Cytogenetic and Molecular Effects of Kaolin’s Foliar Application in Grapevine (Vitis vinifera L.) under Summer’s Stressful Growing Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Kaolin Treatment, and Plant Material
2.2. Cytogenetic Analysis of KL-Treated and Untreated Leaves
2.3. Molecular Analysis of KL-Treated and Untreated Leaves
2.4. Biochemical Analysis of KL-Treated and Untreated Leaves
2.5. Statistical Analysis
3. Results
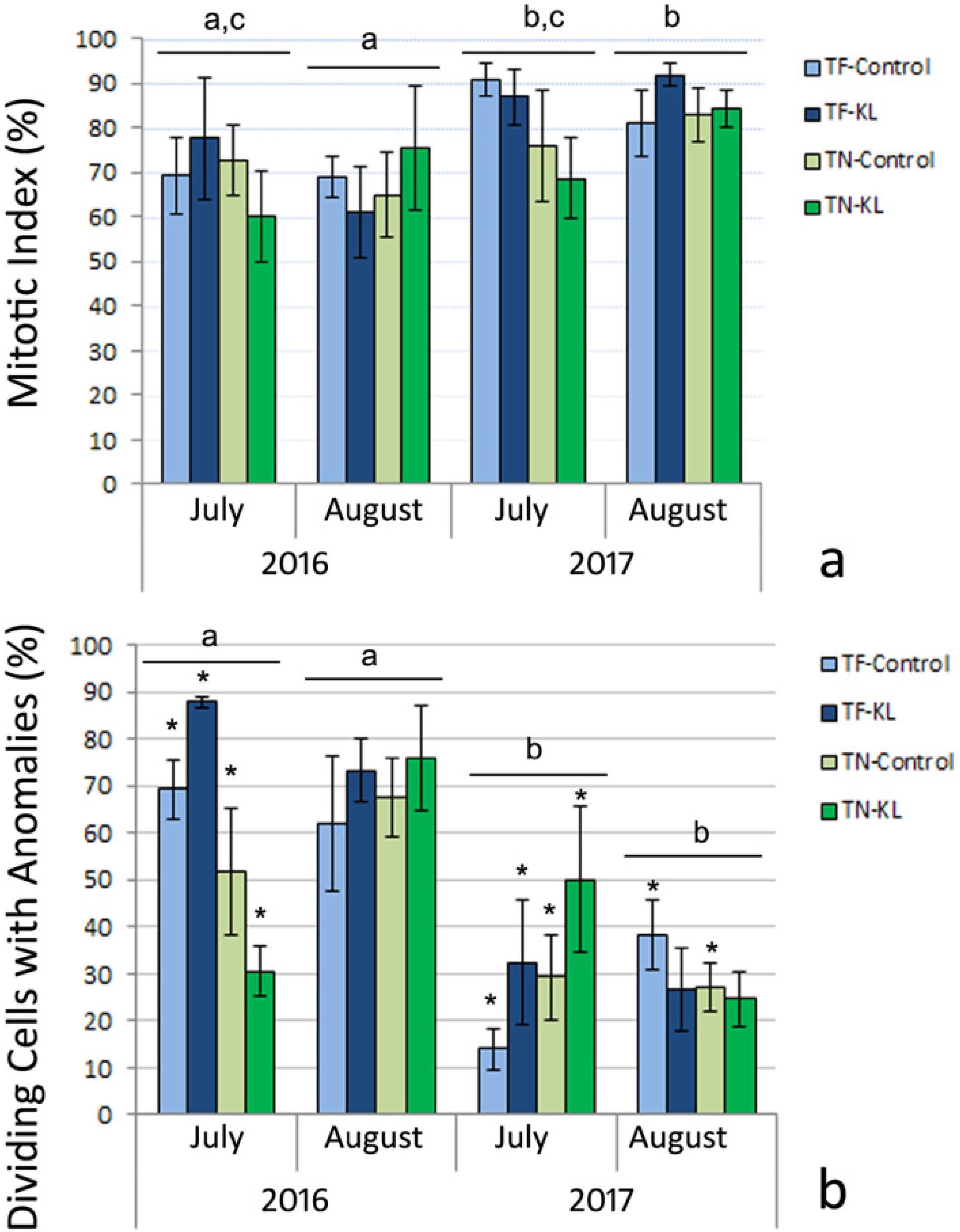
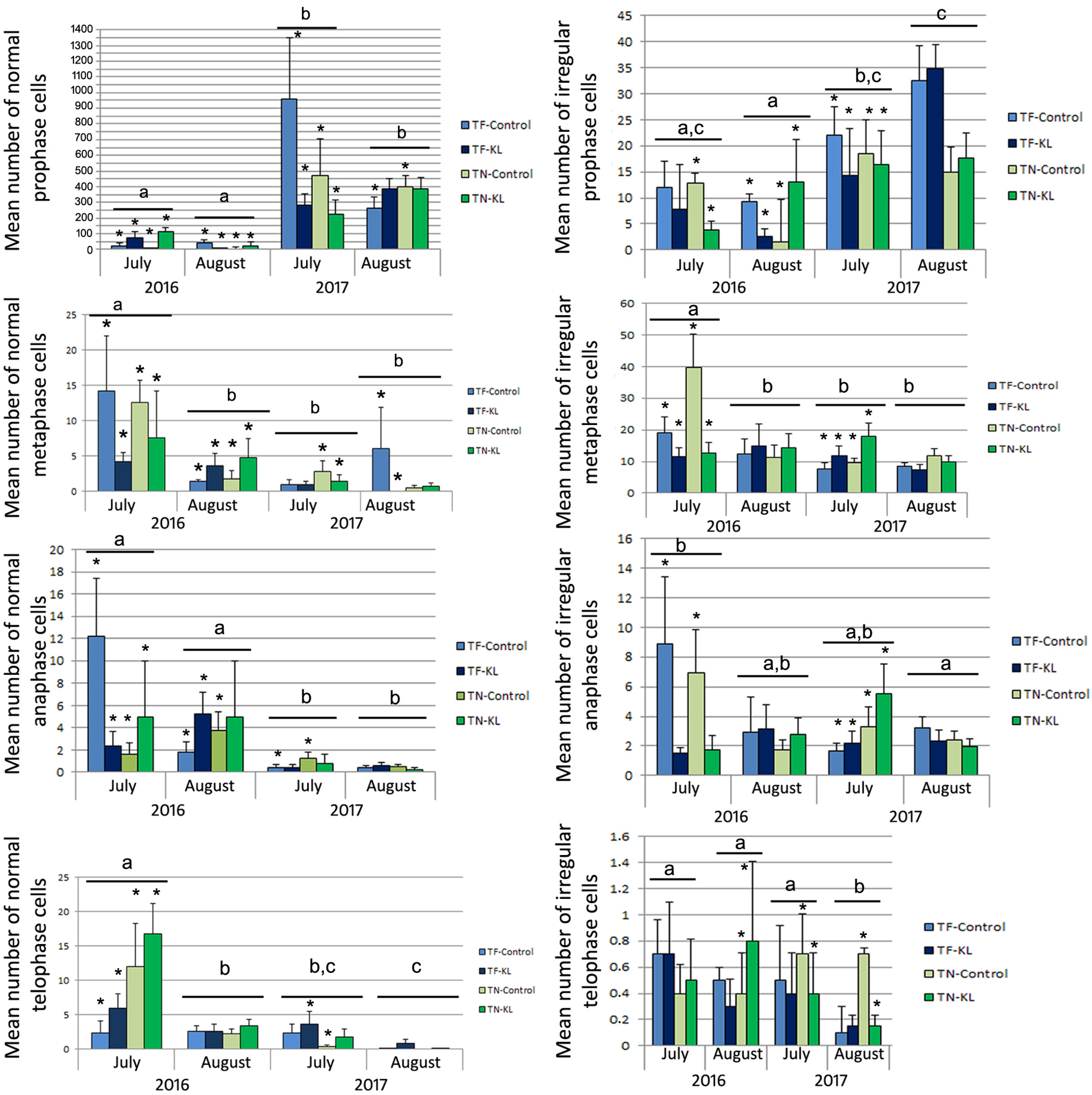
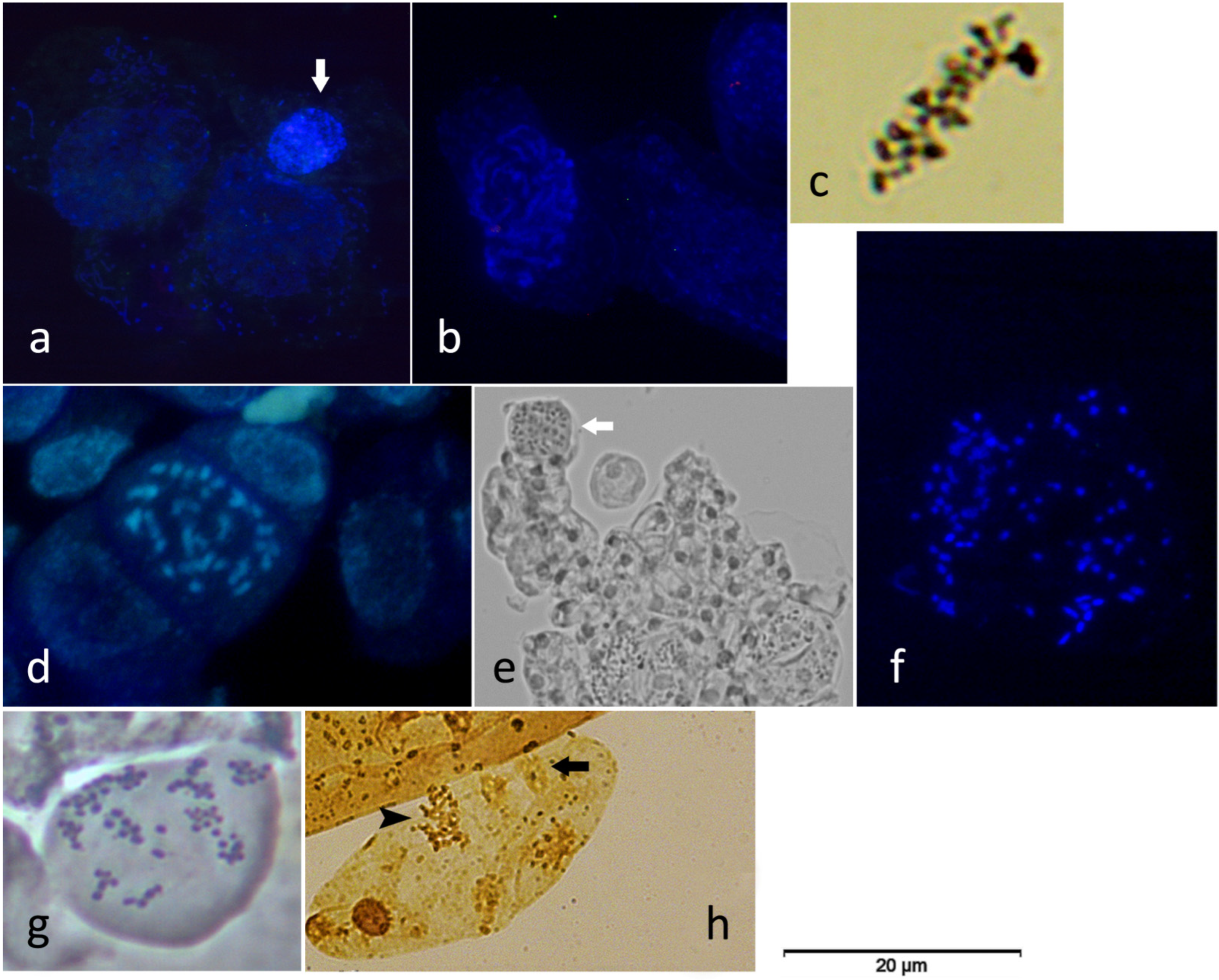
3.1. Cytogenetic Evaluation of the Regularity of the Leaf Mitotic Cell Cycle
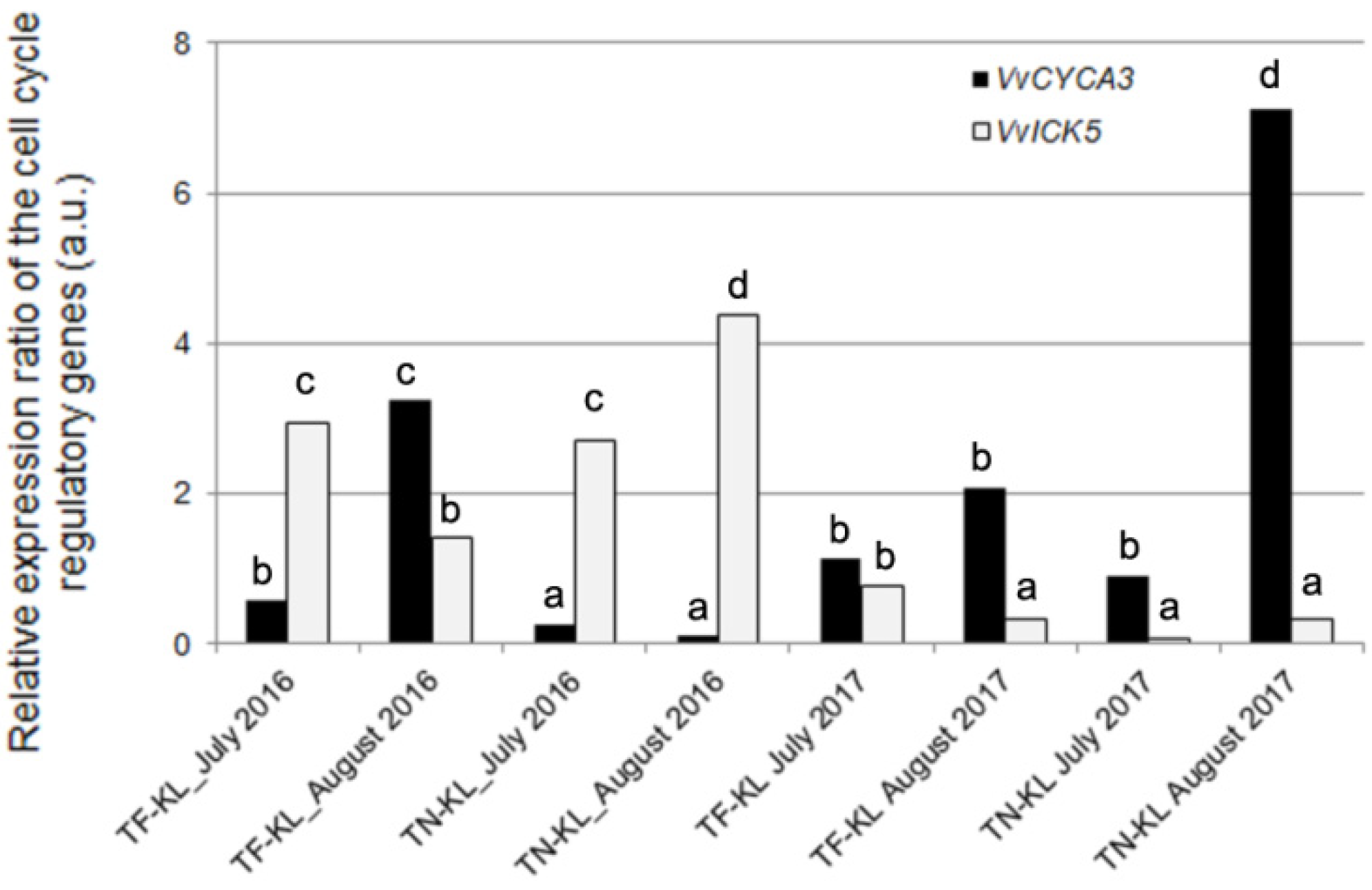
3.2. Regulation of the Leaf Mitotic Cell Cycle Assayed by qPCR
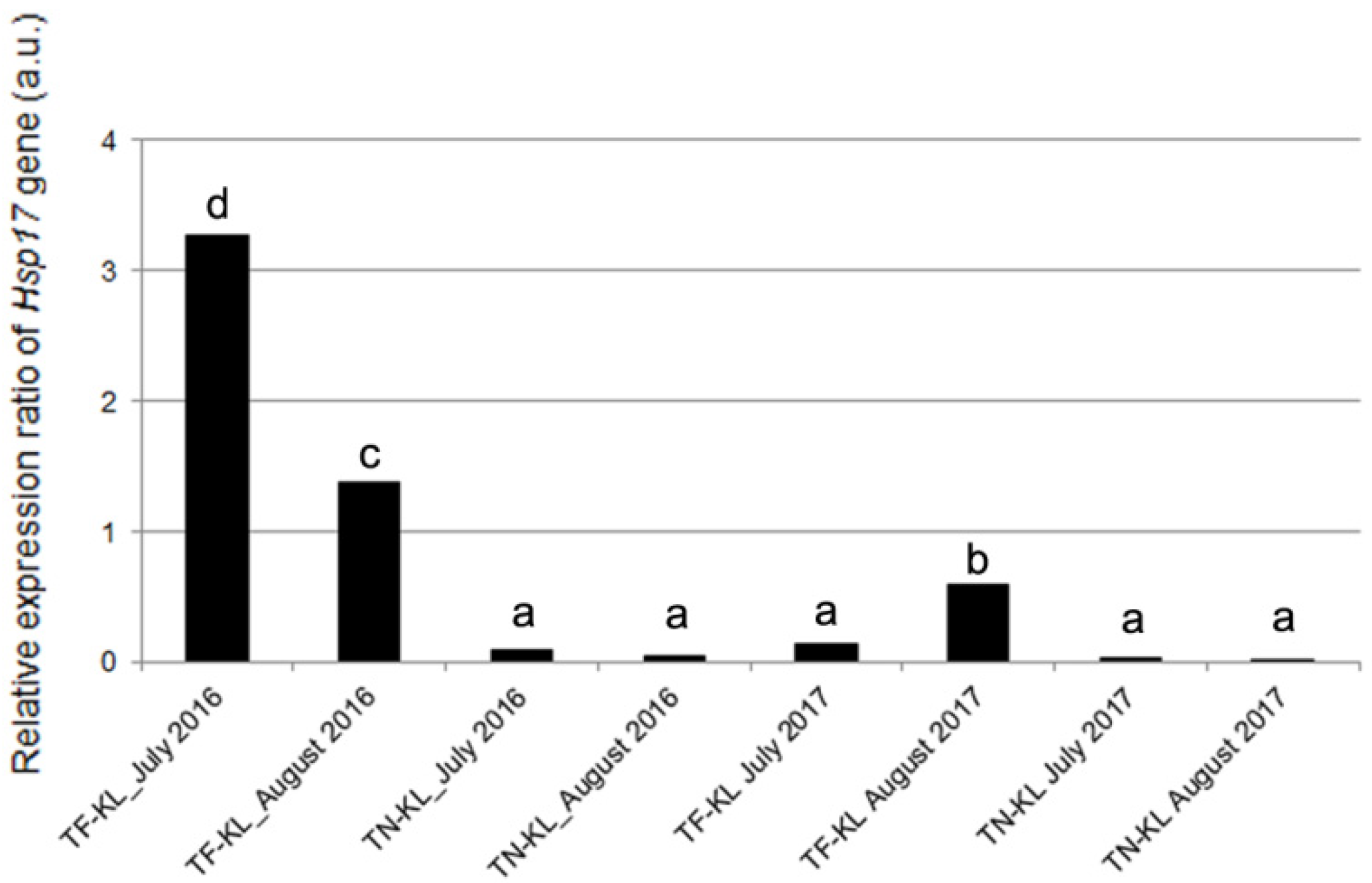
3.3. Expression Profile of the Hsp17.9A Gene
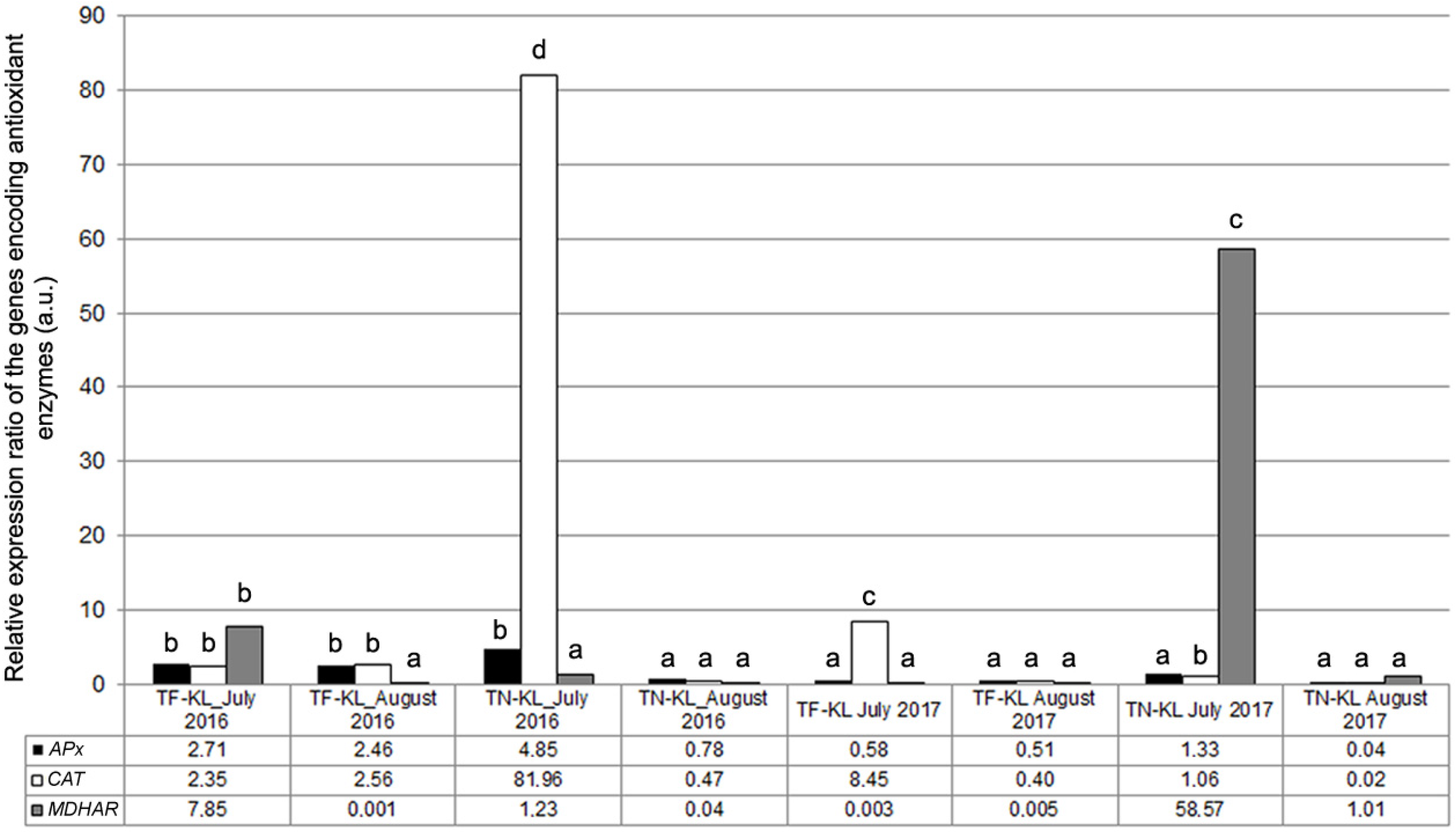
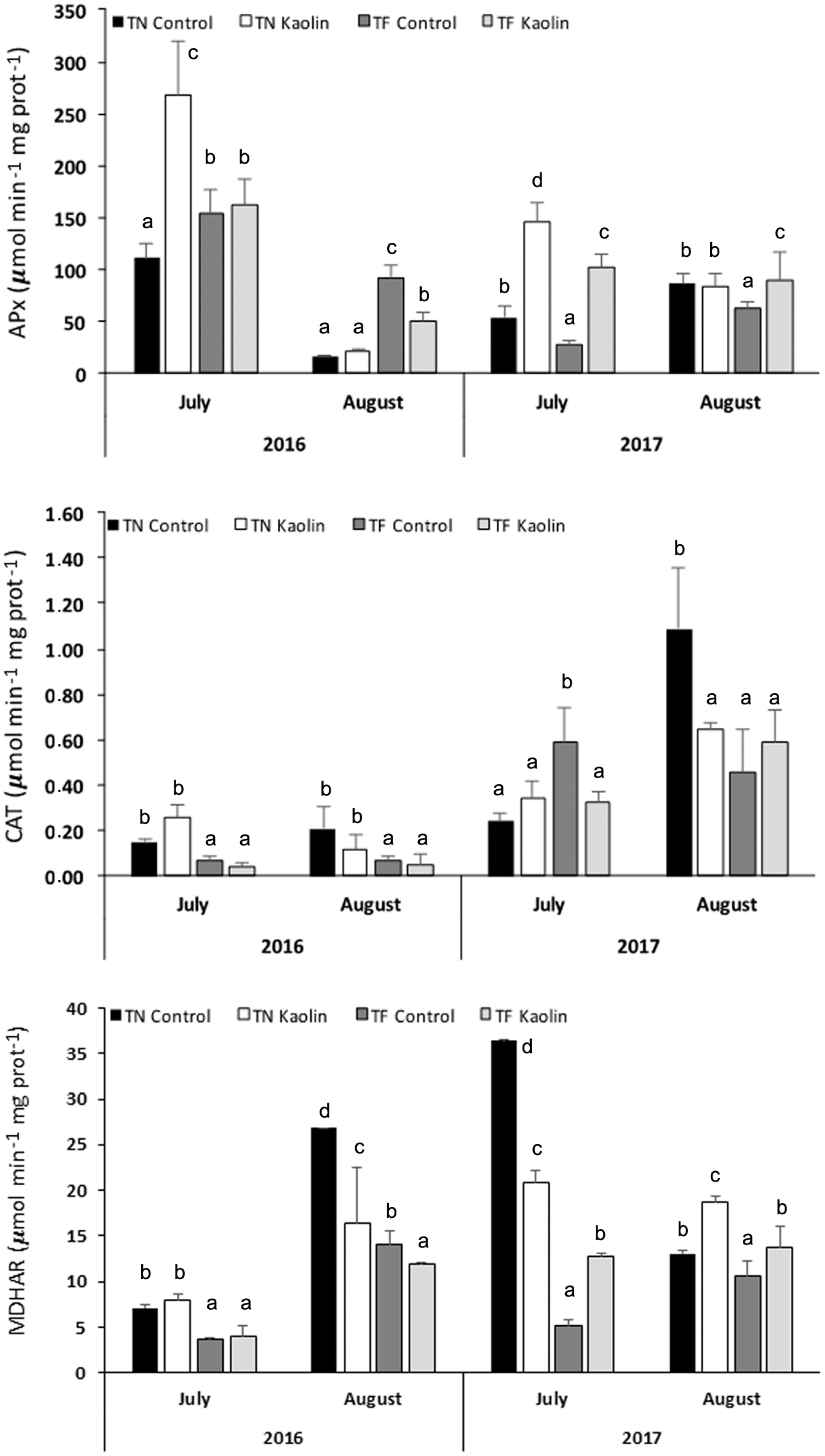
3.4. Molecular and Biochemical Analyses Related to the Antioxidant Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Organization of Vine and Wine (OIV). OIV Statistics Database. Available online: https://oiv.int/what-we-do/global-report?oiv (accessed on 27 May 2024).
- Fraga, H.; García de Cortázar Atauri, I.; Malheiro, A.C.; Santos, J.A. Modelling climate chnage impacts on viticultural yield, phenology and stress conditions in Europe. Glob. Chang. Biol. 2016, 22, 3774–3788. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine (OIV). OIV Statistics Database. Available online: https://www.oiv.int/what-we-do/data-discovery-report?oiv (accessed on 27 May 2024).
- Portaria n.º 380/2012, Diário da República, 1ª Série—N.º 226—22 de Novembro de 2012; Ministério da Agricultura, do Mar, do Ambiente e do Ordenamento do Território: Lisboa, Portugal, 2012; pp. 6712–6715. (In Portuguese)
- Touriga Franca—Vine and Wine Cluster, COLAB Vines & Wines, 6p. Available online: https://www.advid.pt/uploads/TourigaNacional_Final_25.10.pdf (accessed on 27 May 2024). (In Portuguese).
- Touriga Nacional—Vine and Wine Cluster, COLAB Vines & Wines, 7p. Available online: https://www.advid.pt/uploads/TourigaNacional_Final_25.10.pdf (accessed on 27 May 2024). (In Portuguese).
- Portaria n.º 383/2017, Diário da República, 1.ª série—N.º 243—20 de Dezembro de 2017; Agricultura, Florestas e Desenvolvimento Rural: Lisboa, Portugal, 2017; pp. 6659–6660. (In Portuguese)
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field. Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Chaki, M.; Begara-Morales, J.C.; Barroso, J.B. Oxidative Stress in Plants. Antioxidants 2020, 9, 481. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Jha, M.; Tuteja, N. DNA damage and repair in plants under ultraviolet and ionizing radiations. Sci. World J. 2015, 2015, 250158. [Google Scholar] [CrossRef]
- Duchêne, É. How can grapevine genetics contribute to the adaptation to climate change? OENO One 2016, 50, 113–124. [Google Scholar] [CrossRef]
- Bita, C.A.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Extreme Temperature Responses, Oxidative Stress and Antioxidant Defense in Plants. Chapter 6, In: Abiotic Stress—Plant Responses and Applications in Agriculture. Intech Open 2013, 13, 169–205. [Google Scholar]
- Dinis, L.T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Dinis, L.T.; Bernardo, S.; Conde, A.; Pimentel, D.; Ferreira, D.; Félix, L.; Gerós, H.; Correia, C.M.; Moutinho-Pereira, J. Kaolin exogenous application boosts antioxidant capacity and phenolic content in berries and leaves of grapevine under summer stress. J. Plant Physiol. 2016, 191, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, S.; Dinis, L.-T.; Luzio, A.; Pinto, G.; Meijón, M.; Valledor, L.; Conde, A.; Gerós, H.; Correia, C.M.; Moutinho-Pereira, J. Kaolin particle film application lowers oxidative damage and DNA methylation on grapevine (Vitis vinifera L.). Environ. Exp. Bot. 2017, 139, 39–47. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Barros, A.; Pitarch-Bielsa, M.; Malheiro, A.C.; Gómez-Cadenas, A.; Moutinho-Pereira, J. Uncovering the effects of kaolin on balancing berry phytohormones and quality attributes of Vitis vinifera grown in warm-temperate climate regions. J. Sci. Food Agric. 2022, 102, 782–793. [Google Scholar] [CrossRef]
- Carvalho, A.; Leal, F.; Matos, M.; Lima-Brito, J. Effects of heat stress in the leaf mitotic cell cycle and chromosomes of four wine-producing grapevine varieties. Protoplasma 2018, 255, 1725–1740. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; de Jorge Páscoa, R.N.M.; Espinosa-Mansilla, A.; Martín-Merás, I.D.; Lopes, J.A. Comparative quantification of chlorophyll and polyphenol levels in grapevine leaves sampled from different geographical locations. Sci. Rep. 2020, 10, 6246. [Google Scholar] [CrossRef]
- Carvalho, A.; Leal, F.; Matos, M.; Lima-Brito, J. Heat stress tolerance assayed in four wine-producing grapevine varieties using a cytogenetic approach. Ciencia Tec. Vitiv. 2019, 34, 61–70. [Google Scholar] [CrossRef]
- Schuppler, U.; He, P.-H.; John, P.C.L.; Munns, R. Effect of water stress on cell division and cell-division-cycle 2-like cell-cycle kinase activity in wheat leaves. Plant Physiol. 1998, 117, 667–678. [Google Scholar] [CrossRef]
- Ichihashi, Y.; Tsukaya, H. Behavior of leaf meristems and their modification. Front. Plant Sci. 2015, 6, 1060. [Google Scholar] [CrossRef]
- Ichihashi, Y.; Kawade, K.; Usami, T.; Horiguchi, G.; Takahashi, T.; Tsukaya, H. Key proliferative activity in the junction between the leaf blade and leaf petiole of Arabidopsis. Plant Physiol. 2011, 157, 1151–1162. [Google Scholar] [CrossRef]
- Mondal, S.; Karmakar, S.; Panda, D.; Pramanik, K.; Bose, B.; Singhal, R.K. Crucial plant processes under heat stress and tolerance through heat shock proteins. Plant Stress 2023, 100, 100227. [Google Scholar] [CrossRef]
- Rocheta, M.; Becker, J.D.; Coito, J.L.; Carvalho, L.C.; Amâncio, S. Heat and water stress induce unique transcriptional signatures of heat-shock proteins and transcription factors in grapevine. Funct. Integr. Genom. 2014, 14, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Rocheta, M.; Coito, J.L.; Ramos, M.J.N.; Carvalho, L.; Becker, J.D.; Carbonell-Bejerano, P.; Amâncio, S. Transcriptomic comparison between two Vitis vinifera L. varieties (Trincadeira and Touriga Nacional) in abiotic stress conditions. BMC Plant Biol. 2016, 16, 224. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Colaço, S.; Sangiogo, M.; Amâncio, S. Heat stress in grapevine: The pros and cons of acclimation. Plant Cell Environ. 2015, 38, 777–789. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Gonçalves, E.F.; Chaves, M.M. Differential physiological response of the grapevine varieties Touriga Nacional and Trincadeira to combined heat, drought and light stresses. Plant Biol. 2016, 18, 101–111. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Silva, M.; Coito, J.L.; Rocheta, M.P.; Amâncio, S. Design of a custom RT-qPCR array for assignment of abiotic stress tolerance in traditional Portuguese grapevine varieties. Front. Plant Sci. 2017, 8, 1835. [Google Scholar] [CrossRef]
- Carneiro, A.K.; Montessoro, P.d.F.; Fusaro, A.F.; Araújo, B.G.; Hemerly, A.S. Plant CDKs—Driving the Cell Cycle through Climate Change. Plants 2021, 10, 1804. [Google Scholar] [CrossRef]
- Inzé, D.; De Veylder, L. Cell Cycle Regulation in Plant Development. Annu. Rev. Genet. 2006, 40, 77–105. [Google Scholar] [CrossRef]
- Qi, F.; Zhang, F. Cell cycle regulation in the plant response to stress. Front. Plant Sci. 2020, 10, 1765. [Google Scholar] [CrossRef]
- Lydall, D.; Weinert, T. Yeast checkpoint genes in DNA damage processing: Implications for repair and arrest. Science 1995, 270, 1488–1491. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Dante, R.A.; Larkins, B.A.; Sabelli, P.A. Cell cycle control and seed development. Front. Plant Sci. 2014, 5, 493. [Google Scholar] [CrossRef]
- Noriega, X.; Pérez, F.J. Cell cycle genes are activated earlier than respiratory genes during release of grapevine buds from endodormancy. Plant Signal. Behav. 2017, 12, e1321189. [Google Scholar] [CrossRef]
- Vergara, R.; Noriega, X.; Aravena, K.; Prieto, H.; Pérez, F.J. ABA Represses the Expression of Cell Cycle Genes and May Modulate the Development of Endodormancy in Grapevine Buds. Front. Plant Sci. 2017, 8, 812. [Google Scholar] [CrossRef]
- Van Leene, J.; Eeckhout, D.; Persiau, G.; Van De Slijke, E.; Geerinck, J.; Van Isterdael, G.; Witters, E.; De Jaeger, G. Isolation of transcription factor complexes from Arabidopsis cell suspension cultures by tandem affinity purification. Methods Mol. Biol. 2011, 754, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Verkest, A.; Manes, C.L.; Vercruysse, S.; Maes, S.; Van Der Schueren, E.; Beeckman, T.; Genschik, P.; Kuiper, M.; Inzé, D.; De Veylder, L. The cyclin-dependent kinase inhibitor KRP2 controls the onset of the endoreduplication cycle during Arabidopsis leaf development through inhibition of mitotic CDKA;1 kinase complexes. Plant Cell 2005, 17, 1723–1736. [Google Scholar] [CrossRef]
- Vergara, R.; Noriega, X.; Parada, F.; Dantas, D.; Pérez, F.J. Relationship between endodormancy, FLOWERING LOCUS T and cell cycle genes in Vitis vinifera. Planta 2016, 243, 411–419. [Google Scholar] [CrossRef]
- Wang, H.-T.; Cheng, Y.J.; Hsiao, J.-T.; Sheu, F.; Kuan, Y.-C. 17-(Allylamino)-17-demethoxygeldanamycin treatment induces the accumulation of heat shock proteins and alleviates senescence in broccoli. Postharvest Biol. Technol. 2022, 186, 111818. [Google Scholar] [CrossRef]
- Inzé, D. Green light for the cell cycle. EMBO J. 2005, 24, 657–662. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Sabbatini, P.; Squeri, C.; Lavado Rodas, N.; Palliotti, A.; Poni, S. Kaolin reduces ABA biosynthesis through the inhibition of neoxanthin synthesis in grapevines under water deficit. Int. J. Mol. Sci. 2020, 21, 4950. [Google Scholar] [CrossRef]
- FAO and IUSS Working Group WRB. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps—Update 2015; World Soil Resources Reports No. 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- IPMA—Instituto Português do Mar e da Atmosfera. Boletim Climatológico Sazonal—Verão de 2016; Instituto Português do Mar e da Atmosfera: Lisboa, Portugal, 2016; ISSN 2183-1084. (In Portuguese) [Google Scholar]
- IPMA—Instituto Português do Mar e da Atmosfera. Boletim Climatológico Sazonal—Verão de 2017; Instituto Português do Mar e da Atmosfera: Lisboa, Portugal, 2017; ISSN 2183-1084. (In Portuguese) [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Carvalho, A.; Graça, C.; Carocha, V.; Pêra, S.; Lousada, J.L.; Lima-Brito, J.; Paiva, J.A.P. An improved total RNA isolation from secondary tissues of woody species for coding and non-coding gene expression analyses. Wood Sci. Technol. 2015, 49, 647–658. [Google Scholar] [CrossRef]
- Borges, A.F.; Fonseca, C.; Ferreira, R.B.; Lourenço, A.M.; Monteiro, S. Reference gene validation for quantitative RT-PCR during biotic and abiotic stresses in Vitis vinifera. PLoS ONE 2014, 9, e111399. [Google Scholar] [CrossRef]
- Pfaffl, M.; Tichopad, A.; Prgomet, C.; Neuvians, T. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Method Enzymol. 1955, 2, 764–775. [Google Scholar]
- Rao, M.V.; Hale, B.A.; Ormrod, D.P. Amelioration of ozone-induced oxidative damage in wheat plants grown under high carbon dioxide (Role of antioxidant enzymes). Plant Physiol. 1995, 109, 421–432. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef]
- Arrigoni, O.; Dipierro, S.E.; Borraccino, G. Ascorbate free radical reductase: A key enzyme of the ascorbic acid system. FEBS Lett. 1981, 125, 242–244. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.; Dinis, L.-T.; Luzio, A.; Bernardo, S.; Moutinho-Pereira, J.; Lima-Brito, J. Cytogenetic and Molecular Effects of Kaolin’s Foliar Application in Grapevine (Vitis vinifera L.) under Summer’s Stressful Growing Conditions. Genes 2024, 15, 747. https://doi.org/10.3390/genes15060747
Carvalho A, Dinis L-T, Luzio A, Bernardo S, Moutinho-Pereira J, Lima-Brito J. Cytogenetic and Molecular Effects of Kaolin’s Foliar Application in Grapevine (Vitis vinifera L.) under Summer’s Stressful Growing Conditions. Genes. 2024; 15(6):747. https://doi.org/10.3390/genes15060747
Chicago/Turabian StyleCarvalho, Ana, Lia-Tânia Dinis, Ana Luzio, Sara Bernardo, José Moutinho-Pereira, and José Lima-Brito. 2024. "Cytogenetic and Molecular Effects of Kaolin’s Foliar Application in Grapevine (Vitis vinifera L.) under Summer’s Stressful Growing Conditions" Genes 15, no. 6: 747. https://doi.org/10.3390/genes15060747






