Hope on the Horizon? Aptamers in Diagnosis of Invasive Fungal Infections
Abstract
:1. Introduction
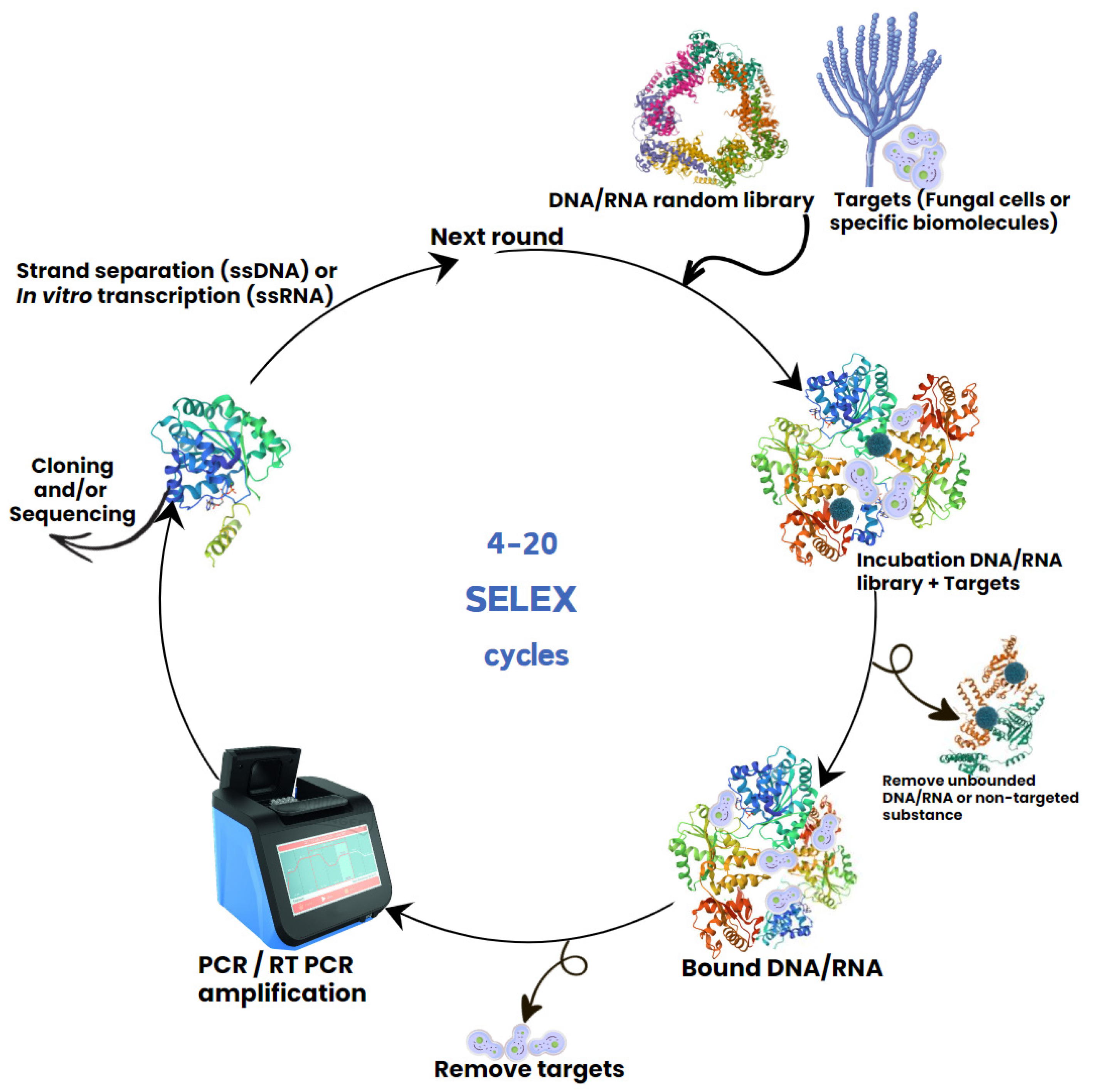
2. Aptamer and SELEX
3. Aptamers’ Applications in Fungal Infections
| Targets | Target Molecules | Aptamer Type/Name | Sequence of the Aptamer (5′ to 3′) | Secondary Structures of the Aptamer | Testing Platform | Specificity (%) | Sensitivity (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Candida albicans | (1→3)-β-D-glucans | dsDNA/AU1 and AD1 | GCGGAATTCGAACAGTCCGAGCC-N60-GGGTCAATGCGTCATA |  | ELONA | 91.94 | 92.31 | Tang et al. [8] |
| Candida albicans | (1→3)-β-D-glucans | dsDNA/A1, A4, A5, and A6 | TCTAGAATCCCAATCCCAATCCCA-50N-ACCCTAAAGCTT | 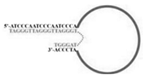 | AGSCS | 89.65 | 92.68 | Hua et al. [19] |
| Candida albicans | (1→3)-β-D-glucans | dsDNA/AD1 | GCGGAATTCGAACAGTCCGAGCCCACACGTGTGAGAAGGGTGTTATCATGTATTTCGTGTTCCTTTCGTCATTCCTTTGTCTGGGGTCAATGCGTCATAGGATCCCGCAAAAAAAAAA | 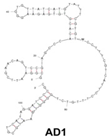 | Aptamer binding to gold nanoparticles | - | - | Clack et al. [20] |
| A. fumigatus and C. albicans | Whole cell | DNA | Multiple aptamers | 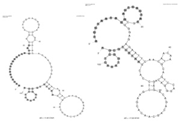 | Fluorescence microscopy | - | - | Milnes [22] |
| A. fumigatus, A. flavus, and A. niger | Spores | ssDNA/Asp-3 | CGTTTGGGCGGTATGAGTTCGGGGGTATACCGCAG | 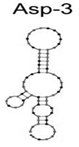 | Cell-SELEX | - | - | Woo Seo et al. [23] |
| Candida species | Various | ssDNA | Polyclonal SELEX aptamer library | - | Flow-cytometry, Fluorometric microtiter plate assays, Fluorescence microscopy | - | - | Kneißle et al. [21] |
| Azole class antifungal drugs | Posaconazole | DNA/Rd 13, Rd 9 | CGGGGGAGGCGGAGGGAGGGAGGACTGGGGCTTCATTGACGTTCTTCACAGTAGGGGTAAGGGCTTAGGTGGTTGGTGCCTG CGCGGGGAGGAGGAGGCAGACTGGGGCTTCTTTGACGTTC |  | GFET | - | - | Wiedman et al. [30] |
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seagle, E.E.; Williams, S.L.; Chiller, T.M. Recent trends in the epidemiology of fungal infections. Infect. Dis. Clin. 2021, 35, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Ahmadikia, K.; Badali, H.; Khodavaisy, S. Opportunistic fungal infections in the epidemic area of COVID-19: A clinical and diagnostic perspective from Iran. Mycopathologia 2020, 185, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Terrero-Salcedo, D.; Powers-Fletcher, M.V. Updates in laboratory diagnostics for invasive fungal infections. J. Clin. Microbiol. 2020, 58, e01487-19. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, A.; Santos, H.; Franco-Duarte, R.; Sampaio, P. Fungal infections diagnosis–past, present and future. Res. Microbiol. 2022, 173, 103915. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Bashardoust, B.; Abdorahimi, M.; Aminizadeh, S.; Salehi, M.; Khodavaisy, S. Emerging Challenges in Diagnosis and Treatment of Invasive Fungal Infections: Addressing the Impact of COVID-19 and New Pathogens. Curr. Fungal Infect. Rep. 2023, 17, 296–308. [Google Scholar] [CrossRef]
- Yang, S.; Rothman, R.E. PCR-based diagnostics for infectious diseases: Uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 2004, 4, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Almirante, B.; Rodríguez, J.G.; Garnacho-Montero, J.; Salavert, M.; Muñoz, P.; Sanguinetti, M. Biomarkers of fungal infection: Expert opinion on the current situation. Rev. Esp. De Quimioter. 2020, 33, 1. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-L.; Hua, Y.; Guan, Q.; Yuan, C.-H. Improved detection of deeply invasive candidiasis with DNA aptamers specific binding to (1→ 3)-β-D-glucans from Candida albicans. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Byun, J. Recent progress and opportunities for nucleic acid aptamers. Life 2021, 11, 193. [Google Scholar] [CrossRef]
- Banerjee, S.; Nilsen-Hamilton, M. Aptamers for infectious disease diagnosis. In E. Coli Infections-Importance of Early Diagnosis and Efficient Treatment; IntechOpen: London, UK, 2019; pp. 1–23. [Google Scholar]
- Deng, B.; Lin, Y.; Wang, C.; Li, F.; Wang, Z.; Zhang, H.; Li, X.-F.; Le, X.C. Aptamer binding assays for proteins: The thrombin example—A review. Anal. Chim. Acta 2014, 837, 1–15. [Google Scholar] [CrossRef]
- Huang, L.; Tian, S.; Zhao, W.; Liu, K.; Ma, X.; Guo, J. Aptamer-based lateral flow assay on-site biosensors. Biosens. Bioelectron. 2021, 186, 113279. [Google Scholar] [CrossRef] [PubMed]
- Davydova, A.; Vorobjeva, M.; Pyshnyi, D.; Altman, S.; Vlassov, V.; Venyaminova, A. Aptamers against pathogenic microorganisms. Crit. Rev. Microbiol. 2016, 42, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, X.; Fu, X.; Li, Z.; Huang, Y.; Liang, C. Advances in aptamer-based biomarker discovery. Front. Cell Dev. Biol. 2021, 9, 659760. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; de Jesus Santos, A.P.; de Sá, V.; Ulrich, H.; Wrenger, C. Aptamer applications in emerging viral diseases. Pharmaceuticals 2021, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Liu, R.; Amini, R.; Salena, B.J.; Li, Y. Discovery and translation of functional nucleic acids for clinically diagnosing infectious diseases: Opportunities and challenges. TrAC Trends Anal. Chem. 2023, 158, 116886. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Pourhajibagher, M.; Raoofian, R.; Tabarzad, M.; Bahador, A. Therapeutic applications of nucleic acid aptamers in microbial infections. J. Biomed. Sci. 2020, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Hu, F.; Ren, X.; Xiong, Y.; Hu, J.; Su, F.; Tang, X.; Wen, Y. A novel aptamer-G-quadruplex/hemin self-assembling color system: Rapid visual diagnosis of invasive fungal infections. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Clack, K.; Sallam, M.; Muyldermans, S.; Sambasivam, P.; Nguyen, C.M.; Nguyen, N.-T. Instant Candida albicans Detection Using Ultra-Stable Aptamer Conjugated Gold Nanoparticles. Micromachines 2024, 15, 216. [Google Scholar] [CrossRef]
- Kneißle, K.; Krämer, M.; Kissmann, A.-K.; Xing, H.; Müller, F.; Amann, V.; Noschka, R.; Gottschalk, K.-E.; Bozdogan, A.; Andersson, J. A Polyclonal SELEX Aptamer Library Allows Differentiation of Candida albicans, C. auris and C. parapsilosis Cells from Human Dermal Fibroblasts. J. Fungi 2022, 8, 856. [Google Scholar] [CrossRef]
- Milnes, B.E. Identification and Validation of Novel Aptamers against Fungal Cells. Master’s Thesis, University of Central Lancashire, Preston, UK, 2019. [Google Scholar]
- Seo, J.-W.; Kim, J.Y.; Oh, J.-J.; Kim, Y.J.; Kim, G.-H. Selection and characterization of toxic Aspergillus spore-specific DNA aptamer using spore-SELEX. RSC Adv. 2021, 11, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, J. Associations between genomic variants and antifungal susceptibilities in the archived global Candida auris population. J. Fungi 2024, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Assessing global fungal threats to humans. mLife 2022, 1, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Santana, D.J.; Anku, J.A.; Zhao, G.; Zarnowski, R.; Johnson, C.J.; Hautau, H.; Visser, N.D.; Ibrahim, A.S.; Andes, D.; Nett, J.E. A Candida auris–specific adhesin, Scf1, governs surface association, colonization, and virulence. Science 2023, 381, 1461–1467. [Google Scholar] [CrossRef]
- Bukkems, L.M.; van Dommelen, L.; Regis, M.; van den Heuvel, E.; Nieuwenhuizen, L. The Use of Galactomannan Antigen Assays for the Diagnosis of Invasive Pulmonary Aspergillosis in the Hematological Patient: A Systematic Review and Meta-Analysis. J. Fungi 2023, 9, 674. [Google Scholar] [CrossRef]
- Bruch, A.; Kelani, A.A.; Blango, M.G. RNA-based therapeutics to treat human fungal infections. Trends Microbiol. 2022, 30, 411–420. [Google Scholar] [CrossRef]
- Wiedman, G.R.; Zhao, Y.; Mustaev, A.; Ping, J.; Vishnubhotla, R.; Johnson, A.C.; Perlin, D.S. An aptamer-based biosensor for the azole class of antifungal drugs. mSphere 2017, 2, e00274-17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodavaisy, S.; Xu, J. Hope on the Horizon? Aptamers in Diagnosis of Invasive Fungal Infections. Genes 2024, 15, 733. https://doi.org/10.3390/genes15060733
Khodavaisy S, Xu J. Hope on the Horizon? Aptamers in Diagnosis of Invasive Fungal Infections. Genes. 2024; 15(6):733. https://doi.org/10.3390/genes15060733
Chicago/Turabian StyleKhodavaisy, Sadegh, and Jianping Xu. 2024. "Hope on the Horizon? Aptamers in Diagnosis of Invasive Fungal Infections" Genes 15, no. 6: 733. https://doi.org/10.3390/genes15060733








