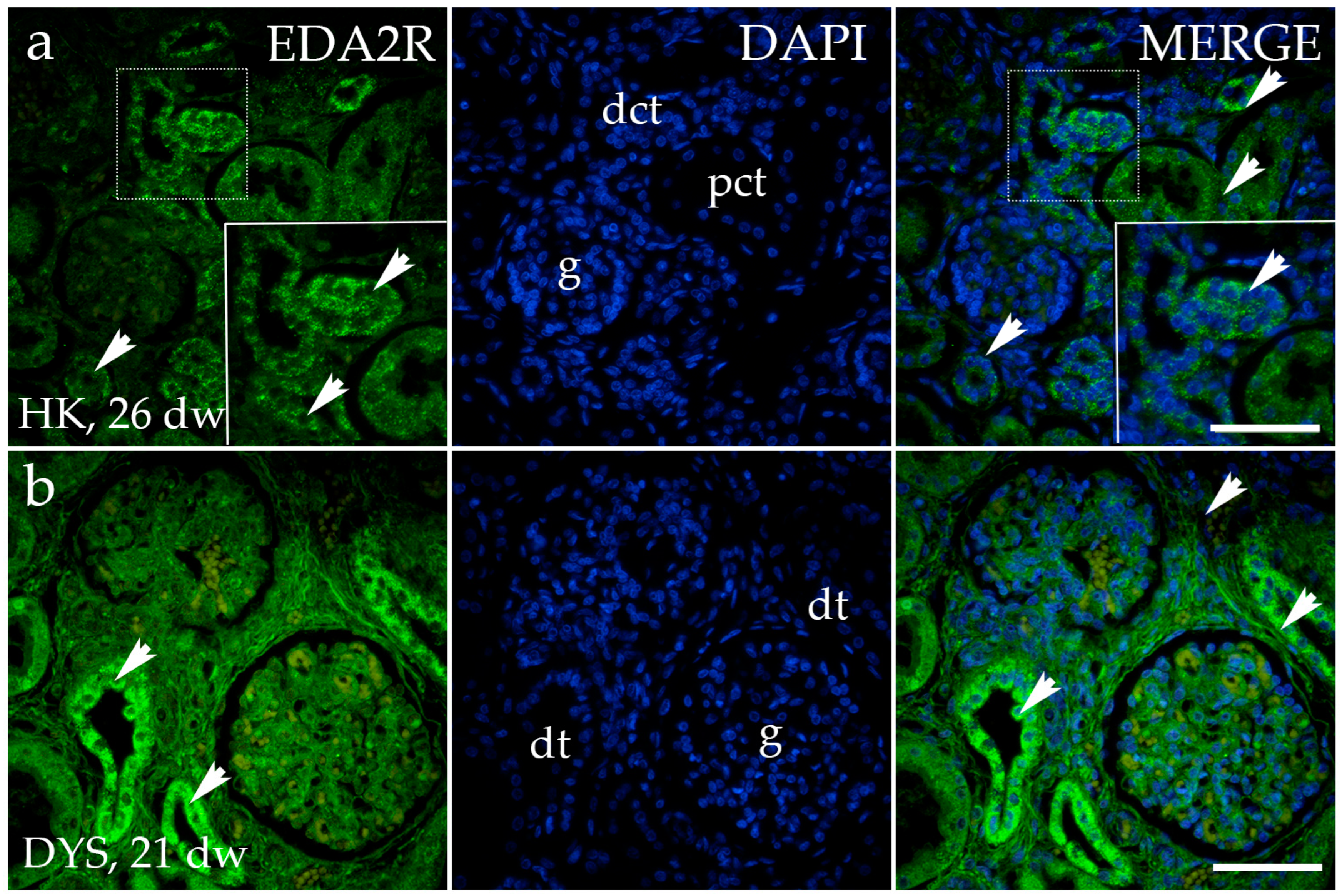
3.2. EDA2R Expression
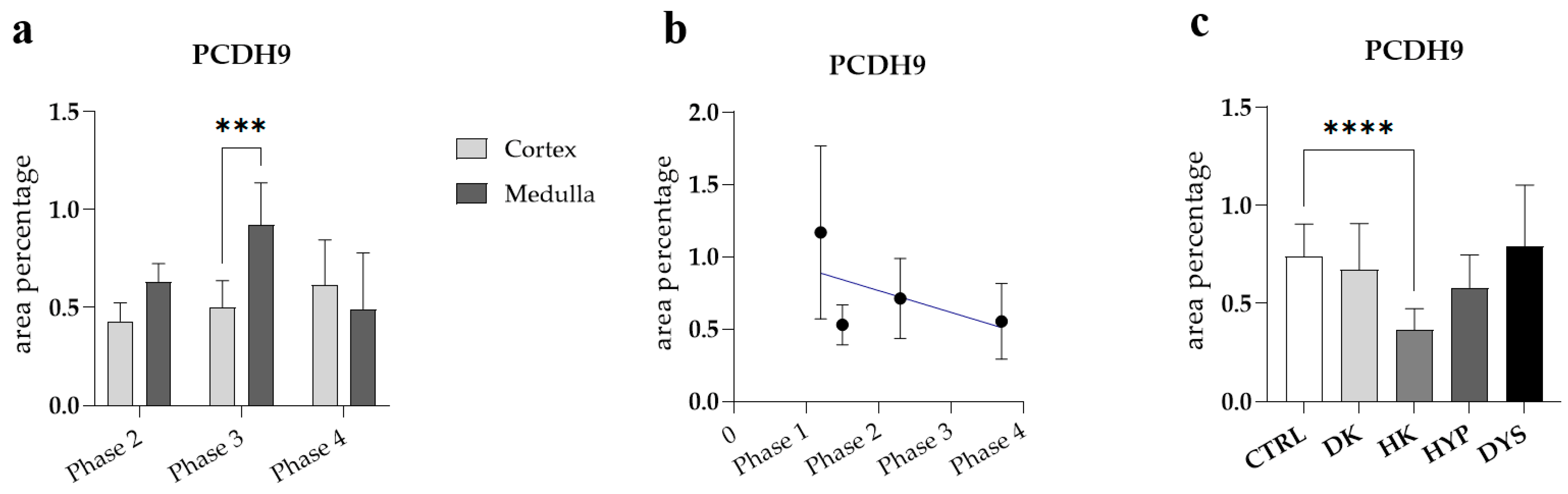
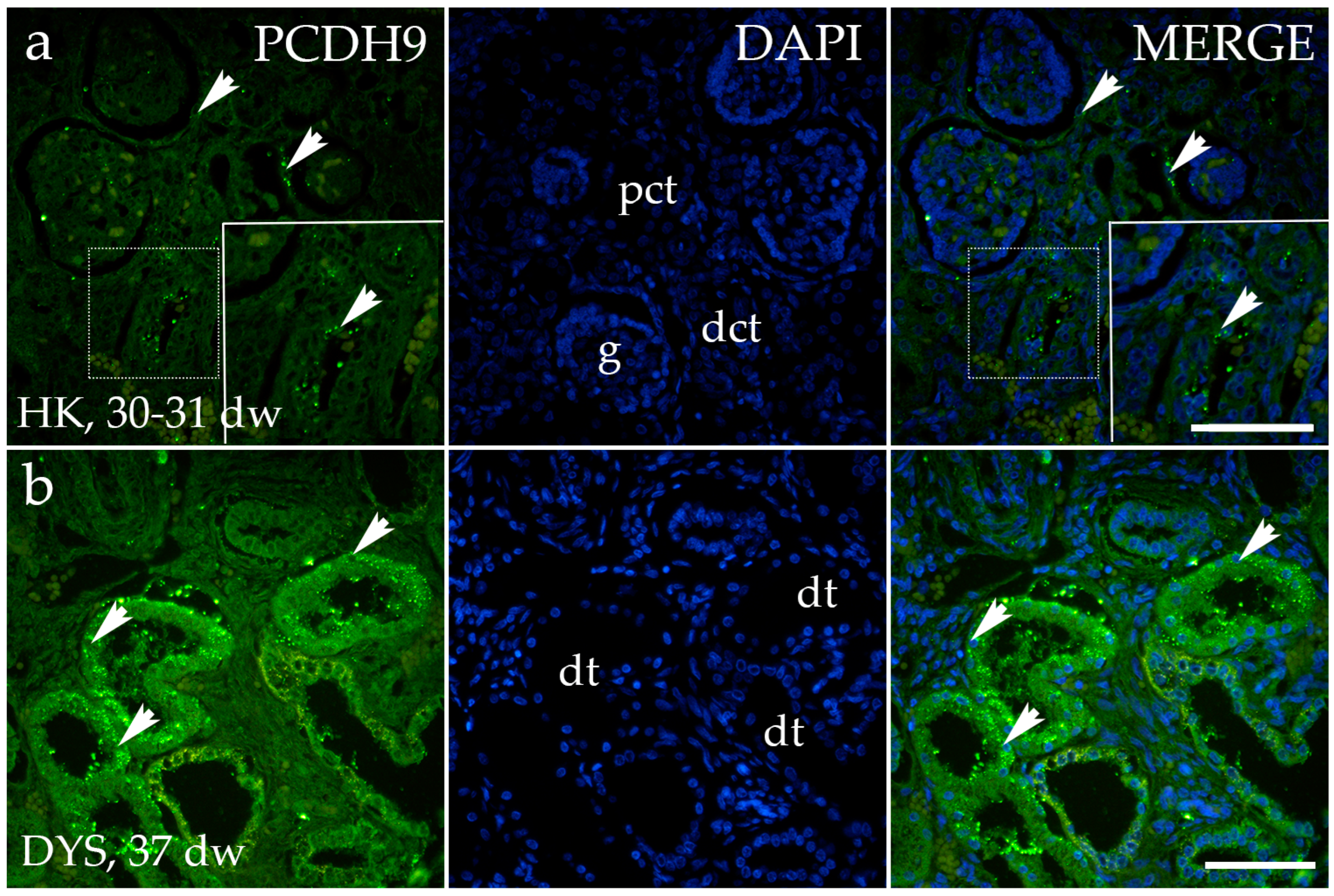
3.3. PCDH9 Expression
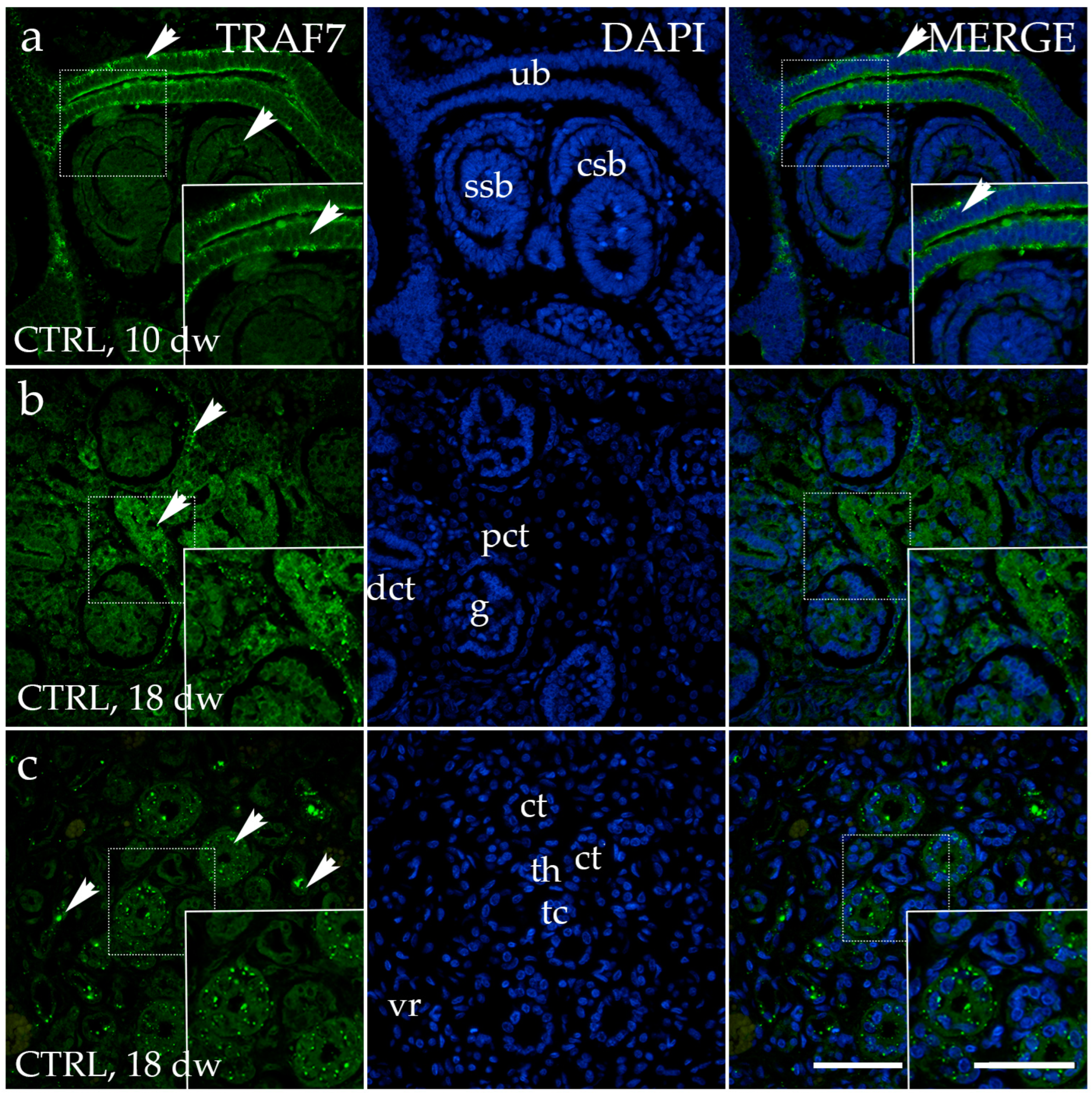
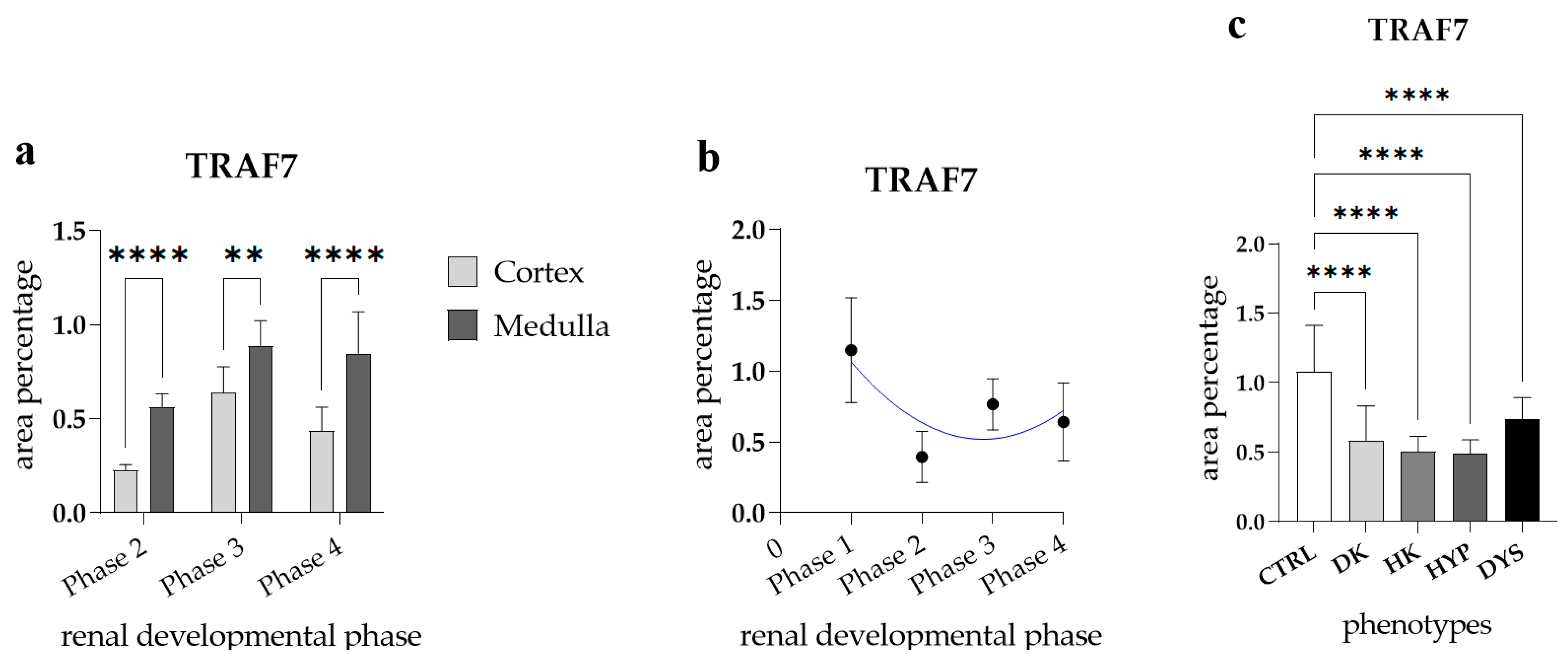
3.4. TRAF7 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Little, M.H.; McMahon, A.P. Mammalian Kidney Development: Principles, Progress, and Projections. Cold Spring Harb. Perspect. Biol. 2012, 4, a008300. [Google Scholar] [CrossRef]
- Faa, G.; Gerosa, C.; Fanni, D.; Monga, G.; Zaffanello, M.; Van Eyken, P.; Fanos, V. Morphogenesis and molecular mechanisms involved in human kidney development. J. Cell. Physiol. 2011, 227, 1257–1268. [Google Scholar] [CrossRef]
- Dressler, G.R. The Cellular Basis of Kidney Development. Annu. Rev. Cell Dev. Biol. 2006, 22, 509–529. [Google Scholar] [CrossRef]
- Rehman, S.; Ahmed, D. Embryology, Kidney, Bladder, and Ureter; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Little, M.H.; Brennan, J.; Georgas, K.; Davies, J.A.; Davidson, D.R.; Baldock, R.A.; Beverdam, A.; Bertram, J.F.; Capel, B.; Chiu, H.S.; et al. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr. Patterns 2007, 7, 680–699. [Google Scholar] [CrossRef]
- Ekblom, P. Renal development. In Kidney Physiology and Pathophysiology; Seldin, D.W., Giebisch, G., Eds.; Raven Press: New York, NY, USA, 1992; pp. 475–501. [Google Scholar]
- Talati, A.N.; Webster, C.M.; Vora, N.L. Prenatal genetic considerations of congenital anomalies of the kidney and urinary tract (CAKUT). Prenat. Diagn. 2019, 39, 679–692. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wang, X.-W.; Liu, C.-H.; Ma, D.; Gao, X.-J.; Jiang, X.-Y.; Mao, J.-H.; Zhu, G.-H.; Zhang, A.-H.; Wang, M.; et al. Genetic spectrum of CAKUT and risk factors for kidney failure: A paediatric multicentre cohort study. Nephrol. Dial. Transplant. 2022, 38, 1981–1991. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yoshikawa, N. Genetic disorders of human congenital anomalies of the kidney and urinary tract (CAKUT). Pediatr. Int. 2003, 45, 610–616. [Google Scholar] [CrossRef]
- Bonthuis, M.; Vidal, E.; Bjerre, A.; Aydog, O.; Baiko, S.; Garneata, L.; Guzzo, I.; Heaf, J.G.; Jahnukainen, T.; Lilien, M.; et al. Ten-year trends in epidemiology and outcomes of pediatric kidney replacement therapy in Europe: Data from the ESPN/ERA-EDTA Registry. Pediatr. Nephrol. 2021, 36, 2337–2348. [Google Scholar] [CrossRef]
- Sanna-Cherchi, S.; Westland, R.; Ghiggeri, G.M.; Gharavi, A.G. Genetic basis of human congenital anomalies of the kidney and urinary tract. J. Clin. Investig. 2018, 128, 4–15. [Google Scholar] [CrossRef]
- Conrad, D.F.; Pinto, D.; Redon, R.; Feuk, L.; Gokcumen, O.; Zhang, Y.; Aerts, J.; Andrews, T.D.; Barnes, C.; Campbell, P.; et al. Origins and functional impact of copy number variation in the human genome. Nature 2010, 464, 704–712. [Google Scholar] [CrossRef]
- Westland, R.; Verbitsky, M.; Vukojevic, K.; Perry, B.J.; Fasel, D.A.; Zwijnenburg, P.J.; Bökenkamp, A.; Gille, J.J.; Saraga-Babic, M.; Ghiggeri, G.M.; et al. Copy number variation analysis identifies novel CAKUT candidate genes in children with a solitary functioning kidney. Kidney Int. 2015, 88, 1402–1410. [Google Scholar] [CrossRef]
- Mikkola, M.L.; Pispa, J.; Pekkanen, M.; Paulin, L.; Nieminen, P.; Kere, J.; Thesleff, I. Ectodysplasin, a protein required for epithelial morphogenesis, is a novel TNF homologue and promotes cell-matrix adhesion. Mech. Dev. 1999, 88, 133–146. [Google Scholar] [CrossRef]
- Bayés, M.; Hartung, A.J.; Ezer, S.; Pispa, J.; Thesleff, I.; Srivastava, A.K.; Kere, J. The Anhidrotic Ectodermal Dysplasia Gene (EDA) Undergoes Alternative Splicing and Encodes Ectodysplasin-A with Deletion Mutations in Collagenous Repeats. Hum. Mol. Genet. 1998, 7, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, C.; Ri, C.; Kumar, V.; Nakamura, Y.; Matsuda, K. Crosstalk of EDA-A2/XEDAR in the p53 Signaling Pathway. Mol. Cancer Res. 2010, 8, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Deng, X.; Jia, J.; Wang, D.; Yuan, G. Ectodysplasin A/Ectodysplasin A Receptor System and Their Roles in Multiple Diseases. Front. Physiol. 2021, 12, 788411. [Google Scholar] [CrossRef]
- Frank, M.; Kemler, R. Protocadherins. Curr. Opin. Cell Biol. 2002, 14, 557–562. [Google Scholar] [CrossRef]
- Strehl, S.; Glatt, K.; Liu, Q.M.; Glatt, H.; Lalande, M. Characterization of Two Novel Protocadherins (PCDH8andPCDH9) Localized on Human Chromosome 13 and Mouse Chromosome 14. Genomics 1998, 53, 81–89. [Google Scholar] [CrossRef]
- Xiao, X.; Zheng, F.; Chang, H.; Ma, Y.; Yao, Y.-G.; Luo, X.-J.; Li, M. The Gene Encoding Protocadherin 9 (PCDH9), a Novel Risk Factor for Major Depressive Disorder. Neuropsychopharmacology 2018, 43, 1128–1137. [Google Scholar] [CrossRef]
- Wang, C.; Yu, G.; Liu, J.; Wang, J.; Zhang, Y.; Zhang, X.; Zhou, Z.; Huang, Z. Downregulation of PCDH9 predicts prognosis for patients with glioma. J. Clin. Neurosci. 2012, 19, 541–545. [Google Scholar] [CrossRef]
- de Tayrac, M.; Etcheverry, A.; Aubry, M.; Saïkali, S.; Hamlat, A.; Quillien, V.; Le Treut, A.; Galibert, M.; Mosser, J. Integrative genome-wide analysis reveals a robust genomic glioblastoma signature associated with copy number driving changes in gene expression. Genes Chromosom. Cancer 2009, 48, 55–68. [Google Scholar] [CrossRef]
- Zotti, T.; Vito, P.; Stilo, R. The seventh ring: Exploring TRAF7 functions. J. Cell. Physiol. 2011, 227, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-G.; Li, L.-Y.; Shu, H.-B. TRAF7 Potentiates MEKK3-induced AP1 and CHOP Activation and Induces Apoptosis. J. Biol. Chem. 2004, 279, 17278–17282. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Jono, H.; Kai, H.; Li, J.-D. The Tumor Suppressor Cylindromatosis (CYLD) Acts as a Negative Regulator for Toll-like Receptor 2 Signaling via Negative Cross-talk with TRAF6 and TRAF7. J. Biol. Chem. 2005, 280, 41111–41121. [Google Scholar] [CrossRef] [PubMed]
- Najm, P.; Zhao, P.; Steklov, M.; Sewduth, R.N.; Baietti, M.F.; Pandolfi, S.; Criem, N.; Lechat, B.; Maia, T.M.; Van Haver, D.; et al. Loss-of-Function Mutations in TRAF7 and KLF4 Cooperatively Activate RAS-Like GTPase Signaling and Promote Meningioma Development. Cancer Res. 2021, 81, 4218–4229. [Google Scholar] [CrossRef] [PubMed]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016, 48, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Goode, B.; Joseph, N.M.; Stevers, M.; Van Ziffle, J.; Onodera, C.; Talevich, E.; Grenert, J.P.; Yeh, I.; Bastian, B.C.; Phillips, J.J.; et al. Adenomatoid tumors of the male and female genital tract are defined by TRAF7 mutations that drive aberrant NF-kB pathway activation. Mod. Pathol. 2017, 31, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.J.; Wu, Y.; Jentoft, M.E.; Mer, G.; Spinner, R.J.; Dyck, P.J.B.; Mauermann, M.L. Genomic analysis reveals frequent TRAF7 mutations in intraneural perineuriomas. Ann. Neurol. 2016, 81, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Visković, D.V.; Lozić, M.; Vukoja, M.; Šoljić, V.; Vukojević, K.; Durdov, M.G.; Filipović, N.; Lozić, B. Spatio-Temporal Expression Pattern of CAKUT Candidate Genes DLG1 and KIF12 during Human Kidney Development. Biomolecules 2023, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Williams, J. The Declaration of Helsinki and public health. Bull. World Health Organ. 2008, 86, 650–651. [Google Scholar] [CrossRef]
- O’Rahilly, R. Guide to the staging of human embryos. Anat. Anz. 1972, 130, 556–559. [Google Scholar]
- Kelam, N.; Racetin, A.; Polović, M.; Benzon, B.; Ogorevc, M.; Vukojević, K.; Durdov, M.G.; Huljev, A.D.; Prusac, I.K.; Čarić, D.; et al. Aberrations in FGFR1, FGFR2, and RIP5 Expression in Human Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). Int. J. Mol. Sci. 2022, 23, 15537. [Google Scholar] [CrossRef] [PubMed]
- Lozic, M.; Minarik, L.; Racetin, A.; Filipovic, N.; Babic, M.S.; Vukojevic, K. CRKL, AIFM3, AIF, BCL2, and UBASH3A during Human Kidney Development. Int. J. Mol. Sci. 2021, 22, 9183. [Google Scholar] [CrossRef] [PubMed]
- Kelam, N.; Racetin, A.; Katsuyama, Y.; Vukojević, K.; Kostić, S. Immunohistochemical Expression Pattern of FGFR1, FGFR2, RIP5, and HIP2 in Developing and Postnatal Kidneys of Dab1−/− (yotari) Mice. Int. J. Mol. Sci. 2022, 23, 2025. [Google Scholar] [CrossRef] [PubMed]
- Racetin, A.; Jurić, M.; Filipović, N.; Šolić, I.; Kosović, I.; Durdov, M.G.; Kunac, N.; Tomaš, S.Z.; Saraga, M.; Šoljić, V.; et al. Expression and localization of DAB1 and Reelin during normal human kidney development. Croat. Med. J. 2019, 60, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Pavic, B.; Ogorevc, M.; Boric, K.; Vukovic, D.; Saraga-Babic, M.; Mardesic, S. Connexin 37, 40, 43 and Pannexin 1 Expression in the Gastric Mucosa of Patients with Systemic Sclerosis. Biomedicines 2023, 11, 2487. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Verhelst, K.; Gardam, S.; Borghi, A.; Kreike, M.; Carpentier, I.; Beyaert, R. XEDAR activates the non-canonical NF-κB pathway. Biochem. Biophys. Res. Commun. 2015, 465, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Zachariah, S.; Quiñones, H.I.; Shindo, M.; Chaudhary, P.M. Role of TRAF3 and -6 in the Activation of the NF-κB and JNK Pathways by X-linked Ectodermal Dysplasia Receptor. J. Biol. Chem. 2002, 277, 44953–44961. [Google Scholar] [CrossRef]
- Kwack, M.H.; Kim, J.C.; Kim, M.K. Ectodysplasin-A2 induces apoptosis in cultured human hair follicle cells and promotes regression of hair follicles in mice. Biochem. Biophys. Res. Commun. 2019, 520, 428–433. [Google Scholar] [CrossRef]
- Chang, B.; Punj, V.; Shindo, M.; Chaudhary, P.M. Adenoviral-mediated gene transfer of ectodysplasin-A2 results in induction of apoptosis and cell-cycle arrest in osteosarcoma cell lines. Cancer Gene Ther. 2007, 14, 927–933. [Google Scholar] [CrossRef]
- Bouchard, M. Transcriptional control of kidney development. Differentiation 2004, 72, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Winyard, P.J.; Nauta, J.; Lirenman, D.S.; Hardman, P.; Sams, V.R.; Risdon, R.A.; Woolf, A.S. Deregulation of cell survival in cystic and dysplastic renal development. Kidney Int. 1996, 49, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Kumar, V.; Jha, A.; Aslam, R.; Wang, H.; Chen, K.; Yu, Y.; He, W.; Chen, F.; Luo, H.; et al. EDA2R mediates podocyte injury in high glucose milieu. Biochimie 2020, 174, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Lv, J.; Yang, Z.; Guo, L.; Zhang, L.; Li, M.; Han, W.; Chen, X.; Zhuang, H.; Lu, F. Protocadherin 9 inhibits epithelial–mesenchymal transition and cell migration through activating GSK-3β in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2014, 452, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, K.; Kirkpatrick, J.; Mirjalili, S. The horseshoe kidney: Surgical anatomy and embryology. J. Pediatr. Urol. 2016, 12, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Evans, D. Urogenital system organs. In Human Malformations and Related Anomalies, 2nd ed.; Stevenson, R., Ed.; Oxford University Press: Oxford, NY, USA, 2005; p. 1161e250. [Google Scholar] [CrossRef]
- Doménech-Mateu, J.M.; Gonzalez-Compta, X. Horseshoe kidney: A new theory on lts embrogenesis based on the study of a 16-mm human embryo. Anat. Rec. 1988, 222, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Pieczynski, J.; Margolis, B.; Koehler, S.; Tellkamp, F.; Niessen, C.M.; Bloch, W.; Kerjaschki, D.; Schermer, B.; Benzing, T.; Brinkkoetter, P.T.; et al. Protein complexes that control renal epithelial polarity. Am. J. Physiol. Ren. Physiol. 2011, 300, F589–F601. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, M.A.; Margolis, B. Apical Lumen Formation in Renal Epithelia. J. Am. Soc. Nephrol. 2009, 20, 1444–1452. [Google Scholar] [CrossRef]
- Boutet, A.; De Frutos, C.A.; Maxwell, P.H.; Mayol, M.J.; Romero, J.; Nieto, M.A. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006, 25, 5603–5613. [Google Scholar] [CrossRef]
- Tissue Expression of TRAF7—Staining in Kidney—The Human Protein Atlas [Internet]. Proteinatlas.org. Available online: https://www.proteinatlas.org/ENSG00000131653-TRAF7/tissue/kidney (accessed on 9 April 2024).
- Tsitsikov, E.N.; Phan, K.P.; Liu, Y.; Tsytsykova, A.V.; Kinter, M.; Selland, L.; Garman, L.; Griffin, C.; Dunn, I.F. TRAF7 is an essential regulator of blood vessel integrity during mouse embryonic and neonatal development. iScience 2023, 26, 107474. [Google Scholar] [CrossRef]
- Ribatti, D.; Ligresti, G.; Nicosia, R.F. Kidney endothelial cell heterogeneity, angiocrine activity and paracrine regulatory mechanisms. Vasc. Pharmacol. 2023, 148, 107139. [Google Scholar] [CrossRef]
- Daniel, E.; Azizoglu, D.B.; Ryan, A.R.; Walji, T.A.; Chaney, C.P.; Sutton, G.I.; Carroll, T.J.; Marciano, D.K.; Cleaver, O. Spatiotemporal heterogeneity and patterning of developing renal blood vessels. Angiogenesis 2018, 21, 617–634. [Google Scholar] [CrossRef]
- Abrahamson, D.R. Development of kidney glomerular endothelial cells and their role in basement membrane assembly. Organogenesis 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Reeves, W.H.; Kanwar, Y.S.; Farquhar, M.G. Assembly of the glomerular filtration surface. Differentiation of anionic sites in glomerular capillaries of newborn rat kidney. J. Cell Biol. 1980, 85, 735–753. [Google Scholar] [CrossRef]
- Mishra-Gorur, K.; Barak, T.; Kaulen, L.D.; Henegariu, O.; Jin, S.C.; Aguilera, S.M.; Yalbir, E.; Goles, G.; Nishimura, S.; Miyagishima, D.; et al. Pleiotropic role of TRAF7 in skull-base meningiomas and congenital heart disease. Proc. Natl. Acad. Sci. USA 2023, 120, e2214997120. [Google Scholar] [CrossRef]
- Bai, Y.; Wei, C.; Li, P.; Sun, X.; Cai, G.; Chen, X.; Hong, Q. Primary cilium in kidney development, function and disease. Front. Endocrinol. 2022, 13, 952055. [Google Scholar] [CrossRef]
- Santoni, M.; Piva, F.; Cimadamore, A.; Giulietti, M.; Battelli, N.; Montironi, R.; Cosmai, L.; Porta, C. Exploring the Spectrum of Kidney Ciliopathies. Diagnostics 2020, 10, 1099. [Google Scholar] [CrossRef]
- McConnachie, D.J.; Stow, J.L.; Mallett, A.J. Ciliopathies and the Kidney: A Review. Am. J. Kidney Dis. 2021, 77, 410–419. [Google Scholar] [CrossRef]
- Kakkar, N.; Menon, S.; Radotra, B.D. Histomorphology of renal dysplasia—An autopsy study. Fetal Pediatr. Pathol. 2006, 25, 73–86. [Google Scholar] [CrossRef]
- Bouwmeester, T.; Bauch, A.; Ruffner, H.; Angrand, P.-O.; Bergamini, G.; Croughton, K.; Cruciat, C.; Eberhard, D.; Gagneur, J.; Ghidelli, S.; et al. A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat. Cell Biol. 2004, 6, 97–105. [Google Scholar] [CrossRef]


| Groups | Developmental Phase | Renal and Associated Pathology | Gestational Phases | Number of Kidney Samples |
|---|---|---|---|---|
| Normal kidneys (CTRL) | Ph1 | N/A | 6 | 1 |
| N/A | 10 | 1 | ||
| N/A | 12 | 1 | ||
| Ph2 | N/A | 15 | 1 | |
| N/A | 16 | 1 | ||
| N/A | 17 | 1 | ||
| N/A | 18 | 2 | ||
| N/A | 21 | 1 | ||
| Ph3 | N/A | 23 | 1 | |
| N/A | 24 | 1 | ||
| N/A | 28 | 3 | ||
| N/A | 29 | 1 | ||
| Ph4 | N/A | 32 | 1 | |
| N/A | 35 | 1 | ||
| N/A | 37 | 1 | ||
| N/A | 38 | 2 | ||
| Horseshoe kidneys (HK) | Ren concreatus arcuatus, cystae multiplices corticales | 22 | 1 | |
| Ren concreatus arcuatus, tetras Fallot | 26 | 1 | ||
| Syndroma Edwards, Ren arcuatus | 30–31 | 1 | ||
| Syndroma Edwards, Ren arcuatus | 34 | 1 | ||
| Dysplastic kidneys (DYS) | Megaureter lateris dextri, Dysplasia renis | 21 | 2 | |
| Renis dysplastica cysticus lateralis sinistri, agenesia renis dextri | ||||
| Dysplasia multicystica renis dextri | 27 | 1 | ||
| Cystes parvae focales | ||||
| Renes dysplastici cystici, Syndroma Potter | 35 | 2 | ||
| Dysplasia renis multicystica bilateralis | ||||
| Agenesis renis dextri et dysplasia renis sinistri cum ureter duplex | 37 | 1 | ||
| Dysplasia hypoplastica, renis bilateralis, syndroma Down, syndroma Potter | 38 | 1 | ||
| Syndroma Potter, Dysplasia renis | 39 | 1 | ||
| Hypoplastic kidneys (HYP) | Hypoplasia renis lateris dextri | 30 | 1 | |
| Hypoplasia renis lateris sinistri | 37 | 1 | ||
| Hypoplasia renis | 38 | 1 | ||
| Duplex kidneys (DK) | Ureter duplex lateris dextri | 24 | 1 | |
| Ureter duplex lateris sinistri | 30 | 1 | ||
| Pyelon et ureter duplex bilateralis | 41 | 1 | ||
| Antibodies | Catalog Number | Host | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | Anti-EDA2R/XEDAR antibody | ab203667 | Rabbit | 1:120 | Abcam (Cambridge, UK) |
| PCDH9 Polyclonal antibody | 25090-1-AP | Rabbit | 1:200 | Proteintech Group, Inc. (Rosemont, IL, USA) | |
| TRAF7 Polyclonal antibody | 11780-1-AP | Rabbit | 1:50 | Proteintech Group, Inc. (Rosemont, IL, USA) | |
| Secondary | Anti-Rabbit IgG, Alexa Fluor ® 488 | 711-545-152 | Donkey | 1:300 | Jackson Immuno Research Laboratories, Inc. (Baltimore, PA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelam, J.; Kelam, N.; Filipović, N.; Komić, L.; Racetin, A.; Komić, D.; Kostić, S.; Kuzmić Prusac, I.; Vukojević, K. Expression of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Candidate Genes EDA2R, PCDH9, and TRAF7 in Normal Human Kidney Development and CAKUT. Genes 2024, 15, 702. https://doi.org/10.3390/genes15060702
Kelam J, Kelam N, Filipović N, Komić L, Racetin A, Komić D, Kostić S, Kuzmić Prusac I, Vukojević K. Expression of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Candidate Genes EDA2R, PCDH9, and TRAF7 in Normal Human Kidney Development and CAKUT. Genes. 2024; 15(6):702. https://doi.org/10.3390/genes15060702
Chicago/Turabian StyleKelam, Jelena, Nela Kelam, Natalija Filipović, Luka Komić, Anita Racetin, Dora Komić, Sandra Kostić, Ivana Kuzmić Prusac, and Katarina Vukojević. 2024. "Expression of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Candidate Genes EDA2R, PCDH9, and TRAF7 in Normal Human Kidney Development and CAKUT" Genes 15, no. 6: 702. https://doi.org/10.3390/genes15060702
APA StyleKelam, J., Kelam, N., Filipović, N., Komić, L., Racetin, A., Komić, D., Kostić, S., Kuzmić Prusac, I., & Vukojević, K. (2024). Expression of Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) Candidate Genes EDA2R, PCDH9, and TRAF7 in Normal Human Kidney Development and CAKUT. Genes, 15(6), 702. https://doi.org/10.3390/genes15060702






