Transcriptional Comparison Reveals Differential Resistance Mechanisms between CMV-Resistant PBC688 and CMV-Susceptible G29
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Virus Strains and Inoculation Method
2.3. Absolute Quantification of CMVs Copy Number
2.3.1. Sampling
2.3.2. RNA Extraction and cDNA Preparation
2.3.3. Plasmid Standard Preparation
2.3.4. Calculation of the CMV Copy Number in Samples
2.4. cDNA Library Construction and Sequencing
2.5. RNA-Seq Data Analysis
2.5.1. Quality Control
2.5.2. Reads Mapping to the Reference Genome
2.5.3. Novel Transcript Prediction
2.5.4. Quantification of Gene Expression Levels
2.5.5. Differential Expression Analysis
2.5.6. GO and KEGG Enrichment Analysis of Differentially Expressed Genes
2.5.7. Heatmap Analysis of Differentially Expressed Genes
3. Results
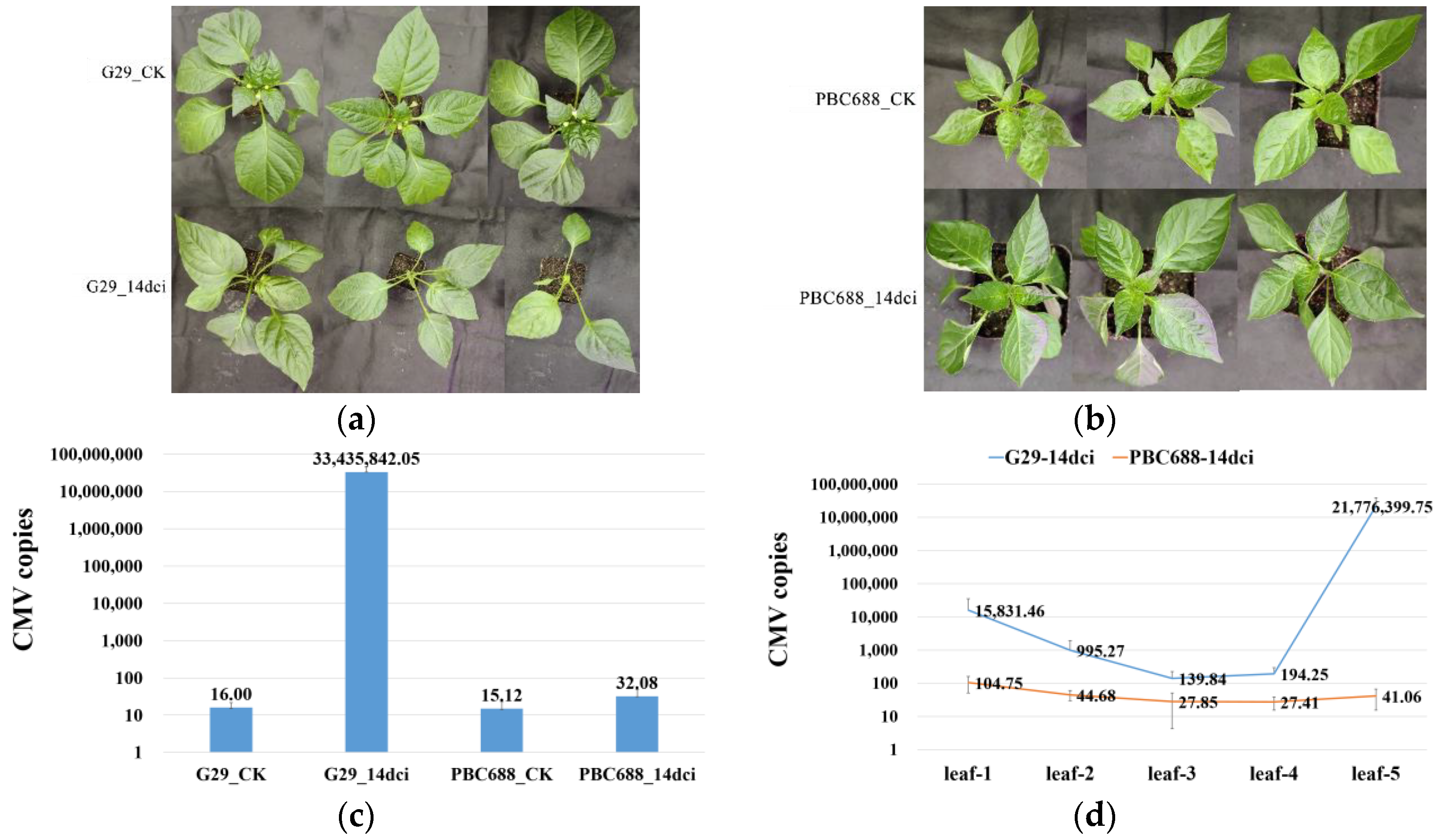
3.1. Absolute Quantification of CMV Copies in CMV-Susceptible G29 and CMV-Resistant PBC688
3.2. Transcription Profile of CMV-Susceptible G29 and CMV-Resistant PBC688
3.3. Transcriptional Response of CMV-Susceptible G29 and CMV-Resistant PBC688
3.4. GO Annotation and Enrichment Analysis of DEGs
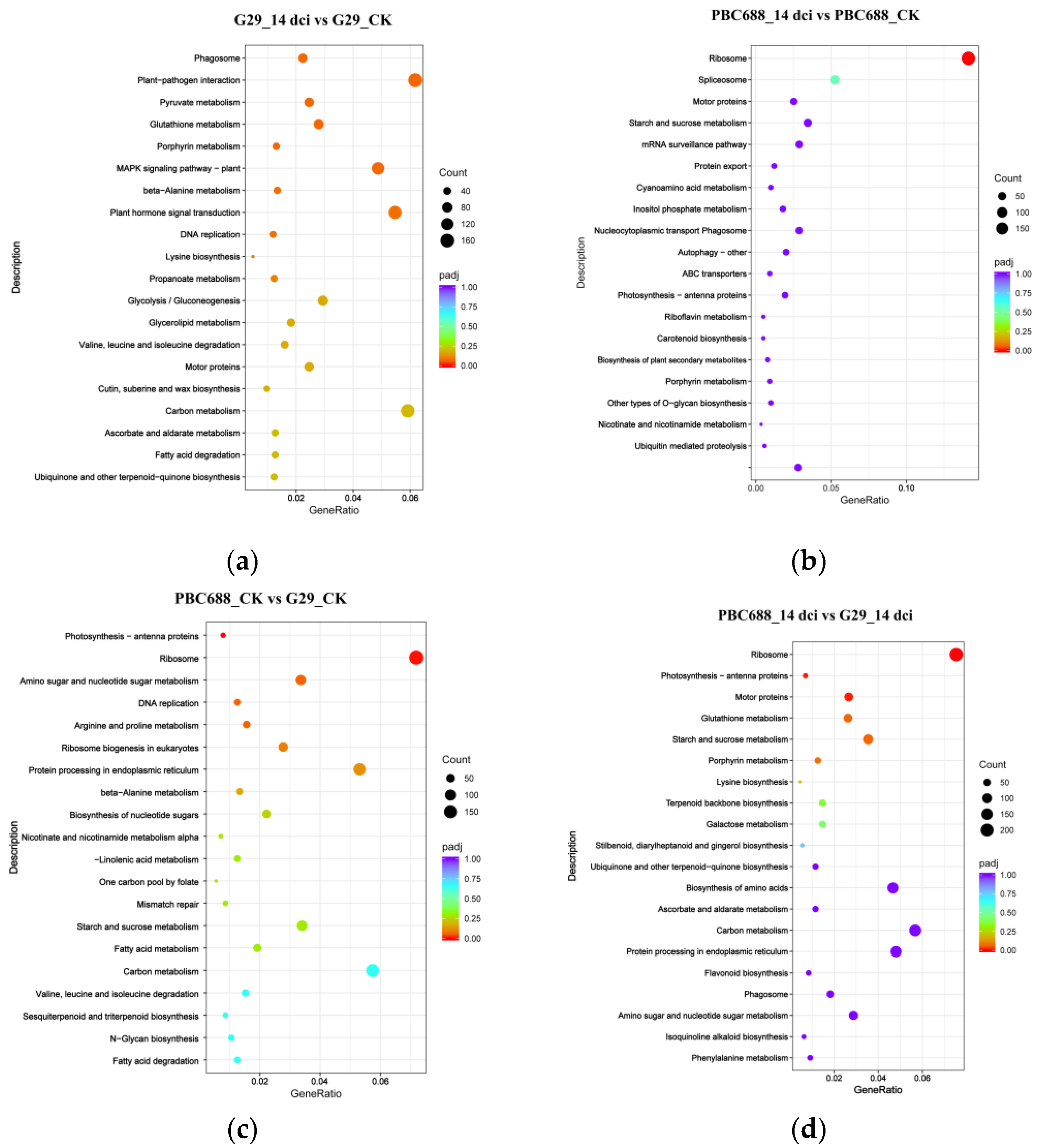
3.5. KEGG Functional Annotations of DEGs
3.6. Annotation of DEGs in Response to CMV Infection of G29
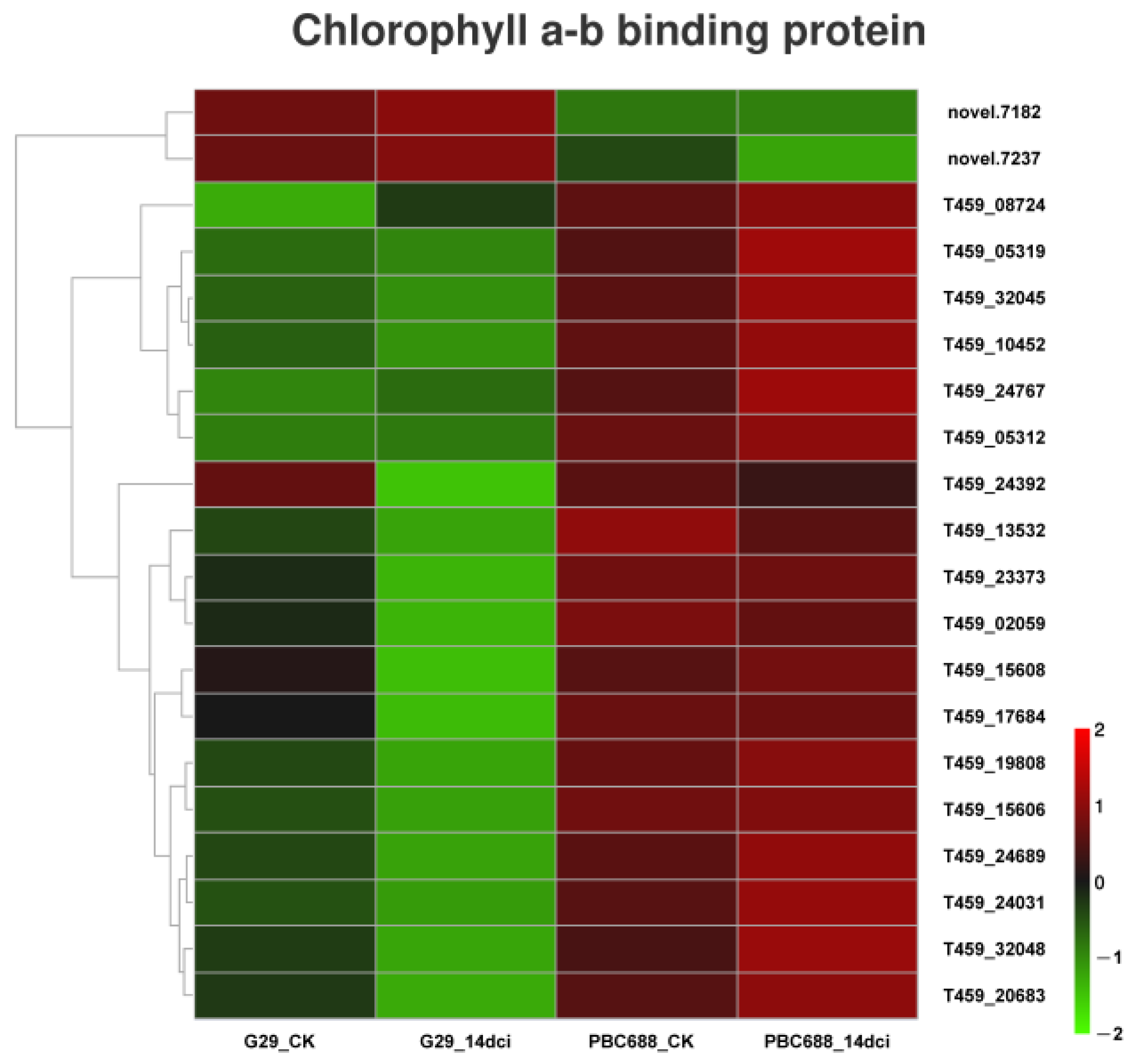
3.7. Annotation of DEGs in Response to CMV Infection of PBC688
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palukaitis, P.; Roossinck, M.J. Cucumber mosaic virus. Adv. Virus Res. 1992, 41, 281–348. [Google Scholar] [CrossRef] [PubMed]
- Jacquemond, M. Cucumber mosaic virus. Adv. Virus Res. 2012, 84, 439–504. [Google Scholar] [CrossRef] [PubMed]
- Chaim, A.B.; Grube, R.; Lapidot, M.; Jahn, M.; Paran, I. Identification of quantitative trait loci associated with resistance to cucumber mosaic virus in Capsicum annuum. Theor. Appl. Genet 2001, 102, 1213–1220. [Google Scholar] [CrossRef]
- Kang, W.H.; Hoang, N.H.; Yang, H.-B.; Kwon, J.-K.; Jo, S.H.; Seo, J.K.; Kim, K.H.; Choi, D.; Kang, B.C. Molecular mapping and characterization of a single dominant gene controlling CMV resistance in peppers (Capsicum annuum L.). Theor. Appl. Genet 2010, 120, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Li, N.; Wang, F.; Ye, Z. Genetic analysis and identification of QTLs for resistance to cucumber mosaic virus in chili pepper (Capsicum annuum L.). Euphytica 2013, 193, 135–145. [Google Scholar] [CrossRef]
- Choi, S.; Lee, J.H.; Kang, W.H.; Kim, J.; Kang, B.C. Identification of Cucumber mosaic resistance 2 (cmr2) that confers resistance to a new Cucumber mosaic virus isolate P1 (CMV-P1) in Pepper (Capsicum spp.). Front. Plant Sci. 2018, 9, 1106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.H.; Li, X.F.; Zheng, J.Y. Transcriptome profiling using Illumina- and SMRT-based RNA-seq of hot pepper for in-depth understanding of genes involved in CMV infection. Gene 2018, 666, 123–133. [Google Scholar] [CrossRef]
- Caranta, C.; Pflieger, S.; Lefebvre, V.; Daubeze, A.; Thabuis, A.; Palloix, A. QTLs involved in the restriction of cucumber mosaic virus (CMV) long-distance movement in pepper. Theor. Appl. Genet. 2002, 104, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kuroda, T.; Miura, Y.; Murai, J. Screening and field trials of virus resistant sources in Capsicum spp. Plant Dis. 2003, 87, 779–783. [Google Scholar] [CrossRef]
- Grube, R.C.; Zhang, Y.; Murphy, J.F.; Loaiza-Figueroa, F.; Lackney, V.K.; Provvidenti, R.; Jahn, M.K. New source of resistance to Cucumber mosaic virus in Capsicum frutescens. Plant Dis. 2000, 84, 885–891. [Google Scholar] [CrossRef]
- Guo, G.J.; Wang, S.B.; Liu, J.B.; Pan, B.G.; Diao, W.P.; Ge, W.; Gao, C.Z.; Snyder, J.C. Rapid identification of QTLs underlying resistance to Cucumber mosaic virus in pepper (Capsicum frutescens). Theor. Appl. Genet. 2017, 130, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Palukaitis, P. Satellite RNAs and satellite viruses. Mol. Plant-Microbe Interact. 2016, 29, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Caranta, C.; Palloix, A.; Lefebvre, V.; Daubeze, A. QTLs for a component of partial resistance to cucumber mosaic virus in pepper: Restriction of virus installation in host-cells. Theor. Appl. Genet. 1997, 94, 431–438. [Google Scholar] [CrossRef]
- Nono-Womdim, R.; Gebre-Selassie, K.; Palloix, A.; Pochard, E.; Marchoux, G. Study of multiplication of cucumber mosaic virus in susceptible and resistant Capsicum annuum lines. J. Ann. Appl. Biol. 1993, 122, 49–56. [Google Scholar] [CrossRef]
- Dufour, O.; Palloix, A.; Selassie, K.G.; Pochard, E.; Marchoux, G. The distribution of cucumber mosaic virus in resistant and susceptible plants of pepper. Can. J. Bot. 1989, 67, 655–660. [Google Scholar] [CrossRef]
- Takahashi, H.; Suzuki, M.; Natsuaki, K.; Shigyo, T.; Hino, K.; Teraoka, T.; Hosokawa, D.; Ehara, Y. Mapping the virus and host genes involved in the resistance response in cucumber mosaic virus-infected Arabidopsis thaliana. Plant Cell Physiol. 2001, 42, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Min, H.E.; Han, J.H.; Yoon, J.B.; Lee, J. QTL mapping of resistance to the Cucumber mosaic virus P1 strain in pepper using a genotyping-by-sequencing analysis. Hortic. Environ. Biote. 2016, 57, 589–597. [Google Scholar] [CrossRef]
- Kang, W.-H.; Seo, J.-K.; Chung, B.N.; Kim, K.-H.; Kang, B.-C. Helicase domain encoded by cucumber mosaic virus RNA1 determines systemic infection of Cmr1 in pepper. PLoS ONE 2012, 7, e43136. [Google Scholar] [CrossRef]
- Rubiales, D.; Fondevilla, S.; Chen, W.; Gentzbittel, L.; Higgins, T.J.V.; Castillejo, M.A.; Singh, K.B.; Rispail, N. Achievements and challenges in legume breeding for pest and disease resistance. Crit. Rev. Plant Sci. 2015, 34, 195–236. [Google Scholar] [CrossRef]
- Lu, J.; Du, Z.X.; Kong, J.; Chen, L.N.; Qiu, Y.H.; Li, G.F.; Meng, X.H.; Zhu, S.F. Transcriptome analysis of Nicotiana tabacum infected by cucumber mosaic virus during systemic symptom development. PLoS ONE 2012, 7, e43447. [Google Scholar] [CrossRef]
- Liu, D.; Gong, M.; Zhao, Q.; Jiang, C.H.; Cheng, L.R.; Ren, M.; Wang, Y.Y. Comparative transcriptome analysis reveals differential gene expression in resistant and susceptible tobacco cultivars in response to infection by cucumber mosaic virus. Crop. J. 2019, 7, 307–321. [Google Scholar] [CrossRef]
- Marathe, R.; Guan, Z.; Anandalakshmi, R.; Zhao, H.; Dinesh-Kumar, S. Study of Arabidopsis thalianaresistome in response to cucumber mosaic virus infection using whole genome microarray. Plant Mol. Biol. 2004, 55, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jo, Y.; Lian, S.; Jo, K.M.; Chu, H.; Yoon, J.Y.; Choi, S.K.; Kim, K.H.; Cho, W.K. Comparative analysis of chrysanthemum transcriptome in response to three RNA viruses: Cucumber mosaic virus, Tomato spotted wilt virus and Potato virus X. Plant Mol. Biol. 2015, 88, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G.O. Gene Ontology Consortium: Going forward. Nucleic Acids Res. 2015, 4343, D1049–D1056. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Nono-Womdim, R.; Marchoux, G.; Pochard, E.; Palloix, A.; Gebre-Selassie, K. Resistance of pepper lines to the movement of cucumber mosaic virus. J. Phytopathol. 1991, 132, 21–32. [Google Scholar] [CrossRef]
- Ouedraogo, R.S.; Pita, J.S.; Somda, I.P.; Traore, O.; Roossinck, M.J. Impact of Cultivated Hosts on the Recombination of Cucumber Mosaic Virus. J. Virol. 2019, 93, e01770-18. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qu, X.; Dong, Z.; Luo, L.; Shao, C.; Forner, J.; Lohmann, J.U.; Su, M.; Xu, M.; Liu, X.; et al. WUSCHEL triggers innate antiviral immunity in plant stem cells. Science 2020, 370, 227–231. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Aerts, N.; Mendes, P.M.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Abscisic acid is involved in chitosan-induced resistance to tobacco necrosis virus (TNV). Plant Physiol. Biochem. 2008, 46, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, K.Y.; Lin, N.S. The abscisic acid pathway has multifaceted effects on the accumulation of Bamboo mosaic virus. Mol. Plant Microbe Interact. 2014, 27, 177–189. [Google Scholar] [CrossRef]
- He, L.; Jin, P.; Chen, X.; Zhang, T.Y.; Zhong, K.L.; Liu, P.; Chen, J.P.; Yang, J. Comparative proteomic analysis of Nicotiana benthamiana plants under Chinese wheat mosaic virus infection. BMC Plant Biol. 2021, 21, 51. [Google Scholar] [CrossRef]
- Xie, K.; Li, L.; Zhang, H.; Wang, R.; Tan, X.; He, Y.; Hong, G.; Li, J.; Ming, F.; Yao, X.; et al. Abscisic acid negatively modulates plant defense against rice black-streaked dwarf virus infection by suppressing the jasmonate pathway and regulating reactive oxygen species levels in rice. Plant Cell Environ. 2018, 41, 2504–2514. [Google Scholar] [CrossRef]
- Wool, I.G.; Chan, Y.L.; Glück, A. Structure and evolution of mammalian ribosomal proteins. Biochem. Cell Biol. 1995, 73, 933–947. [Google Scholar] [CrossRef]
- Wang, B.; Song, N.; Tang, C.; Ma, J.; Wang, N.; Sun, Y.; Kang, Z. PsRPs26, a 40S Ribosomal protein subunit, regulates the growth and pathogenicity of Puccinia striiformis f. sp. Tritici. Front. Microbiol. 2019, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.S.; Senthil-Kumar, M.; Ramu, V.S.; Wang, K.; Mysore, K.S. Plant ribosomal proteins, RPL12 and RPL19, play a role in non-host disease resistance against bacterial pathogens. Front. Plant Sci. 2016, 6, 1192. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Yang, Z.; Wang, X.; Butt, H.I.; Chen, E.; He, S.; Zhang, C.; Zhang, X.; Li, F. Salicylic acid-related cotton (Gossypium arboreum) ribosomal protein GaRPL18 contributes to resistance to Verticillium dahliae. BMC Plant Biol. 2017, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Du, Z.; Bai, Z.; Liang, Z. The interaction between endogenous 30S ribosomal subunit protein S11 and Cucumber mosaic virus LS2b protein affects viral replication, infection and gene silencing suppressor activity. PLoS ONE 2017, 12, e0182459. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Zhou, Y. Ribosomal protein L18 is an essential factor that promote rice stripe virus accumulation in small brown planthopper. Virus Res. 2018, 247, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.J.; Xu, X.J.; Yan, Z.Y.; Tettey, C.K.; Fang, L.; Yang, G.L.; Geng, C.; Tian, Y.P.; Li, X.D. The chloroplast ribosomal protein large subunit 1 interacts with viral polymerase and promotes virus infection. Plant Physiol. 2021, 187, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; El-Sappah, A.H.; Ali, H.M.; Zandi, P.; Huang, Q.; Soaud, S.A.; Alazizi, E.M.Y.; Wafa, H.A.; Hossain, M.A.; Liang, Y. Pathogenesis-related proteins (PRs) countering environmental stress in plants: A review. S. Afr. J. Bot 2023, 160, 414–427. [Google Scholar] [CrossRef]
- Xu, Y.H.; Liu, R.; Yan, L.; Liu, Z.Q.; Jiang, S.C.; Shen, Y.Y.; Wang, X.F.; Zhang, D.P. Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis. J. Exp. Bot. 2012, 63, 1095–1106. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.H.; Jiang, S.C.; Lu, K.; Lu, Y.F.; Feng, X.J.; Wu, Z.; Liang, S.; Yu, Y.T.; Wang, X.F.; et al. Light-harvesting chlorophyll a/b-binding proteins, positively involved in abscisic acid signalling, require a transcription repressor, WRKY40, to balance their function. J. Exp. Bot. 2013, 64, 5443–5456. [Google Scholar] [CrossRef]
- Deng, Y.-S.; Kong, F.Y.; Zhou, B.; Zhang, S.; Yue, M.M.; Meng, Q.W. Heterology expression of the tomato LeLhcb2 gene confers elevated tolerance to chilling stress in transgenic tobacco. Plant Physiol. Biochem. 2014, 80, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant 2016, 158, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Zhang, C.M.; Su, Y.Q.; Ma, J.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. Environ. Exp. Bot. 2017, 135, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Ma, J.; Wu, N.; Su, Y.Q.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Zeng, X.Y.; Yuan, S. The roles of Arabidopsis proteins of Lhcb4, Lhcb5 and Lhcb6 in oxidative stress under natural light conditions. Plant Physiol. Biochem. 2018, 130, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, H.; Luo, J.; Wang, H.; Dong, Q.; Wang, Y.; Yang, K.; Mao, K.; Ma, F. Genome-wide analysis of the light-harvesting chlorophyll a/b-binding gene family in apple (Malus domestica) and functional characterization of MdLhcb4.3, which confers tolerance to drought and osmotic stress. Plant Physiol. Biochem. 2020, 154, 517–529. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, C.; Xiang, B.; Niu, J.; Zheng, Y. Processing tomato chlorophyll a/b-binding protein 1C interacts with CMV 2b protein. Physiol. Mol. Plant P 2022, 120, 101857. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Pan, B.; Gong, C.; Wang, S.; Liu, J.; Gao, C.; Diao, W. Transcriptional Comparison Reveals Differential Resistance Mechanisms between CMV-Resistant PBC688 and CMV-Susceptible G29. Genes 2024, 15, 731. https://doi.org/10.3390/genes15060731
Guo G, Pan B, Gong C, Wang S, Liu J, Gao C, Diao W. Transcriptional Comparison Reveals Differential Resistance Mechanisms between CMV-Resistant PBC688 and CMV-Susceptible G29. Genes. 2024; 15(6):731. https://doi.org/10.3390/genes15060731
Chicago/Turabian StyleGuo, Guangjun, Baogui Pan, Chengsheng Gong, Shubin Wang, Jinbing Liu, Changzhou Gao, and Weiping Diao. 2024. "Transcriptional Comparison Reveals Differential Resistance Mechanisms between CMV-Resistant PBC688 and CMV-Susceptible G29" Genes 15, no. 6: 731. https://doi.org/10.3390/genes15060731




