PC Gene Affects Milk Production Traits in Dairy Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Phenotypic Data
2.2. DNA Extraction
2.3. SNP Identification and Genotyping
2.4. Association Analyses
2.5. Functional Prediction and Verification of Mutation Sites in 5′ Region of PC
3. Results
3.1. SNPs Identification
3.2. Associations between SNPs and Five Milk Production Traits
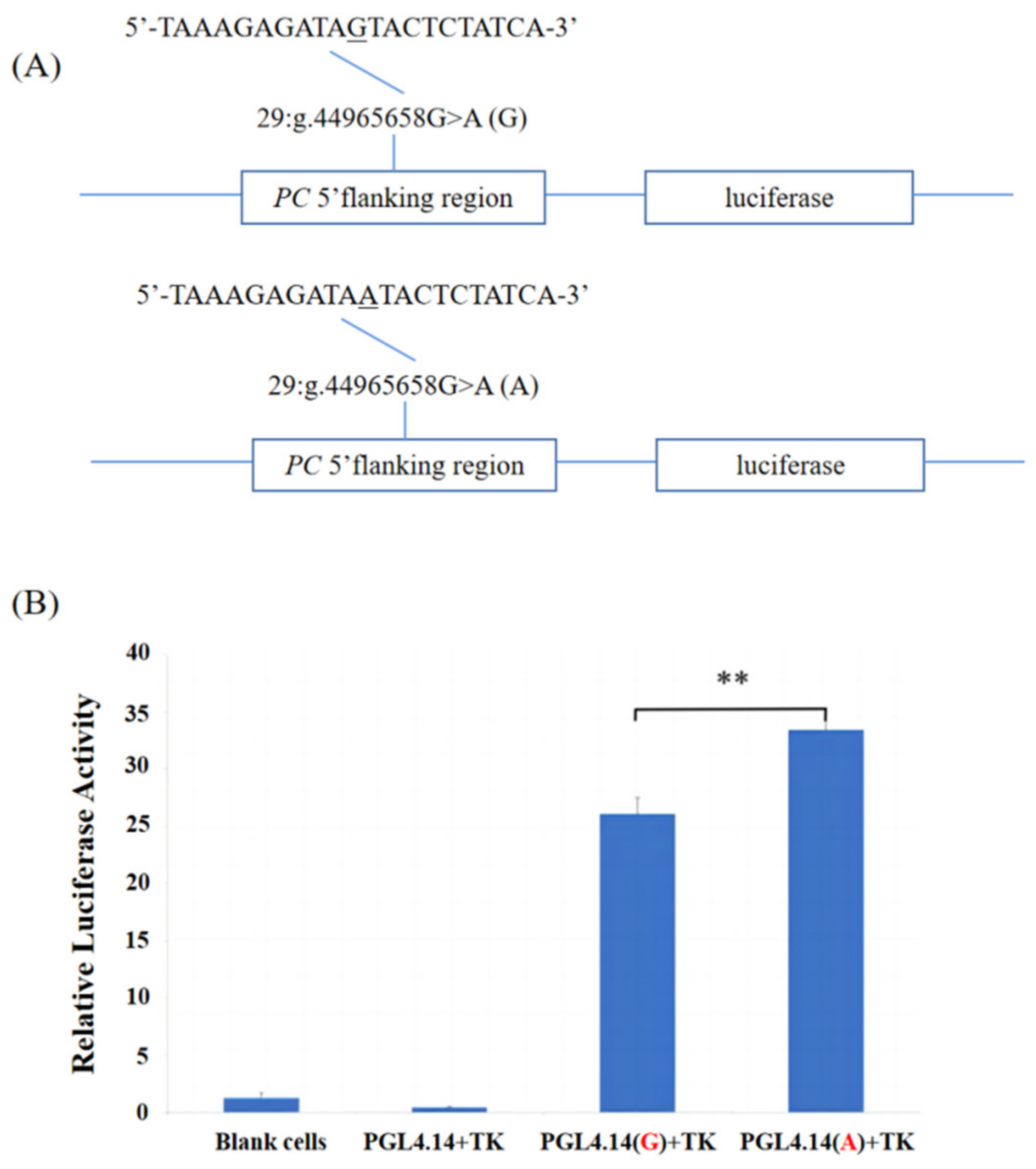
3.3. The Regulation of the 5′ Region SNPs on Transcriptional Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osorio, J.S.; Lohakare, J.; Bionaz, M. Biosynthesis of milk fat, protein, and lactose: Roles of transcriptional and posttranscriptional regulation. Physiol. Genom. 2016, 48, 231–256. [Google Scholar] [CrossRef]
- Spelman, R.J.; Coppieters, W.; Karim, L.; van Arendonk, J.A.; Bovenhuis, H. Quantitative trait loci analysis for five milk production traits on chromosome six in the Dutch Holstein-Friesian population. Genetics 1996, 144, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.; Georges, M. Domestic-animal genomics: Deciphering the genetics of complex traits. Nat. Rev. Genet. 2004, 5, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Wiggans, G.R.; Vanraden, P.M.; Cooper, T.A. The genomic evaluation system in the United States: Past, present, future. J. Dairy Sci. 2011, 94, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Stock, K.F.; Reents, R. Genomic selection: Status in different species and challenges for breeding. Reprod. Domest. Anim. 2013, 48 (Suppl. S1), 2–10. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, L.R. Strategy for applying genome-wide selection in dairy cattle. J. Anim. Breed. Genet. 2006, 123, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Guinan, F.L.; Wiggans, G.R.; Norman, H.D.; Durr, J.W.; Cole, J.B.; Van Tassell, C.P.; Misztal, I.; Lourenco, D. Changes in genetic trends in US dairy cattle since the implementation of genomic selection. J. Dairy Sci. 2023, 106, 1110–1129. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras-Saldana, S.; Lopez, B.I.; Moghaddar, N.; Park, W.; Park, J.E.; Chung, K.Y.; Lim, D.; Lee, S.H.; Shin, D.; van der Werf, J.H.J. Use of gene expression and whole-genome sequence information to improve the accuracy of genomic prediction for carcass traits in Hanwoo cattle. Genet. Sel. Evol. 2020, 52, 54. [Google Scholar] [CrossRef]
- Brondum, R.F.; Su, G.; Janss, L.; Sahana, G.; Guldbrandtsen, B.; Boichard, D.; Lund, M.S. Quantitative trait loci markers derived from whole genome sequence data increases the reliability of genomic prediction. J. Dairy Sci. 2015, 98, 4107–4116. [Google Scholar] [CrossRef]
- Zhang, Z.; Erbe, M.; He, J.; Ober, U.; Gao, N.; Zhang, H.; Simianer, H.; Li, J. Accuracy of whole-genome prediction using a genetic architecture-enhanced variance-covariance matrix. G3 Genes Genomes Genet. 2015, 5, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Shi, L.; Liu, L.; Liang, R.; Li, Q.; Li, J.; Han, B.; Sun, D. Analysis of Liver Proteome and Identification of Critical Proteins Affecting Milk Fat, Protein, and Lactose Metabolism in Dariy Cattle with iTRAQ. Proteomics 2019, 19, e1800387. [Google Scholar] [CrossRef]
- Valle, M. Pyruvate Carboxylase, Structure and Function. Subcell. Biochem. 2017, 83, 291–322. [Google Scholar] [PubMed]
- Hughey, C.C.; Crawford, P.A. Pyruvate Carboxylase Wields a Double-Edged Metabolic Sword. Cell Metab. 2019, 29, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- White, H.M.; Koser, S.L.; Donkin, S.S. Characterization of bovine pyruvate carboxylase promoter 1 responsiveness to serum from control and feed-restricted cows. J. Anim. Sci. 2011, 89, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, R.B.; Cecava, M.J.; Donkin, S.S. Changes in mRNA expression for gluconeogenic enzymes in liver of dairy cattle during the transition to lactation. J. Dairy Sci. 2000, 83, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Lillehammer, M.; Arnyasi, M.; Lien, S.; Olsen, H.G.; Sehested, E.; Odegard, J.; Meuwissen, T.H. A genome scan for quantitative trait locus by environment interactions for production traits. J. Dairy Sci. 2007, 90, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Viitala, S.M.; Schulman, N.F.; de Koning, D.J.; Elo, K.; Kinos, R.; Virta, A.; Virta, J.; Maki-Tanila, A.; Vilkki, J.H. Quantitative trait loci affecting milk production traits in Finnish Ayrshire dairy cattle. J. Dairy Sci. 2003, 86, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Tiezzi, F.; Maltecca, C. Accounting for trait architecture in genomic predictions of US Holstein cattle using a weighted realized relationship matrix. Genet. Sel. Evol. 2015, 47, 24. [Google Scholar] [CrossRef]
- Jolma, A.; Yan, J.; Whitington, T.; Toivonen, J.; Nitta, K.R.; Rastas, P.; Morgunova, E.; Enge, M.; Taipale, M.; Wei, G.; et al. DNA-binding specificities of human transcription factors. Cell 2013, 152, 327–339. [Google Scholar] [CrossRef]
- Spivakov, M.; Akhtar, J.; Kheradpour, P.; Beal, K.; Girardot, C.; Koscielny, G.; Herrero, J.; Kellis, M.; Furlong, E.E.; Birney, E. Analysis of variation at transcription factor binding sites in Drosophila and humans. Genome Biol. 2012, 13, R49. [Google Scholar] [CrossRef]
- Kasowski, M.; Grubert, F.; Heffelfinger, C.; Hariharan, M.; Asabere, A.; Waszak, S.M.; Habegger, L.; Rozowsky, J.; Shi, M.; Urban, A.E.; et al. Variation in transcription factor binding among humans. Science 2010, 328, 232–235. [Google Scholar] [CrossRef] [PubMed]
- McDaniell, R.; Lee, B.K.; Song, L.; Liu, Z.; Boyle, A.P.; Erdos, M.R.; Scott, L.J.; Morken, M.A.; Kucera, K.S.; Battenhouse, A.; et al. Heritable individual-specific and allele-specific chromatin signatures in humans. Science 2010, 328, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Van Handel, B.; Montel-Hagen, A.; Sasidharan, R.; Nakano, H.; Ferrari, R.; Boogerd, C.J.; Schredelseker, J.; Wang, Y.; Hunter, S.; Org, T.; et al. Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell 2012, 150, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Schones, D.E.; Cui, K.; Ybarra, R.; Northrup, D.; Tang, Q.; Gattinoni, L.; Restifo, N.P.; Huang, S.; Zhao, K. Regulation of nucleosome landscape and transcription factor targeting at tissue-specific enhancers by BRG1. Genome Res. 2011, 21, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Morrissey, C.S.; Keller, C.A.; Mishra, T.; Pimkin, M.; Blobel, G.A.; Weiss, M.J.; Hardison, R.C. Dynamic shifts in occupancy by TAL1 are guided by GATA factors and drive large-scale reprogramming of gene expression during hematopoiesis. Genome Res. 2014, 24, 1945–1962. [Google Scholar] [CrossRef] [PubMed]
- Grosveld, F.; Rodriguez, P.; Meier, N.; Krpic, S.; Pourfarzad, F.; Papadopoulos, P.; Kolodziej, K.; Patrinos, G.P.; Hostert, A.; Strouboulis, J. Isolation and Characterization of Hematopoietic Transcription Factor Complexes by in Vivo Biotinylation Tagging and Mass Spectrometry. Ann. N. Y. Acad. Sci. 2005, 1054, 55–67. [Google Scholar] [CrossRef]
- Li, Y.; Lou, W.; Raja, V.; Denis, S.; Yu, W.; Schmidtke, M.W.; Reynolds, C.A.; Schlame, M.; Houtkooper, R.H.; Greenberg, M.L. Cardiolipin-induced activation of pyruvate dehydrogenase links mitochondrial lipid biosynthesis to TCA cycle function. J. Biol. Chem. 2019, 294, 11568–11578. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, V.B.; Schenkel, F.S.; Chen, S.Y.; Oliveira, H.R.; Casey, T.M.; Melka, M.G.; Brito, L.F. Genomewide Association Analyses of Lactation Persistency and Milk Production Traits in Holstein Cattle Based on Imputed Whole-Genome Sequence Data. Genes 2021, 12, 1830. [Google Scholar] [CrossRef]
- Walker, C.G.; Crookenden, M.A.; Henty, K.M.; Handley, R.R.; Kuhn-Sherlock, B.; White, H.M.; Donkin, S.S.; Snell, R.G.; Meier, S.; Heiser, A.; et al. Epigenetic regulation of pyruvate carboxylase gene expression in the postpartum liver. J. Dairy Sci. 2016, 99, 5820–5827. [Google Scholar] [CrossRef]
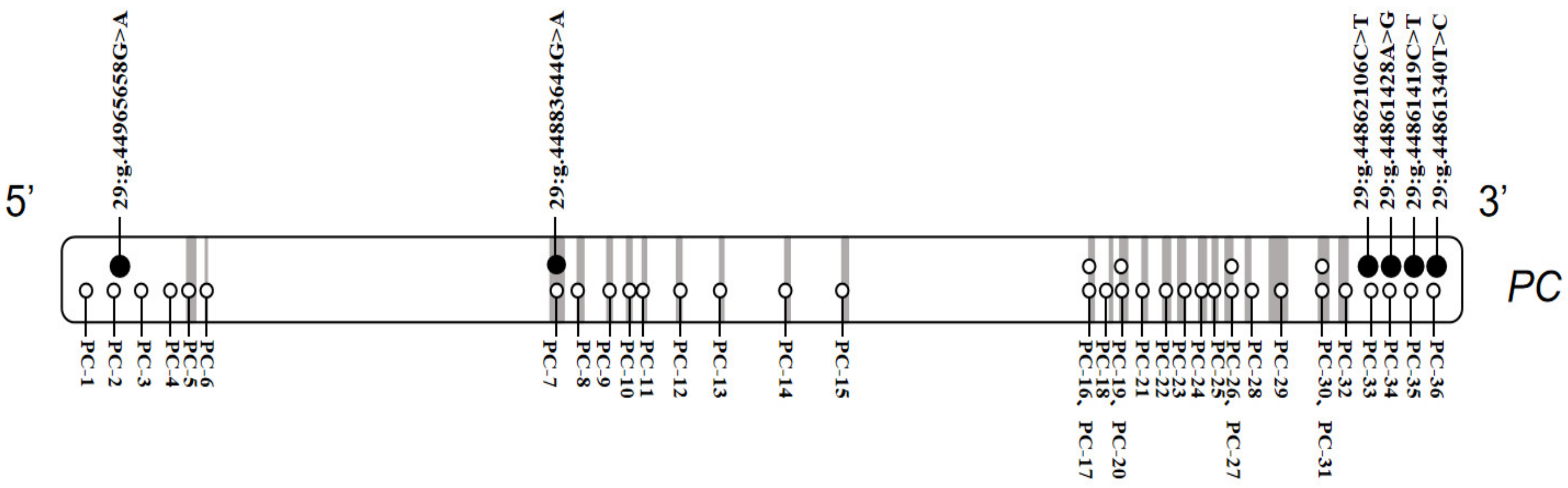
| SNP Name | GenBank No. | Location | Genotype | Genotypic Frequency | Allele | Allelic Frequency |
|---|---|---|---|---|---|---|
| 29:g.44965658G>A | rs42193753 | 5′ regulatory region | GG | 0.0908 | G | 0.3103 |
| AG | 0.4389 | A | 0.6897 | |||
| AA | 0.4703 | |||||
| 29:g.44883644G>A | rs42195007 | exon 3 | GG | 0.0303 | G | 0.1773 |
| AG | 0.2941 | A | 0.8227 | |||
| AA | 0.6757 | |||||
| 29:g.44862106C>T | rs110381742 | 3′ regulatory region | CC | 0.7038 | C | 0.8405 |
| CT | 0.2735 | T | 0.1595 | |||
| TT | 0.0227 | |||||
| 29:g.44861428A>G | rs109519857 | 3′ regulatory region | AA | 0.7038 | A | 0.8405 |
| AG | 0.2735 | G | 0.1595 | |||
| GG | 0.0227 | |||||
| 29:g.44861419C>T | rs133706500 | 3′ regulatory region | CC | 0.3838 | C | 0.6211 |
| CT | 0.4746 | T | 0.3789 | |||
| TT | 0.1416 | |||||
| 29:g.44861340T>C | rs110386158 | 3′ regulatory region | TT | 0.7049 | T | 0.8411 |
| CT | 0.2724 | C | 0.1589 | |||
| CC | 0.0227 |
| SNP Name | Lactation | Genotype (No.) | Milk Yield (kg) | Fat Yield (kg) | Fat Percentage (%) | Protein Yield (kg) | Protein Percentage (%) |
|---|---|---|---|---|---|---|---|
| 29:g.44965658G>A | 1 | GG (84) | 10,053 A ± 188.17 | 325.58 ± 7.89 | 3.26 ± 0.08 | 302.03 A ± 5.75 | 3.02 A ± 0.05 |
| AG (406) | 10,051 A ± 177.94 | 326.14 A ± 7.54 | 3.27 ± 0.07 | 297.18 B ± 5.5 | 2.97 B ± 0.05 | ||
| AA (435) | 9876.16 B ± 178.56 | 320.52 B ± 7.57 | 3.27 ± 0.07 | 293.6 C ± 5.52 | 2.99 ± 0.05 | ||
| P | 0.001 | 0.0089 | 0.8672 | 0.0005 | 0.0165 | ||
| 2 | GG (62) | 10,415 A ± 112.87 | 373.96 A ± 4.69 | 3.62 ± 0.05 | 311.14 A ± 3.42 | 3 ± 0.03 | |
| AG (245) | 10,692 B ± 70.11 | 385.1 B ± 3.04 | 3.61 ± 0.03 | 315.1 A ± 2.21 | 2.96 ± 0.02 | ||
| AA (296) | 10,792 B ± 64.48 | 384.72 B ± 2.83 | 3.58 ± 0.03 | 319.99 B ± 2.06 | 2.97 ± 0.02 | ||
| P | 0.004 | 0.0509 | 0.4316 | 0.007 | 0.3234 | ||
| 29:g.44883644G>A | 1 | GG (28) | 10,178 ± 221.89 | 339.1 A ± 9.11 | 3.35 ± 0.09 | 303.76 ± 6.64 | 3.00 ± 0.05 |
| AG (272) | 10,038 ± 179.99 | 324.44 B ± 7.62 | 3.26 ± 0.07 | 296.84 ± 5.55 | 2.97 ± 0.05 | ||
| AA (625) | 9971.64 ± 176.75 | 324.5 B ± 7.5 | 3.27 ± 0.07 | 296.95 ± 5.47 | 2.99 ± 0.05 | ||
| P | 0.1752 | 0.0146 | 0.1773 | 0.1743 | 0.1503 | ||
| 2 | GG (23) | 10,499 ± 174.8 | 357.71 A ± 7.14 | 3.44 A ± 0.07 | 315.89 ± 5.21 | 3.02 ± 0.04 | |
| AG (173) | 10,635 ± 77.47 | 378.92 B ± 3.31 | 3.58 ± 0.03 | 314.64 ± 2.41 | 2.97 ± 0.02 | ||
| AA (407) | 10,756 ± 60.82 | 387.01 C ± 2.69 | 3.61 B ± 0.07 | 318.3 ± 1.96 | 2.97 ± 0.02 | ||
| P | 0.1313 | <0.0001 | 0.0425 | 0.2506 | 0.5181 | ||
| 29:g.44862106C>T | 1 | CC (651) | 9987.86 A ± 176.71 | 324.89 A ± 7.5 | 3.27 ± 0.07 | 297.34 A ± 5.47 | 2.99 ± 0.05 |
| CT (253) | 10,016 A ± 180.39 | 323.46 A ± 7.63 | 3.25 ± 0.07 | 296.19 A ± 5.56 | 2.97 ± 0.05 | ||
| TT (21) | 10,337 B ± 234.65 | 339.82 B ± 9.57 | 3.30 ± 0.09 | 307.37 B ± 6.98 | 2.99 ± 0.06 | ||
| P | 0.0705 | 0.0209 | 0.4895 | 0.0331 | 0.2059 | ||
| 2 | CC (427) | 10,781 A ± 60.26 | 387.89 A ± 2.67 | 3.61 A ± 0.02 | 318.81 A ± 1.95 | 2.97 ± 0.02 | |
| CT (159) | 10,548 B ± 80.12 | 375.88 B ± 3.41 | 3.58 A ± 0.03 | 313.44 B ± 2.48 | 2.98 ± 0.02 | ||
| TT (17) | 10,495 ± 203.88 | 353.35C ± 8.29 | 3.4 B ± 0.08 | 311.29 ± 6.05 | 2.98 ± 0.05 | ||
| P | 0.0064 | <0.0001 | 0.0314 | 0.0402 | 0.7269 | ||
| 29:g.44861428A>G | 1 | AA (651) | 9983.94 A ± 176.7 | 324.84 A ± 7.5 | 3.27 ± 0.07 | 297.21 A ± 5.47 | 2.99 ± 0.05 |
| AG (253) | 10,028 A ± 180.4 | 323.62 A ± 7.63 | 3.25 ± 0.07 | 296.56 A ± 5.56 | 2.97 ± 0.05 | ||
| GG (21) | 10,339 B ± 234.65 | 339.86 B ± 9.57 | 3.3 ± 0.09 | 307.45 B ± 6.98 | 2.99 ± 0.06 | ||
| P | 0.058 | 0.0224 | 0.4197 | 0.0407 | 0.2204 | ||
| 2 | AA (428) | 10,775 ± 60.22 A | 387.92 ± 2.67 A | 3.61 ± 0.02 A | 318.67 ± 1.94 A | 2.97 ± 0.02 | |
| AG (158) | 10,562 ± 80.28 B | 375.71 ± 3.41 B | 3.57 ± 0.03 A | 313.78 ± 2.49 B | 2.98 ± 0.02 | ||
| GG (17) | 10,496 ± 203.89 | 353.29 ± 8.29 C | 3.4 ± 0.08 B | 311.32 ± 6.05 | 2.98 ± 0.05 | ||
| P | 0.0137 | <0.0001 | 0.0225 | 0.0647 | 0.7735 | ||
| 29:g.44861419C>T | 1 | CC (355) | 9898.04 A ± 179.13 | 322.33 ± 7.59 | 3.28 ± 0.07 | 294.45 A ± 5.53 | 2.99 ± 0.05 |
| CT (439) | 10,002 B ± 177.59 | 324.18 ± 7.53 | 3.27 ± 0.07 | 296.72 ± 5.49 | 2.98 ± 0.05 | ||
| TT (131) | 10,089 B ± 183.81 | 326.08 ± 7.74 | 3.25 ± 0.07 | 300.11 B ± 5.64 | 2.99 ± 0.05 | ||
| P | 0.0168 | 0.3576 | 0.6528 | 0.0166 | 0.7129 | ||
| 2 | CC (245) | 10,786 A ± 68.41 | 385.42 ± 2.97 | 3.59 ± 0.03 | 319.68 A ± 2.17 | 2.97 ± 0.02 | |
| CT (273) | 10,697 ± 67.39 | 382.81 ± 2.94 | 3.59 ± 0.03 | 316.81 ± 2.14 | 2.97 ± 0.02 | ||
| TT (85) | 10,553 B ± 97.13 | 381 ± 4.07 | 3.62 ± 0.04 | 311.51 B ± 2.96 | 2.96 ± 0.02 | ||
| P | 0.0623 | 0.4761 | 0.7483 | 0.0217 | 0.8088 | ||
| 29:g.44861340T>C | 1 | TT (652) | 9986.08 A ± 176.7 | 324.9 A ± 7.5 | 3.27 ± 0.07 | 297.3 A ± 5.47 | 2.99 ± 0.05 |
| CT (252) | 10,022 A ± 180.43 | 323.43 A ± 7.63 | 3.25 ± 0.07 | 296.31 A ± 5.56 | 2.97 ± 0.05 | ||
| CC (21) | 10,338 B ± 234.65 | 339.82 B ± 9.57 | 3.3 ± 0.09 | 307.39 B ± 6.98 | 2.99 ± 0.06 | ||
| P | 0.0652 | 0.0207 | 0.422 | 0.0359 | 0.1949 | ||
| 2 | TT (428) | 10,775 A ± 60.22 | 387.92 A ± 2.67 | 3.61 A ± 0.02 | 318.67 A ± 1.94 | 2.97 ± 0.02 | |
| CT (158) | 10,562 B ± 80.28 | 375.71 B ± 3.41 | 3.57 A ± 0.03 | 313.78 B ± 2.49 | 2.98 ± 0.02 | ||
| CC (17) | 10,496 ± 203.89 | 353.29 C ± 8.29 | 3.4 B ± 0.08 | 311.32 ± 6.05 | 2.98 ± 0.05 | ||
| P | 0.0137 | <0.0001 | 0.0225 | 0.0647 | 0.7735 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, A.; Guo, Z.; Chen, A.; Xu, L.; Sun, D.; Han, B. PC Gene Affects Milk Production Traits in Dairy Cattle. Genes 2024, 15, 708. https://doi.org/10.3390/genes15060708
Du A, Guo Z, Chen A, Xu L, Sun D, Han B. PC Gene Affects Milk Production Traits in Dairy Cattle. Genes. 2024; 15(6):708. https://doi.org/10.3390/genes15060708
Chicago/Turabian StyleDu, Aixia, Zijiao Guo, Ao Chen, Lingna Xu, Dongxiao Sun, and Bo Han. 2024. "PC Gene Affects Milk Production Traits in Dairy Cattle" Genes 15, no. 6: 708. https://doi.org/10.3390/genes15060708





