Genetic Differentiation of Reed Canarygrass (Phalaris arundinacea L.) within Eastern Europe and Eurasia
Abstract
:1. Introduction
2. Results
2.1. Genetic Diversity of Populations of P. arundinacea
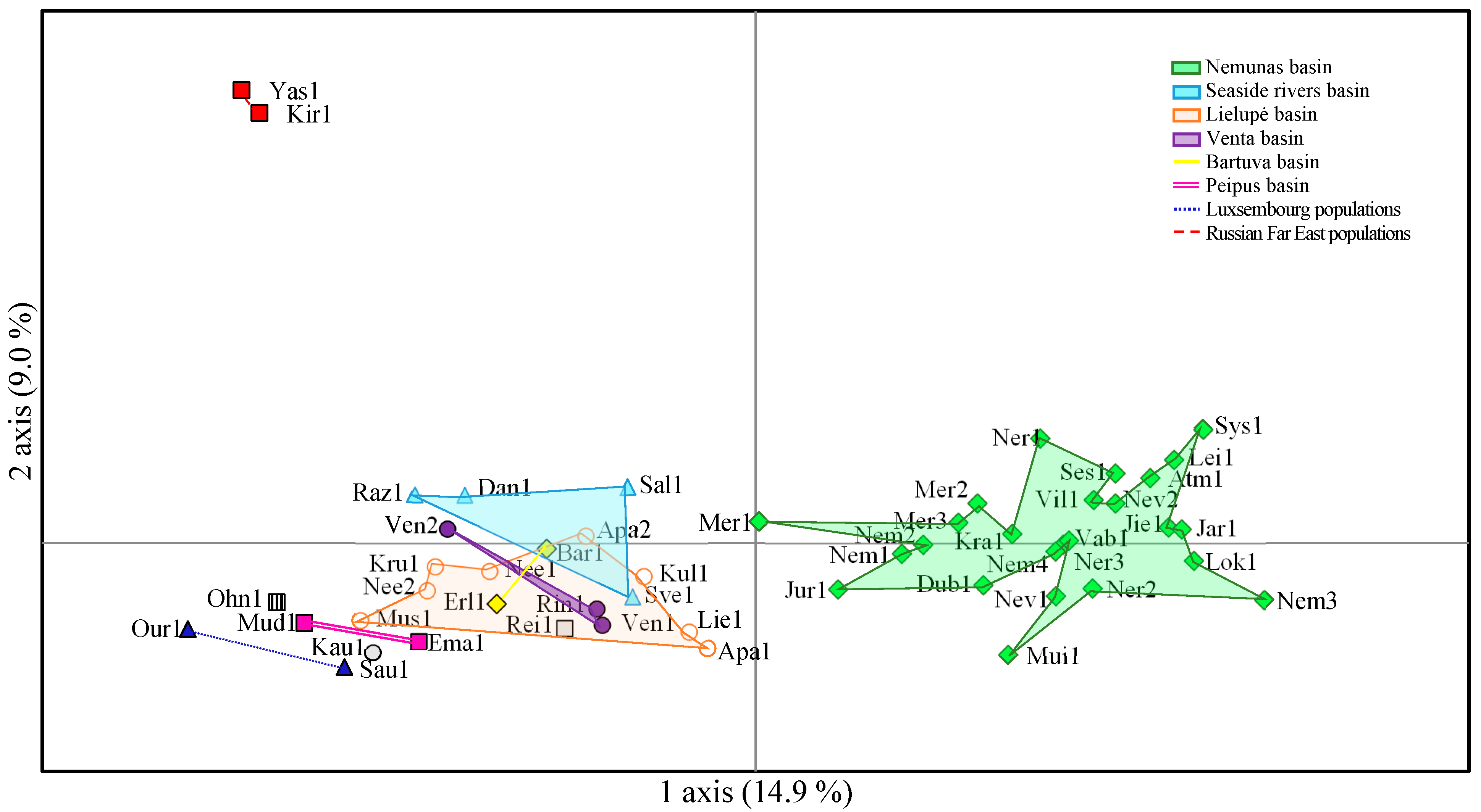
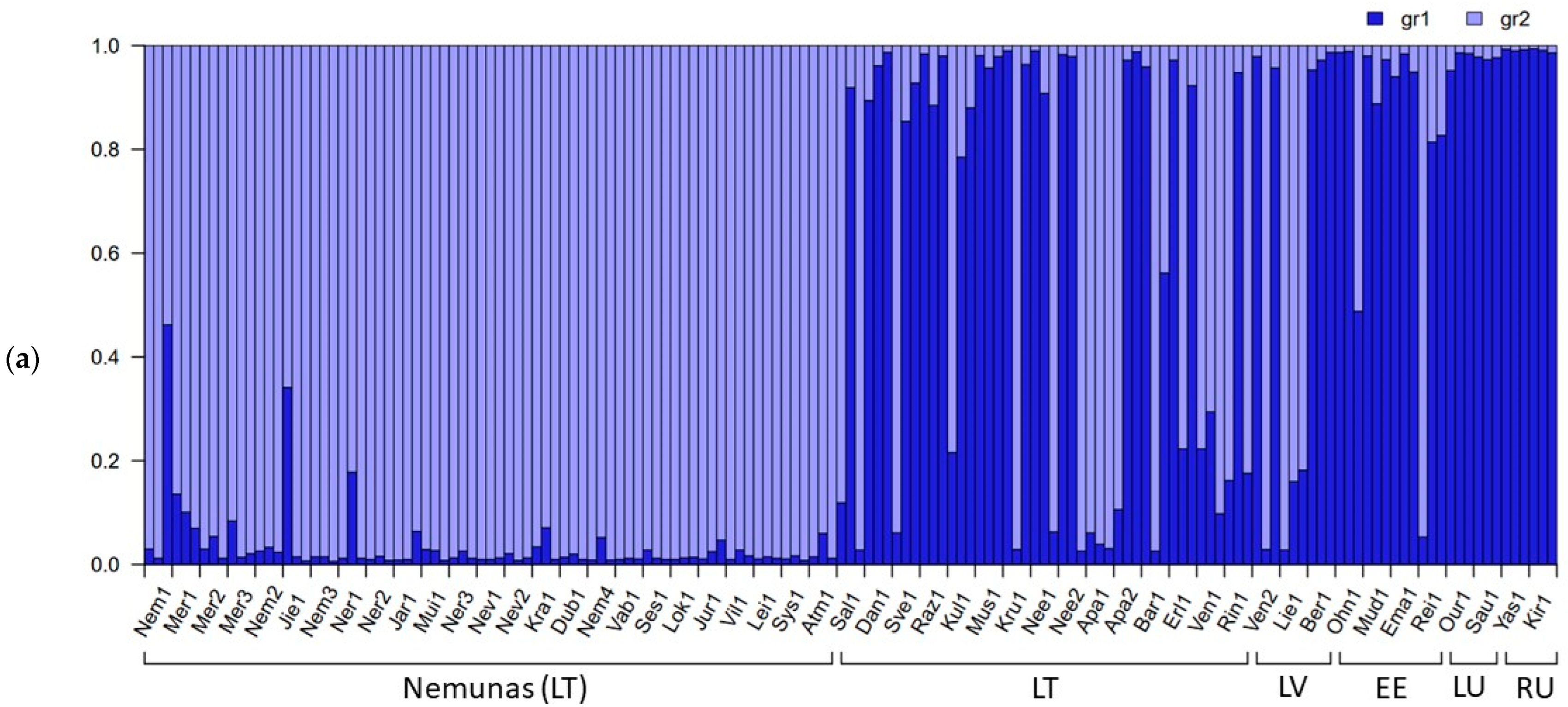
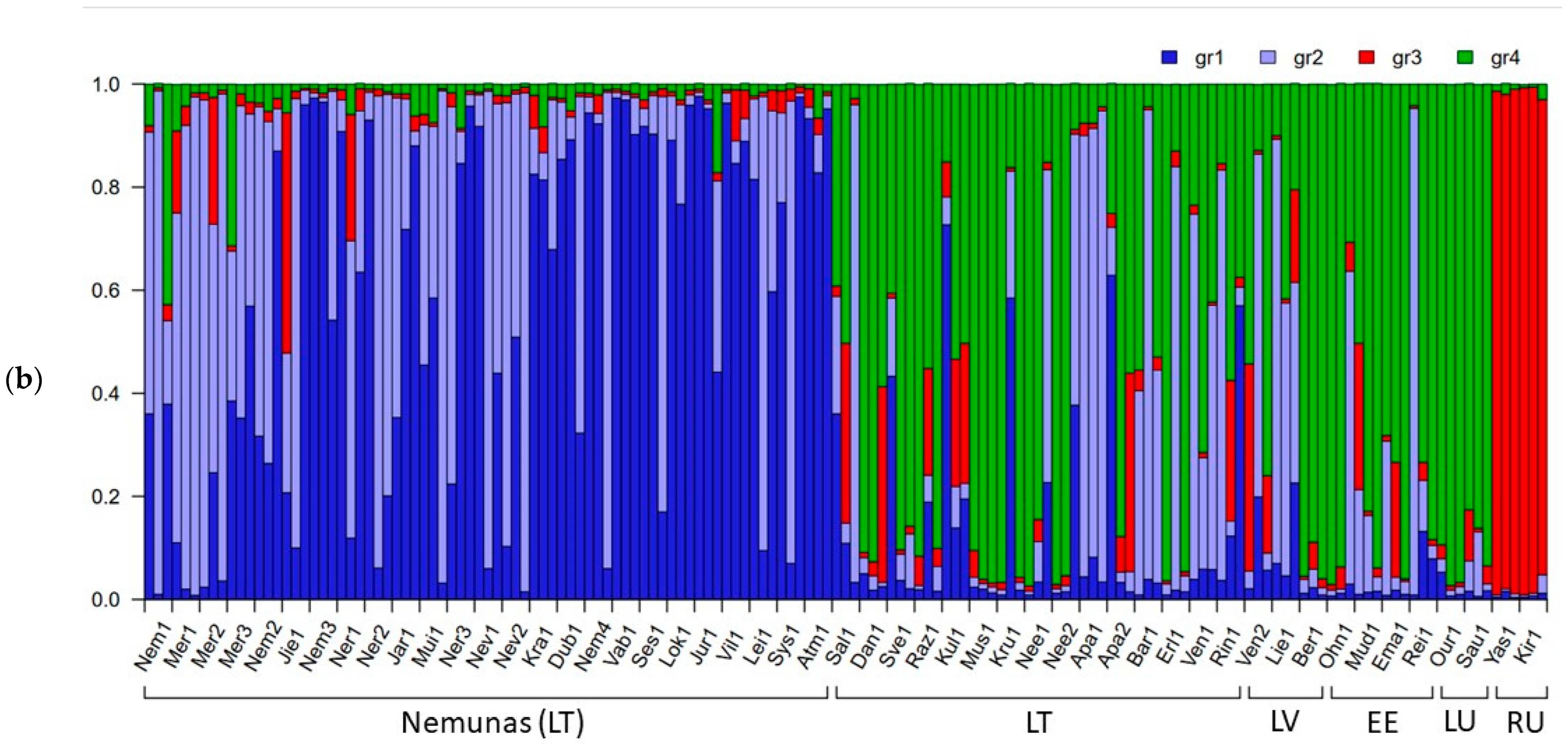
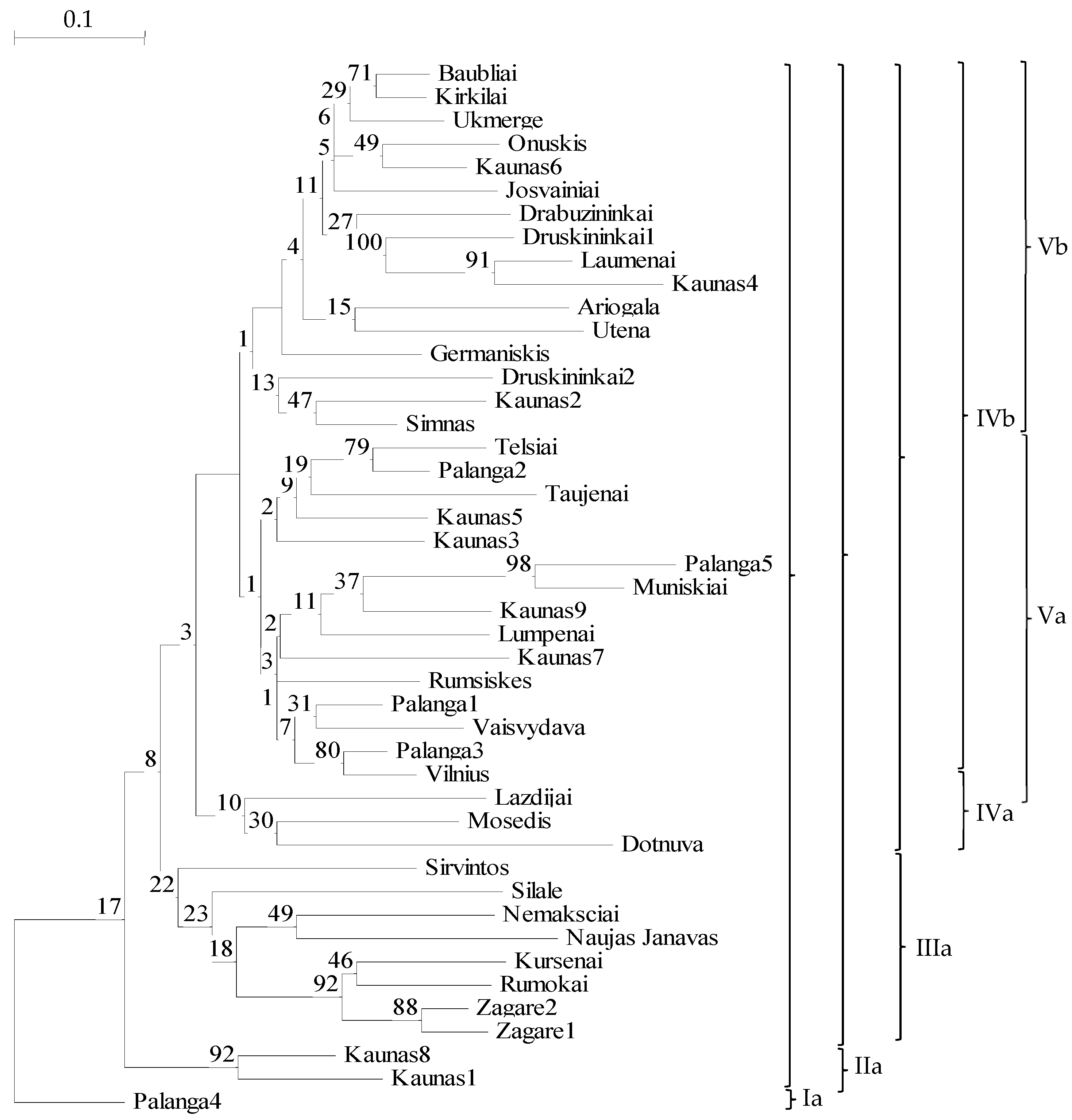
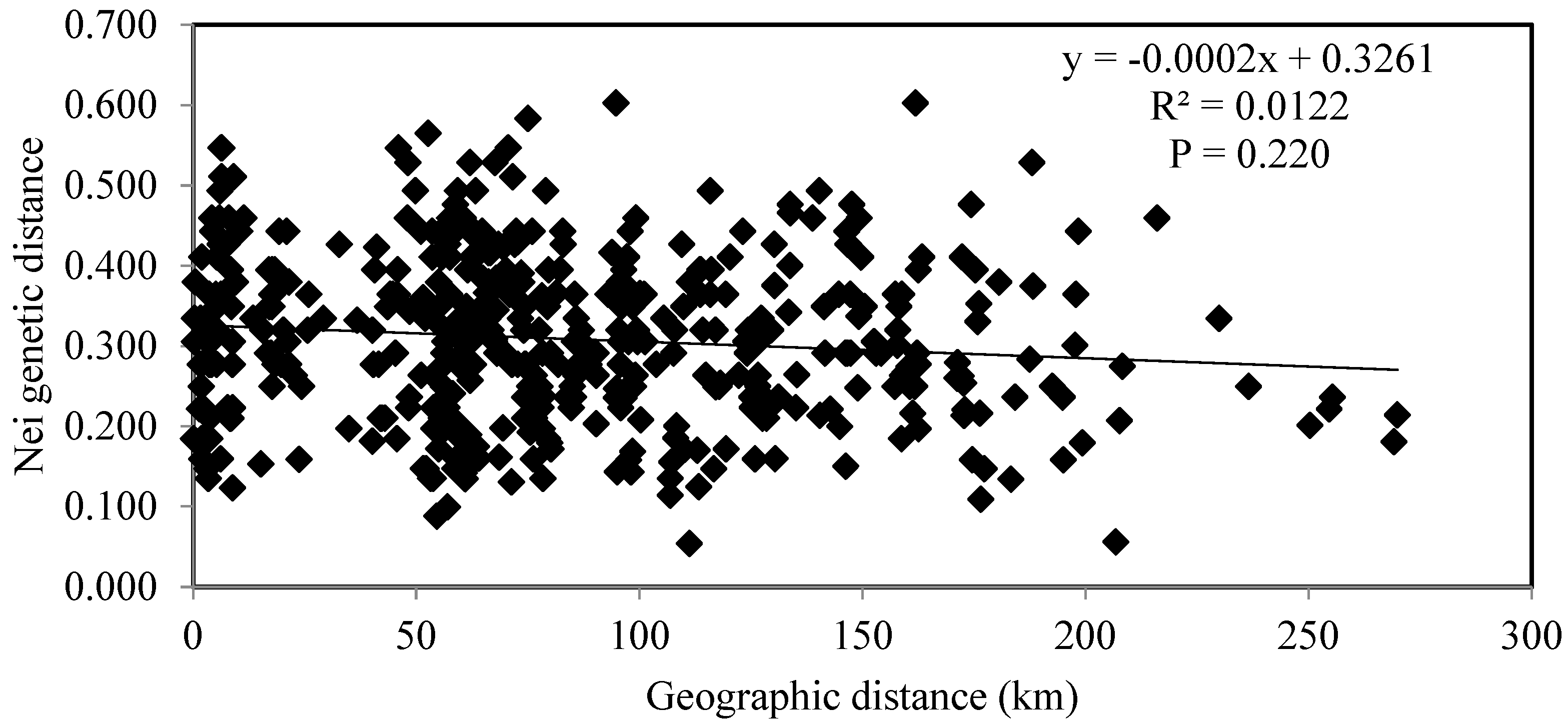
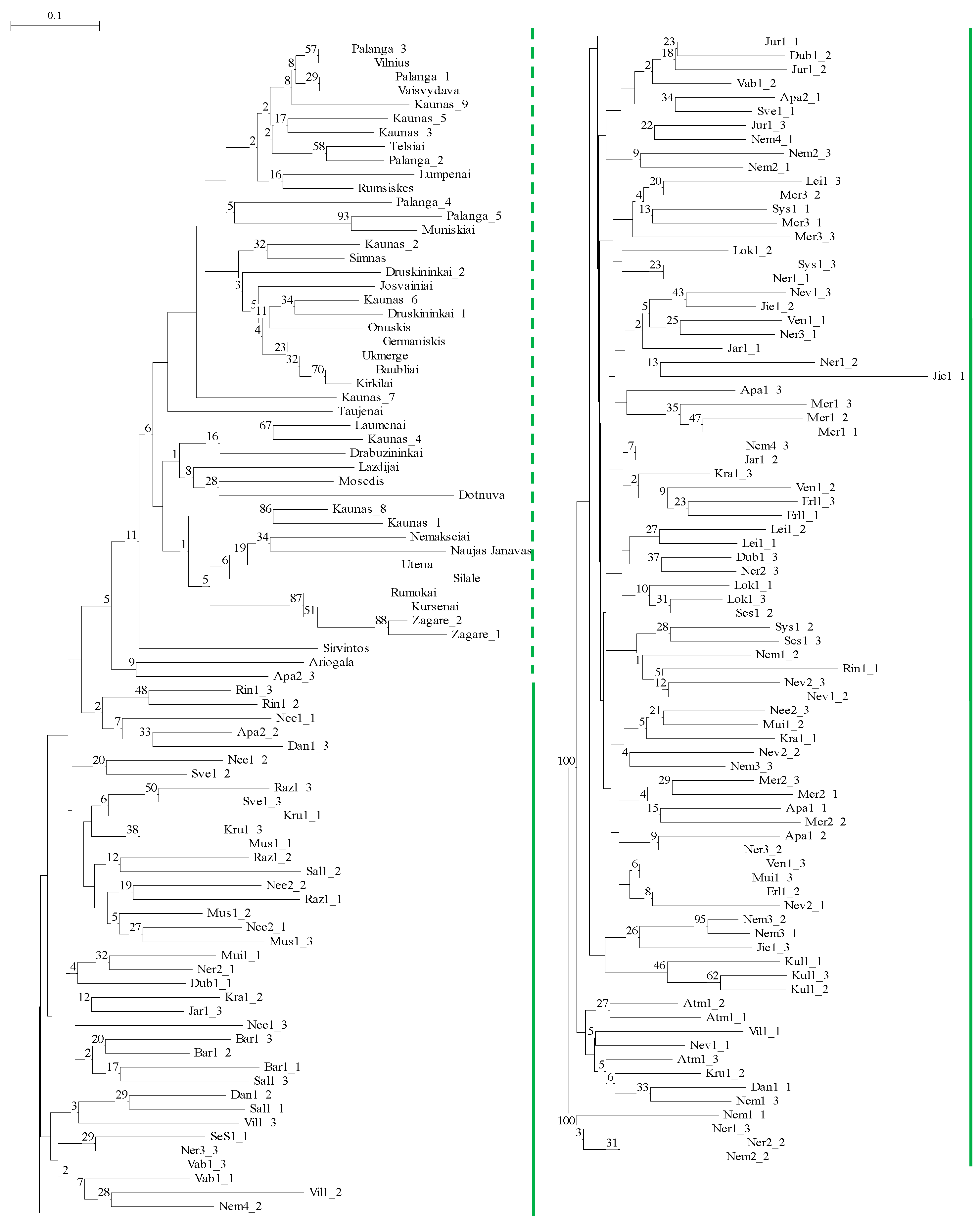
2.2. Genetic Differentiation of Populations of P. arundinacea
2.3. Genetic Diversity of P. arundinacea var. picta Grown in Lithuania
3. Discussion
3.1. Genetic Diversity of Populations
3.2. Interpopulation Variability
3.3. Genetic Features of Ornamental GenotypesAccessions of Lithuanian P. arundinacea
3.4. River Basin Population Genetic Variation (SSRs, SNPs)
4. Materials and Methods
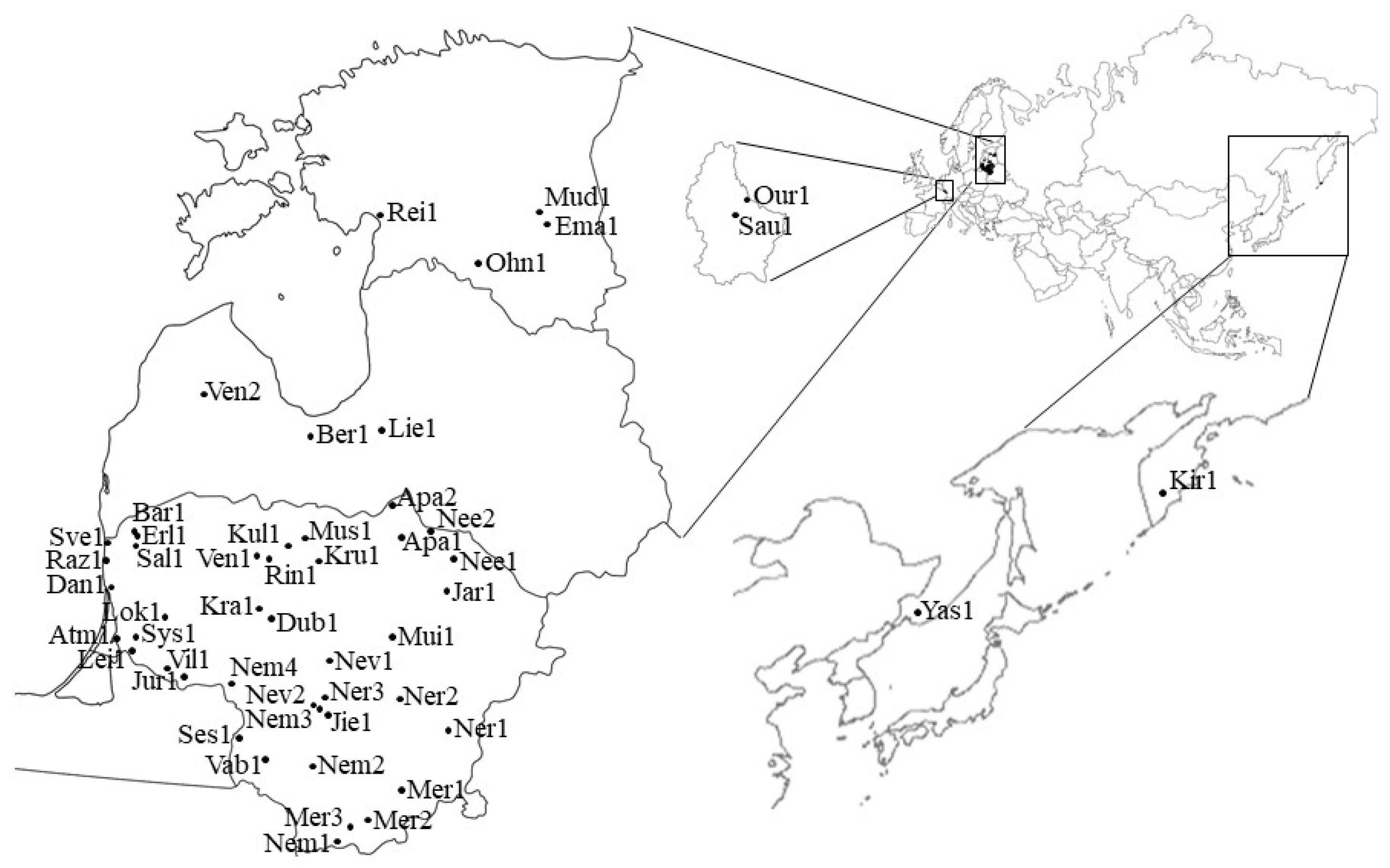
4.1. Sites and Sample Collection
4.2. Environmental Variables
4.3. Molecular Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Union for Conservation of Nature. The Ramsar Convention: A Legal Review. In The 5th Conference on the Convention of Wetlands for International Importance Especially as Waterfowl Habitat; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland; Caliari, Italy, 1980; pp. 24–29. [Google Scholar]
- Ramsar Convention Secretariat. The Fourth Ramsar Strategic Plan 2016–2024. Ramsar Handbooks for the Wise Use of Wetlands, 5th ed.; Ramsar Convention Secretariat: Caliari, Italy; Gland, Switzerland, 2016. [Google Scholar]
- Douglas, E. Building the European Union‘s Natura 2000 network. Nat. Conserv. 2012, 12, 11–26. [Google Scholar]
- Gailiušis, B.; Jablonskis, J.; Kovalenkovienė, M. Lithuanian Rivers: Hydrography and Runoff (Lietuvos upės: Hidrografija ir Nuotėkis); Lietuvos Energetikos Institutas: Kaunas, Lithuania, 2001. [Google Scholar]
- Noticias del mundo ecológico. Ecological Sustainability: Engineering Change. In The 5th International EcoSummit Congress; EcoSummit: Montpellier, France, 2016; Available online: http://www.ecosummit2016.org/ (accessed on 16 December 2023).
- Mathews, S.; Tsai, R.C.; Kellogg, E.A. Phylogenetic structure in the grass family (Poaceae): Evidence from the nuclear gene phytochrome B. Am. J. Bot. 2000, 87, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Bouchenak-Khelladi, Y.; Salamin, N.; Savolainen, V.; Forest, F.; van der Bank, M.; Chase, M.W.; Hodkinson, T.R. Large multi-gene phylogenetic trees of the grasses (Poaceae): Progress towards complete tribal and generic level sampling. Mol. Phylogenet. Evol. 2008, 47, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Teisher, J.K.; Clark, L.G.; Barbera, P.; Gillespie, L.J.; Zuloaga, F.O. A worldwide phylogenetic classification of the Poaceae (Gramineae) II: An update and a comparison of two 2015 classifications. J. Syst. Evol. 2017, 55, 259–290. [Google Scholar] [CrossRef]
- Centre for Agriculture and Biosciences International [CABI]. Phalaris arundinacea. In Invasive Species Compendium; The Centre for Agriculture and Bioscience International: Wallingford, UK, 2021; Available online: www.cabi.org/isc (accessed on 11 December 2023).
- Weinmann, F.; Boule, M.; Brunner, K.; Yoshino, V. Wetland Plants of the Pacific Northwest; U.S. Army Corps of Engineers: Seattle, WA, USA, 1984. [Google Scholar]
- Snarskis, P. An Identification Guide to the Plants of Lithuania SSR (Vadovas Lietuvos TSR Augalams Pažinti); Valstybinė politinės ir mokslinės literatūros leidykla: Vilnius, Lithuania, 1954. [Google Scholar]
- Nelson, F.M.; Anderson, N.O.; Casler, D.M.; Jakubowski, R.A. Population genetic structure of N. American and European Phalaris arundinacea L. as inferred from inter-simple sequence repeat markers. Biol. Inv. 2014, 16, 353–363. [Google Scholar] [CrossRef]
- Voshell, S.M.; Hilu, W.H. Canary grasses (Phalaris, Poaceae): Biogeography, molecular dating and the role of floret structure in dispersal. Mol. Ecol. 2014, 23, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Gifford, A.L.; Ferdy, J.B.; Molofsky, J. Genetic composition and morphological variation among populations of the invasive grass, Phalaris arundinacea. Can. J. Bot. 2002, 80, 779–785. [Google Scholar] [CrossRef]
- Ito, M.; Sato, E.; Goto, H.; Hattori, Y. Growth morphology of reed canarygrass (Phalaris arundinacea L.) tillers under sward conditions. Nippon Sochi Gakkaishi 1990, 36, 254–262. [Google Scholar]
- Grime, R.J.; Hodgson, G.J.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species, 2nd ed.; MacMillan Publishers (Castlepoint Press): Colvend, UK, 2007. [Google Scholar]
- Bloju, O.; Boldura, O.M.; Samfira, I.; Popescu, S. Evaluation of genetic diversity of some Phalaris sp. using microsatellites. J. Hortic. For. Biotechnol. 2014, 18, 135–140. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Indicator values of plants in Central Europe. Scripta Geobotanica 1992, 18, 77–153. [Google Scholar]
- Valk, A.V.D.; Bliss, L.C. Hydrarch succession and net primary production of oxbow lakes in central Alberta. Can. J. Bot. 1971, 49, 1177–1199. [Google Scholar] [CrossRef]
- Long, C.J.; Whitlock, C. Fire and vegetation history from the coastal rain forest of the western Oregon Coast Range. Quat. Res. 2002, 58, 215–225. [Google Scholar] [CrossRef]
- Morin, E.; Bouchard, A.; Jutras, P. Ecological analysis of disturbed riverbanks in the Montreal area of Quebec. Environ. Manag. 1989, 13, 215–225. [Google Scholar] [CrossRef]
- Foster, R.D.; Wetzel, P.R. Invading monotypic stands of Phalaris arundinacea: A test of fire, herbicide, and woody and herbaceous native plant groups. Restor. Ecol. 2005, 13, 318–324. [Google Scholar] [CrossRef]
- Brooks, M.L. Effects of fire suppression and postfire management activities on plant invasions. In Wildland Fire in Ecosystems: Fire and Nonnative Invasive Plants; General Technical Report; Zouhar, K., Smith, J.K., Sutherland, S.M., Brooks, L., Eds.; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2008; pp. 269–280. [Google Scholar]
- Hansen, P.L.; Pfister, R.D.; Boggs, K.C.; Bradley, J.; Joy, J.; Hinckley, D.K. Classification and management of Montana’s riparian and wetland sites. In Miscellaneous Publication, No. 54; Montana Forest and Conservation Experiment Station: Missoula, MT, USA, 1995. [Google Scholar]
- Green, E.K.; Galatowitsch, S.M. Effects of Phalaris arundinacea and nitrate-N addition on the establishment of wetland plant communities. J. Appl. Ecol. 2002, 39, 134–144. [Google Scholar] [CrossRef]
- Natkevičiūtė-Ivanauskienė, M. Varpiniai—Gramineae. In Lietuvos TSR Flora; Minkevičius, A., Jankevičius, K., Brunza, K., Dagys, J., Lekavičius, A., Pipinys, J., Eds.; Valstybinė politinės ir mokslinės literatūros leidykla: Vilnius, Lithuania, 1963; Volume 2. [Google Scholar]
- Larson, G.E. Aquatic and Wetland Vascular Plants of the Northern Great Plains; Gen. Tech. Rep., RM 238; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1993. [Google Scholar]
- Merigliano, M.F.; Lesica, P. The native status of reed canarygrass (Phalaris arundinacea L.) in the inland northwest, USA. Nat. Areas J. 1998, 18, 223–230. [Google Scholar]
- Noyszewski, A.K.; Anderson, N.O.; Smith, A.G.; Kilian, A.; Dalbotten, D.; Ito, E.; Timm, A.; Pellerin, H.; Kubatova, B.; Januš, V.; et al. Riparian populations of Minnesota reed canarygrass (Phalaris arundinacea) are most likely native, based on SNPs (DArTseqLD). Wetl. Ecol. Manag. 2021, 29, 467–494. [Google Scholar] [CrossRef]
- Harrison, R.D.; Chatterton, N.J.; Page, R.J.; Curto, M.; Asay, K.H.; Jensen, K.B.; Horton, W.H. Effects of nine introduced grasses on ecological biodiversity in the Columbia basin. In Rangelands in a Sustainable Biosphere, Proceedings of the Fifth International Rangeland Congress, Salt Lake City, UT, USA, 23–28 July 1995; Society for Range Management: Salt Lake City, UT, USA, 1996; pp. 211–212. [Google Scholar]
- Lesica, P. Spread of Phalaris arundinacea adversely impacts the endangered plant Howellia aquatilis. Great Basin Nat. 1997, 57, 366–368. [Google Scholar]
- Swearingen, J.; Bargeron, C. Invasive Plant Atlas of the United States; Center for Invasive Species and Ecosystem Health, University of Georgia: Athens, GA, USA, 2016; Available online: http://www.invasiveplantatlas.org/ (accessed on 16 December 2023).
- Lavergne, S.; Molofsky, J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc. Nat. Acad. Sci. USA 2007, 104, 3883–3888. [Google Scholar] [CrossRef]
- Jakubowski, A.R.; Casler, M.D.; Jackson, R.D. Landscape context predicts reed canarygrass invasion: Implications for management. Wetlands 2010, 30, 685–692. [Google Scholar] [CrossRef]
- Hitchcock, C.L.; Cronquist, A.; Ownbey, M. Vascular Plants of the Pacific Northwest. Part 1: Vascular Cryptogams, Gymnosperms, and Monocotyledons; University of Washington Press: Seattle, WA, USA, 1969. [Google Scholar]
- Kline, P.; Broersma, K. The yield, nitrogen and nitrate content of reed canarygrass, meadow foxtail and timothy fertilized with nitrogen. Can. J. Plant Sci. 1983, 63, 943–950. [Google Scholar] [CrossRef]
- Galatowitsch, S.M.; Anderson, N.O.; Ascher, P. Invasiveness in wetland plants in temperate north America. Wetlands 1999, 19, 733–755. [Google Scholar] [CrossRef]
- Tasi, J.; Barcsak, Z. Relationship between the phenological phase of grass and the quality of fodder. Novenytermeles 2001, 50, 31–42. [Google Scholar]
- Struzhkina, T.M. Phalaris arundinacea, a valuable fodder crop for the Kamchatka region, Russia. Kormoproizvodstvo 2002, 8, 11–15. [Google Scholar]
- Casler, M.D. Genetics, breeding, and ecology of reed canarygrass. Int. J. Plant Breed. 2010, 4, 30–36. [Google Scholar]
- Lefor, M.W. Phalaris arundinacea L. (reed canary grass—Gramineae) as a hydrophyte in Essex, Connecticut, USA. Environ. Manag. 1987, 11, 771–773. [Google Scholar] [CrossRef]
- Essl, F.; Rabitsch, W.; Breuss, O. Neobiota in Österreich; Umweltbundesamt: Wien, Germany, 2002. [Google Scholar]
- Verloove, F. Catalogue of neophytes in Belgium (1800–2005). Scr. Bot. Belg. 2006, 39, 1–89. [Google Scholar]
- Medvecká, J.; Kliment, J.; Májeková, J.; Halada, Ľ.; Zaliberová, M.; Gojdičová, E.; Feráková, V.; Jarolímek, I. Inventory of the alien flora of Slovakia. Preslia 2012, 84, 257–309. [Google Scholar]
- Pyšek, P.; Danihelka, J.; Sádlo, J.; Chrtek, J., Jr.; Chytrý, M.; Jarošík, V.; Kaplan, Z.; Krahulec, F.; Moravcová, L.; Pergl, J.; et al. Catalogue of alien plants of the Czech Republic: Checklist update, taxonomic diversity and invasion patterns, 2nd ed. Preslia 2012, 84, 155–255. [Google Scholar]
- Petrova, A.; Vladimirov, V. Reports of some ornamental plants as aliens for the Bulgarian flora. Phytol. Balc. 2019, 25, 387–394. [Google Scholar]
- Hübner, T.M.; Tischer, S.; Tanneberg, H.; Kuschk, P. Influence of phenol and phenanthrene on the growth of Phalaris arundinacea and Phragmites australis. Int. J. Phytoremediation 2000, 2, 331–342. [Google Scholar] [CrossRef]
- Polechońska, L.; Klink, A. Trace metal bioindication and phytoremediation potentialities of Phalaris arundinacea L. (reed canary grass). J. Geochem. Explor. 2014, 146, 27–33. [Google Scholar] [CrossRef]
- Dzantor, E.K.; Chekol, T.; Vough, L.R. Feasibility of using forage grasses and legumes for phytoremediation of organic pollutants. J. Environ. Sci. Health A 2000, 35, 1645–1661. [Google Scholar] [CrossRef]
- Wrobel, C.; Coulman, E.B.; Smith, L.D. The potential use of reed canarygrass (Phalaris arundinacea L.) as a biofuel crop. Acta Agric. Scand. 2009, 59, 1–18. [Google Scholar]
- Ustak, S.; Šinko, J.; Munoz, J. Reed canary grass (Phalaris arundinacea L.) as a promising energy crop. J. Cent. Eur. Agric. 2019, 20, 1143–1168. [Google Scholar] [CrossRef]
- Casler, M.D.; Phillips, M.M.; Krohn, A.L. DNA polymorphisms reveal geographic races of reed canary grass. Crop Sci. 2009, 49, 2139–2148. [Google Scholar] [CrossRef]
- Anderson, N.O.; Kavova, T.; Bastlova, D.; Čurn, V.; Kubatova, B.; Edwards, K.R.; Vojtech, J.; Kvet, J. Phenotypic and genotypic variation in Czech forage, ornamental and wild populations of reed canarygrass. Crop Sci. 2016, 56, 2421–2435. [Google Scholar] [CrossRef]
- Kávová, T.; Kubátová, B.; Curn, V.; Anderson, N.O. Genetic variability of US and Czech Phalaris arundinacea L. wild and cultivated populations. In New Perspective in Forage Crops; Evan, R.L., Bezerra, L.R., Eds.; Intech: London, UK, 2018; pp. 169–186. [Google Scholar] [CrossRef]
- Jakubowski, A.R.; Casler, M.D.; Jackson, R.D. Has selection for improved agronomic traits made reed canarygrass invasive? PLoS ONE 2011, 6, e25757. [Google Scholar] [CrossRef]
- Jakubowski, A.R.; Jackson, R.D.; Johnson, R.C.; Hu, J.; Casler, M.D. Genetic diversity and population structure of Eurasian populations of reed canarygrass: Cytotypes, cultivars, and interspecific hybrids. Crop Pasture Sci. 2011, 62, 982–991. [Google Scholar] [CrossRef]
- Jakubowski, A.R.; Casler, M.D.; Jackson, R.D. Genetic evidence suggests a widespread distribution of native North American populations of reed canarygrass. Biol. Inv. 2012, 15, 261–268. [Google Scholar] [CrossRef]
- Jakubowski, A.R.; Jackson, R.D.; Casler, M.D. The history of reed canarygrass in North America: Persistence of natives among invading Eurasian populations. Crop Sci. 2013, 54, 210–219. [Google Scholar] [CrossRef]
- Barth, S.; Jankowska, M.J.; Hodkinson, T.R.; Vellani, T.; Klaas, M. Variation in sequences containing microsatellite motifs in the perennial biomass and forage grass, Phalaris arundinacea (Poaceae). BMC Res. Notes 2016, 9, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Kettenring, K.M.; Menuz, D.R.; Mock, K.E. The nativity and distribution of the cryptic invader Phalaris arundinacea (reed canarygrass) in riparian areas of the Columbia and Missouri River Basins. Wetlands 2019, 39, 55–66. [Google Scholar] [CrossRef]
- Graper, A.; Noyszewski, A.; Anderson, N.; Smith, A. Variability in ITS1 and ITS2 sequences of historic (herbaria) and extant (fresh) Phalaris species. BMC Plant Biol. 2021, 21, 515. [Google Scholar] [CrossRef] [PubMed]
- Perdereau, A.; Klaas, M.; Barth, S.; Hodkinson, T.R. Plastid genome sequencing reveals biogeographical structure and extensive population genetic variation in wild populations of Phalaris arundinacea L. in north-western Europe. Glob. Chang. Biol. Bioenergy 2017, 9, 46–56. [Google Scholar] [CrossRef]
- Anderson, N.O.; Jocienė, L.; Krokaitė, E.; Rekašius, T.; Paulauskas, A.; Kupčinskienė, E. Genetic diversity of Phalaris arundinacea populations in relation to river regulation in the Merkys basin, Lithuania. River Res. Appl. 2018, 34, 300–309. [Google Scholar] [CrossRef]
- Anderson, N.O. Throwing out the bathwater but keeping the baby: Lessons learned from purple loosestrife and reed canarygrass. HortTechnology 2019, 29, 539–548. [Google Scholar] [CrossRef]
- Anderson, N.O.; Smith, A.G.; Noyszewski, A.K.; Ito, E.; Dalbotten, D.; Pellerin, H. Management and control issues for native, invasive species (reed canarygrass): Evaluating philosophical, management, and legislative Issues. HortTechnology 2021, 31, 354–366. [Google Scholar] [CrossRef]
- Lekavičius, A. A Plant Guide (Vadovas augalams Pažinti); Mokslas: Vilnius, Lithuania, 1989. [Google Scholar]
- Zviedre, E.; Vītola, I.; Vizule-Kahovska, L.; Upena, I. Evaluation of Phytobenthos and Macrophytes of the Inland Surface Waters and Ecological Status Defined by Macrophyte Reference Index. Report, Part II, Rivers; Latvian Environment, Geology and Meteorology Center: Ryga, Latvia, 2015. [Google Scholar]
- Pocienė, L.; Šarūnaitė, L.; Tilvikienė, V.; Šlepetys, J.; Kadžiulienė, Ž. The yield and composition of reed canary grass biomass as raw material for combustion. Biologija 2013, 59, 195–200. [Google Scholar] [CrossRef]
- Amaleviciute-Volunge, K.; Slepetiene, A.; Butkute, B. The suitability of perennial grasses for combustion as influenced by chemical composition and plant growth stage. Zemdirbyste 2020, 107, 317–322. [Google Scholar] [CrossRef]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Nat. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef] [PubMed]
- Krokaitė, E.; Shakeneva, D.; Juškaitytė, E.; Tomas, R.; Nemaniūtė-Gužienė, J.; Butkuvienė, J.; Patamsytė, J.; Rančelienė, V.; Vyšniauskienė, R.; Duchovskienė, L.; et al. Nitrogen concentration of the aquatic plant species in relation to land cover type and other variables of the environment. Zemdirbyste 2019, 106, 203–212. [Google Scholar] [CrossRef]
- Vyšniauskienė, R.; Rančelienė, V.; Naugžemys, D.; Rudaitytė-Lukošienė, E.; Patamsytė, J.; Butkauskas, D.; Kupčinskienė, E.; Žvingila, D. Genetic diversity of Nuphar lutea in Lithuanian river populations. Aquat. Bot. 2020, 161, 103–173. [Google Scholar] [CrossRef]
- Jocienė, L.; Krokaitė, E.; Shakeneva, D.; Rekašius, T.; Stanys, V.; Šikšnianienė, J.B.; Žvingila, D.; Paulauskas, A.; Kupčinskienė, E. Relationship between genetic and environmental characteristics of Lithuanian populations of purple loosestrife (Lythrum salicaria). J. Environ. Eng. Landsc. Manag. 2022, 30, 81–93. [Google Scholar] [CrossRef]
- Li, J.; Baga, M.; Hucl, P.; Chibbar, N.R. Developement of microsatellite markers in canary seed (Phalaris canariensis L.). Mol. Breed. 2011, 28, 611–621. [Google Scholar] [CrossRef]
- Lawrence, C.J.; Dong, Q.; Polacco, M.L.; Seigfried, T.E.; Brendel, V. MaizeGDB, the community database for maize genetics and genomics. Nucleic Acids Res. 2004, 32, D393–D397. [Google Scholar] [CrossRef] [PubMed]
- Jocienė, L.; Krokaitė, E.; Rekašius, T.; Juškaitytė, E.; Ielciu, I.; Galanina, O.; Kupčinskienė, E. The molecular evidence for invasive climber Echinocystis lobata (Michx.) Torr. & A. Gray in eastern and central Europe. Diversity 2023, 15, 1084. [Google Scholar] [CrossRef]
- Kupcinskiene, E.; Zybartaite, L.; Paulauskas, A. Comparison of molecular diversity of three Impatiens species from Central Europe and Baltic region. Zemdirbyste-Agriculture 2015, 102, 87–94. [Google Scholar] [CrossRef]
- CORINE Land Cover. CORINE Land Cover Nomenclature Conversion to Land Cover Classification System; European Environment Agency: Vienna, Austria, 2012; Available online: http://www.igeo.pt/gdr/pdf/CLC2012_nomenclature_addendum.pdf (accessed on 8 December 2023).
- European Union. 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; European Parliament, Council of the European Union: Brussels, Belgium, 2000; Volume 327, pp. 1–73. [Google Scholar]
- Aplinkos Apsaugos Agentura. Agriculture and Lithuanian Waters. Impact of Agricultural Activities on the State of Lithuanian Rivers and Pollution Loads to the Baltic Sea (Žemės ūkis ir Lietuvos Vandenys. Žemės ūkio Veiklos Poveikis Lietuvos upių Būklei ir Taršos Apkrovoms į Baltijos Jūrą); Aplinkos Apsaugos Agentura: Vilnius, Lithuania, 2018; Available online: https://vanduo.old.gamta.lt/files/%C5%BDem%C4%97s_%C5%ABkio_poveikio_ataskaita1539180129871.pdf (accessed on 2 December 2023).
- Tumas, R. Regularities of river water quality under the interactions of physical geography factors and farming intensity. In Proceedings of the ÕÕ Nordic Hydrological Conference, Helsinki, Finland, 10–13 August 1998; pp. 100–108. [Google Scholar]
- Jociene, L.; Stravinskaite, K.; Krokaite, E.; Janulioniene, R.; Rekašius, T.; Paulauskas, A.; Marozas, V.; Kupcinskiene, E. AFLP-based genetic structure of Lithuanian populations of small balsam (Impatiens parviflora DC.) in relation to habitat characteristics. Forests 2022, 13, 1228. [Google Scholar] [CrossRef]
- Nelson, M.F.; Anderson, N.O. How many marker loci are necessary? Analysis of dominant marker data sets using two popular population genetic algorithms. Ecol. Evol. 2013, 3, 3455–3470. [Google Scholar] [CrossRef]
- Guo, W.; Wang, R.; Zhou, S.; Zhang, S.; Zhang, Z. Genetic diversity and clonal structure of Phragmites australis in the Yellow river delta of China. Biochem. Syst. Ecol. 2003, 31, 1093–1109. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 3–15. [Google Scholar]
- Kupcinskiene, E.; Zybartaite, L.; Janulioniene, R.; Zukauskiene, J.; Paulauskas, A. Molecular diversity of small balsam populations in relation to site characteristics. Cent. Eur. J. Biol. 2013, 8, 1048–1061. [Google Scholar] [CrossRef]
- Štorchová, H.; Hrdličková, R.; Chrtek, J., Jr.; Tetera, M.; Fitze, D.; Fehrer, J. An improved method of DNA isolation from plants collected in the field and conserved in saturated NaCl/CTAB solution. Taxon 2000, 49, 79–84. [Google Scholar] [CrossRef]
- Juškaitytė, E.; Krokaitė, E.; Jocienė, L.; Paulauskas, A.; Kupčinskienė, E. Methodical search for molecular studies of some riparian species of herbaceous plants. In Human and Nature Safety, Proceedings of the 22nd International Scientific-Practice Conference, Kaunas, Lithuania, 4–6 May 2016, ; Academy, LUA: Kaunas, Lithuania, 2016; Volume 22, pp. 186–189. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Nat. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 2001, 27, 379–423. [Google Scholar] [CrossRef]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Yeh, F.C.; Yang, R.C.; Boyle, T. POPGENE Version 1.32: Microsoft Windows-Based Freeware for Population Genetic Analysis, Quick User Guide; Center for International Forestry Research: Edmonton, AB, Canada, 1999. [Google Scholar]
- Van de Peer, Y.; De Wachter, R. Construction of evolutionary distance trees with TREECON for Windows: Accounting for variation in nucleotide substitution rate among sites. Bioinformatics 1997, 13, 227–230. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Pohlert, T. The pairwise multiple comparison of mean ranks package (PMCMR). R Package 2014, 27, 9. [Google Scholar]
- Subirana, I.; Sanz, H.; Vila, J. Building bivariate tables: The compare groups package for R. J. Stat. Softw. 2014, 57, 1–16. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and visualize the results of multivariate data analyses. R Package Ver. 2017, 1, 337–354. [Google Scholar]
- Gruber, B.; Unmack, P.J.; Berry, O.F.; Georges, A. DArTR: An R package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Mol. Ecol. Resour. 2018, 18, 691–699. [Google Scholar] [CrossRef]




| Primer | Number of Alleles | Range of Allele Size (bp) |
|---|---|---|
| csm045 | 9 | 90–106 |
| csm049 | 3 | 144–160 |
| csm071 | 5 | 126–160 |
| csm074 | 5 | 115–151 |
| csm075 | 11 | 133–161 |
| csm090 | 4 | 97–105 |
| csm101 | 5 | 113–149 |
| csm104 | 4 | 104–110 |
| csm106 | 13 | 131–163 |
| csm111 | 5 | 107–138 |
| csm122 | 5 | 121–149 |
| phi071 | 10 | 126–154 |
| umc2185 | 10 | 104–155 |
| umc2779 | 6 | 121–147 |
| Total | 95 |
| Populations | % P | I | h | ||
|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | ||||
| Europe | 30.0 | 0.166 | ±0.027 | 0.112 | ±0.018 |
| Russian Far East | 25.0 | 0.139 | ±0.025 | 0.093 | ±0.017 |
| Baltic countries | 30.1 | 0.169 | ±0.027 | 0.114 | ±0.019 |
| Lithuanian | 29.5 | 0.166 | ±0.027 | 0.112 | ±0.018 |
| Nemunas basin (LT) | 29.5 | 0.166 | ±0.027 | 0.122 | ±0.019 |
| Seaside river basin (LT) | 32.2 | 0.176 | ±0.027 | 0.117 | ±0.018 |
| Lielupė basin (LT-LV) | 28.6 | 0.163 | ±0.027 | 0.110 | ±0.019 |
| Venta basin (LV) | 33.0 | 0.199 | ±0.029 | 0.135 | ±0.020 |
| Bartuva (LT) | 22.9 | 0.130 | ±0.025 | 0.088 | ±0.018 |
| Peipus (EE) | 33.5 | 0.182 | ±0.027 | 0.121 | ±0.018 |
| Rein (LU) | 33.0 | 0.182 | ±0.028 | 0.122 | ±0.019 |
| Average | 29.9 | 0.168 | ±0.004 | 0.113 | ±0.003 |
| Source | df | SS | Est. Var. | % | Φ |
|---|---|---|---|---|---|
| 1. | |||||
| Among groups of populations of Nemunas and Lielupė basins | 1 | 49 | 0.97 | 8 | ΦCT = 0.083 *** |
| Among populations within groups | 31 | 429 | 1.56 | 13 | ΦSC = 0.145 *** |
| Within populations | 66 | 605 | 9.16 | 78 | ΦST = 0.216 *** |
| Total | 98 | 1082 | 11.69 | 100 | |
| 2. | |||||
| Among groups of populations of Nemunas and Seaside basins | 1 | 35 | 1.03 | 9 | ΦCT = 0.088 *** |
| Among populations withing groups | 27 | 360 | 1.33 | 11 | ΦSC = 0.125 *** |
| Within populations | 58 | 542 | 9.35 | 80 | ΦST = 0.201 *** |
| Total | 86 | 937 | 11.70 | 100 | |
| 3. | |||||
| Among groups of populations of Nemunas and Venta basin | 1 | 25 | 0.70 | 6 | ΦCT = 0.061 *** |
| Among populations within groups | 26 | 348 | 1.32 | 12 | ΦSC = 0.123 *** |
| Within populations | 56 | 527 | 9.42 | 82 | ΦST = 0.176 *** |
| Total | 83 | 900 | 11.44 | 100 | |
| 4. | |||||
| Among groups of populations of Nemunas and Bartuva basins | 1 | 28 | 1.35 | 11 | ΦCT = 0.113 *** |
| Among populations within groups | 25 | 336 | 1.45 | 12 | ΦSC = 0.138 *** |
| Within populations | 54 | 490 | 9.07 | 76 | ΦST = 0.236 *** |
| Total | 80 | 854 | 11.87 | 100 | |
| 5. | |||||
| Among groups of populations of different Baltic States river basins (Nemunas, Seaside rivers, Lielupė, Bartuva, Venta, Dauguva, Vorstjarv, Peipus, Pernu) | 8 | 185 | 0.79 | 7 | ΦCT = 0.068 *** |
| Among populations within groups | 38 | 523 | 1.46 | 12 | ΦSC = 0.134 *** |
| Within populations | 94 | 884 | 9.40 | 81 | ΦST = 0.193 *** |
| Total | 140 | 1592 | 11.65 | 100 |
| Source | df | SS | MS | Est. Var. | % | Φ |
|---|---|---|---|---|---|---|
| 1. | ||||||
| Among groups of populations beside different land cover types | 2 | 43 | 21.7 | 0.197 | 2 | ΦCT = 0.017 *** |
| Among populations | 37 | 550 | 14.9 | 1.882 | 17 | ΦSC = 0.169 *** |
| Within populations | 80 | 738 | 9.2 | 9.225 | 82 | ΦST = 0.184 *** |
| Total | 119 | 1332 | 11.304 | 100 | ||
| 2. | ||||||
| Among groups of populations beside different river state | 3 | 54 | 17.8 | 0.106 | 1 | ΦCT = 0.009 * |
| Among populations | 36 | 540 | 15.0 | 1.925 | 17 | ΦSC = 0.173 *** |
| Within populations | 80 | 738 | 9.2 | 9.225 | 82 | ΦST = 0.180 *** |
| Total | 119 | 1332 | 11.256 | 100 | ||
| 3. | ||||||
| Among groups of populations from different areas based on N concentrations within 1991–1996 | 2 | 45 | 22.3 | 0.197 | 2 | ΦCT = 0.017 *** |
| Among populations | 37 | 549 | 14.8 | 1.870 | 17 | ΦSC = 0.169 *** |
| Within populations | 80 | 738 | 9.2 | 9.225 | 82 | ΦST = 0.183 *** |
| Total | 119 | 1332 | 11.192 | 100 | ||
| 4. | ||||||
| Among groups of populations beside different size rivers | 3 | 47 | 15.6 | 0.014 | 0 | ΦCT = 0.001 NS |
| Among populations | 36 | 547 | 15.2 | 1.989 | 18 | ΦSC = 0.177 *** |
| Within populations | 80 | 738 | 9.2 | 9.225 | 82 | ΦST = 0.178 *** |
| Total | 119 | 1332 | 11.228 | 100 | ||
| 5. | ||||||
| Among groups of populations beside different riverbed origins | 1 | 13 | 12.5 | 0.000 | 0 | ΦCT = −0.006 NS |
| Among populations | 38 | 581 | 15.3 | 2.022 | 18 | ΦSC = 0.180 *** |
| Within populations | 80 | 738 | 9.2 | 9.225 | 82 | ΦST = 0.175 *** |
| Total | 119 | 1332 | 11.247 | 100 |
| Source | df | SS | Est. Var. | % | Φ |
|---|---|---|---|---|---|
| 1. >8000 km transect | |||||
| Among groups of populations of Europe and Asia continents (49 + 2) | 1 | 59 | 3.77 | 25 | ΦCT = 0.247 *** |
| Among populations within Europe and Asia population groups | 49 | 769 | 2.10 | 14 | ΦSC = 0.183 *** |
| Within populations | 102 | 957 | 9.38 | 62 | ΦST = 0.385 *** |
| Total | 152 | 1784 | 15.25 | 100 | |
| 2. >8000 km transect | |||||
| Among groups of populations of Luxembourg and Russian Far East (2 + 2) | 1 | 39 | 3.58 | 23 | ΦCT = 0.230 * |
| Among populations within LU and Russian Far East population groups | 2 | 36 | 2.92 | 19 | ΦSC = 0.243 * |
| Within populations | 8 | 73 | 9.08 | 58 | ΦST = 0.417 *** |
| Total | 11 | 148 | 15.58 | 100 | |
| 3. >7000 km transect | |||||
| Among groups of populations of Nemunas and Russian Far East (25 + 2) | 1 | 66 | 4.66 | 30 | ΦCT = 0.303 *** |
| Among populations within Nemunas and Far East population groups | 25 | 348 | 1.60 | 10 | ΦSC = 0.149 *** |
| Within populations | 54 | 493 | 9.12 | 59 | ΦST = 0.407 *** |
| Total | 80 | 906 | 15.38 | 100 | |
| 4. >1000 km transect | |||||
| Among groups of populations of Baltic region and Luxembourg (47 + 2) | 1 | 25 | 0.81 | 7 | ΦCT = 0.066 * |
| Among populations within Baltic countries and LU population groups | 47 | 719 | 1.95 | 16 | ΦSC = 0.171 *** |
| Within populations | 98 | 925 | 9.44 | 77 | ΦST = 0.226 *** |
| Total | 146 | 1669 | 12.20 | 100 |
| Source | df | SS | Est. Var. | % | Φ |
|---|---|---|---|---|---|
| Among groups of grown and naturally growing individuals | 1 | 349 | 348.6 | 31 | 0.306 *** |
| Within populations | 163 | 1902 | 11.7 | 69 | |
| Total | 164 | 2251 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, N.O.; Krokaitė-Kudakienė, E.; Jocienė, L.; Rekašius, T.; Chernyagina, O.A.; Paulauskas, A.; Kupčinskienė, E. Genetic Differentiation of Reed Canarygrass (Phalaris arundinacea L.) within Eastern Europe and Eurasia. Genes 2024, 15, 734. https://doi.org/10.3390/genes15060734
Anderson NO, Krokaitė-Kudakienė E, Jocienė L, Rekašius T, Chernyagina OA, Paulauskas A, Kupčinskienė E. Genetic Differentiation of Reed Canarygrass (Phalaris arundinacea L.) within Eastern Europe and Eurasia. Genes. 2024; 15(6):734. https://doi.org/10.3390/genes15060734
Chicago/Turabian StyleAnderson, Neil O., Edvina Krokaitė-Kudakienė, Lina Jocienė, Tomas Rekašius, Olga A. Chernyagina, Algimantas Paulauskas, and Eugenija Kupčinskienė. 2024. "Genetic Differentiation of Reed Canarygrass (Phalaris arundinacea L.) within Eastern Europe and Eurasia" Genes 15, no. 6: 734. https://doi.org/10.3390/genes15060734
APA StyleAnderson, N. O., Krokaitė-Kudakienė, E., Jocienė, L., Rekašius, T., Chernyagina, O. A., Paulauskas, A., & Kupčinskienė, E. (2024). Genetic Differentiation of Reed Canarygrass (Phalaris arundinacea L.) within Eastern Europe and Eurasia. Genes, 15(6), 734. https://doi.org/10.3390/genes15060734






