Effects of Drought Stress on Abscisic Acid Content and Its Related Transcripts in Allium fistulosum—A. cepa Monosomic Addition Lines
Abstract
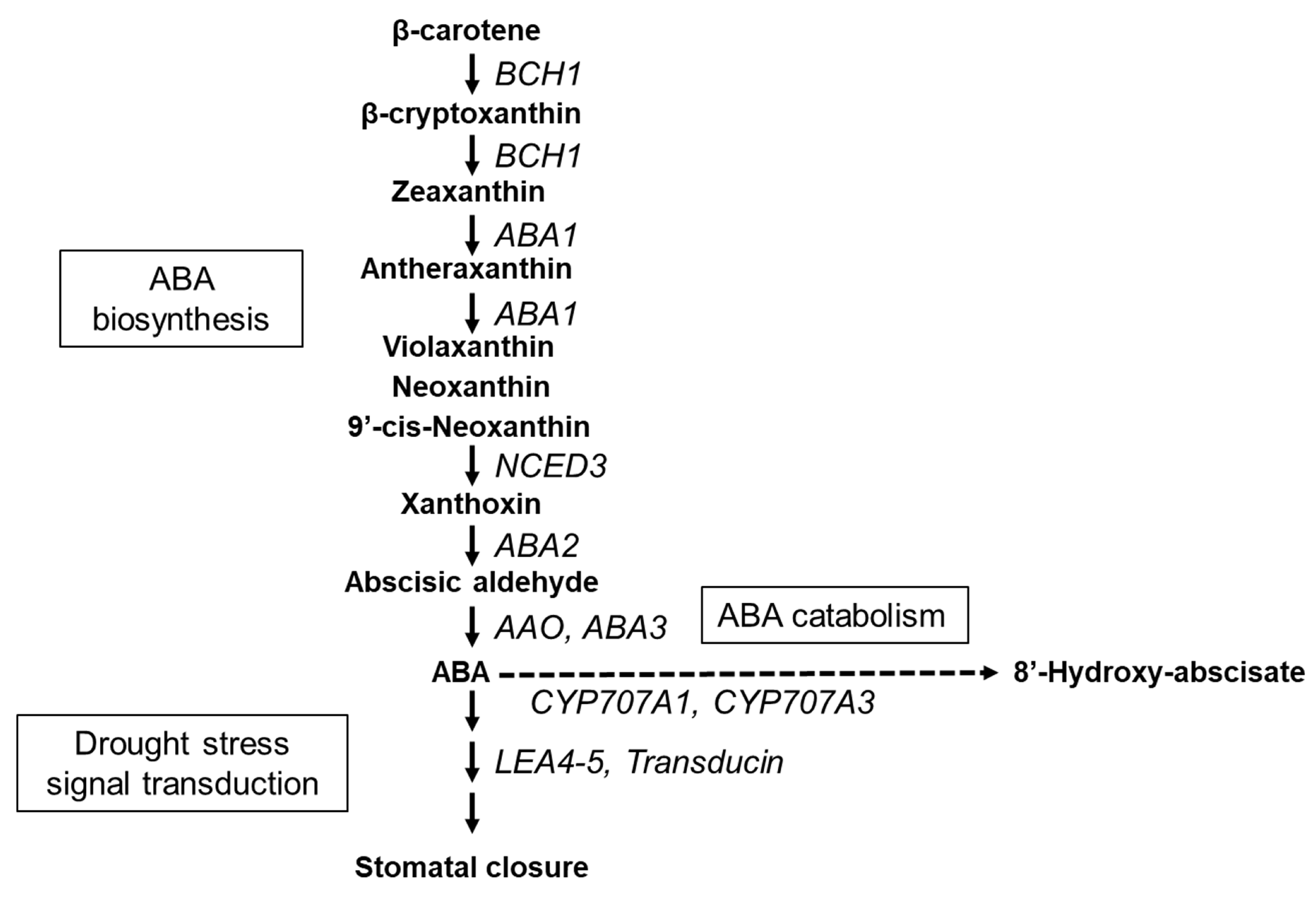
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Drought Treatment
2.2. -Carotene, Violaxanthin, and Neoxanthin Measurement
2.3. ABA Measurement
2.4. qPCR
2.5. Primer Design
2.6. Ascorbic Acid Measurement
2.7. Statistical Analysis
3. Results
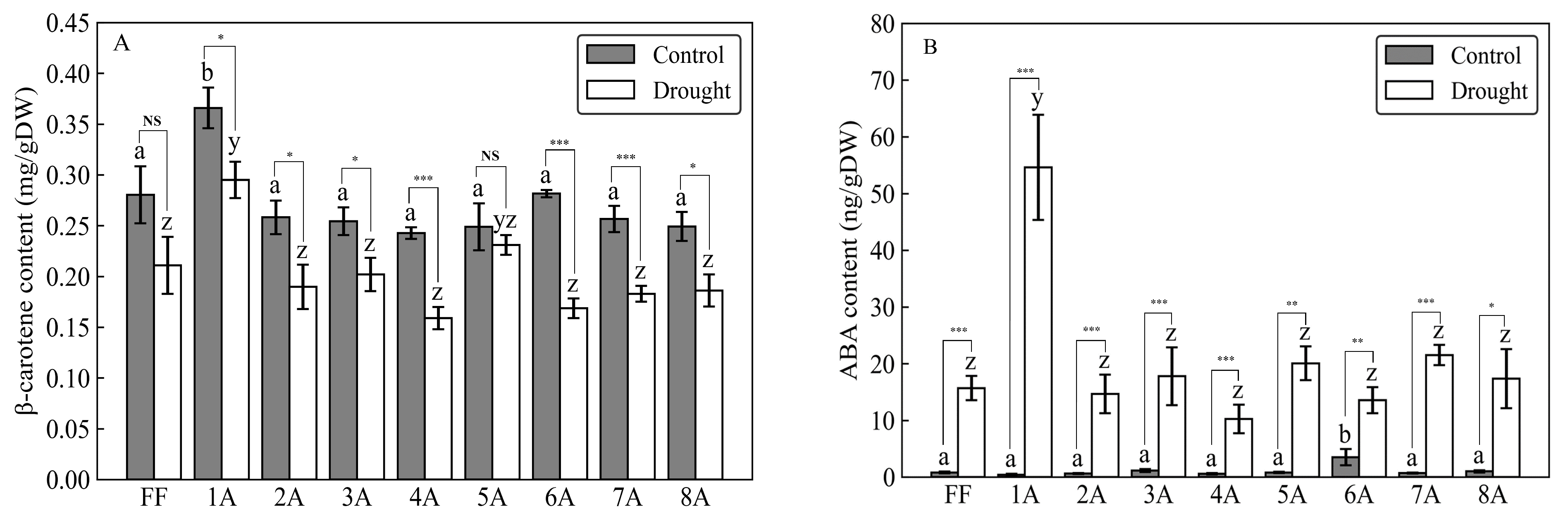
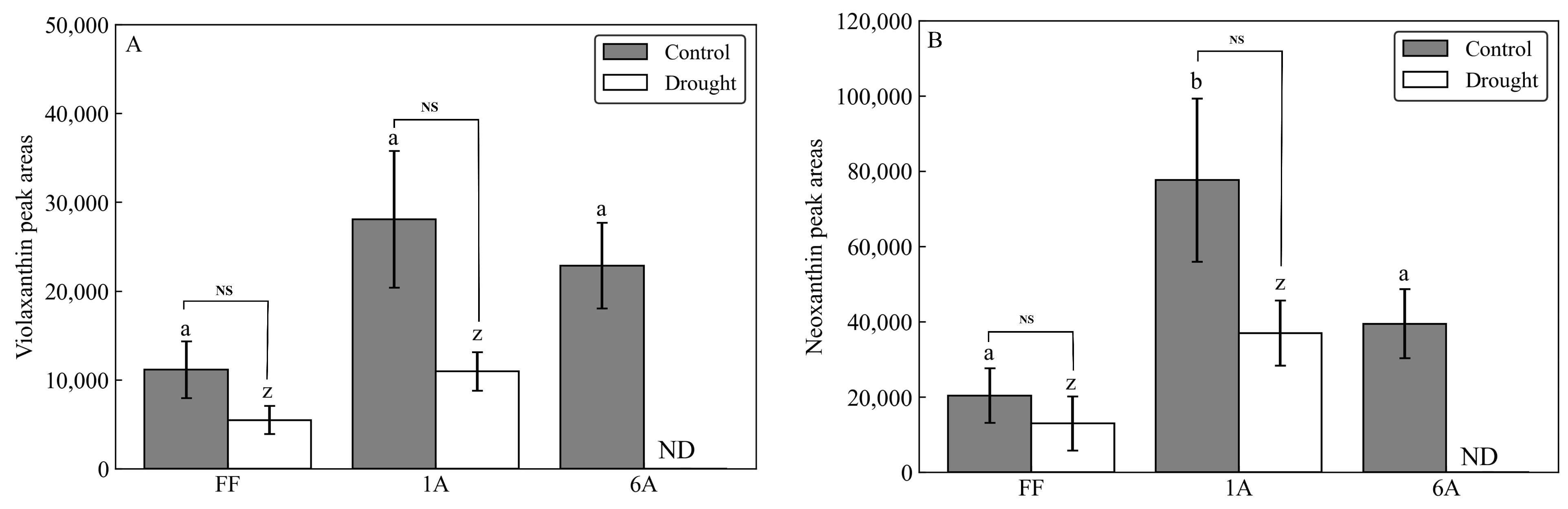
3.1. Carotenoid and ABA Content in AMALs under Drought Conditions
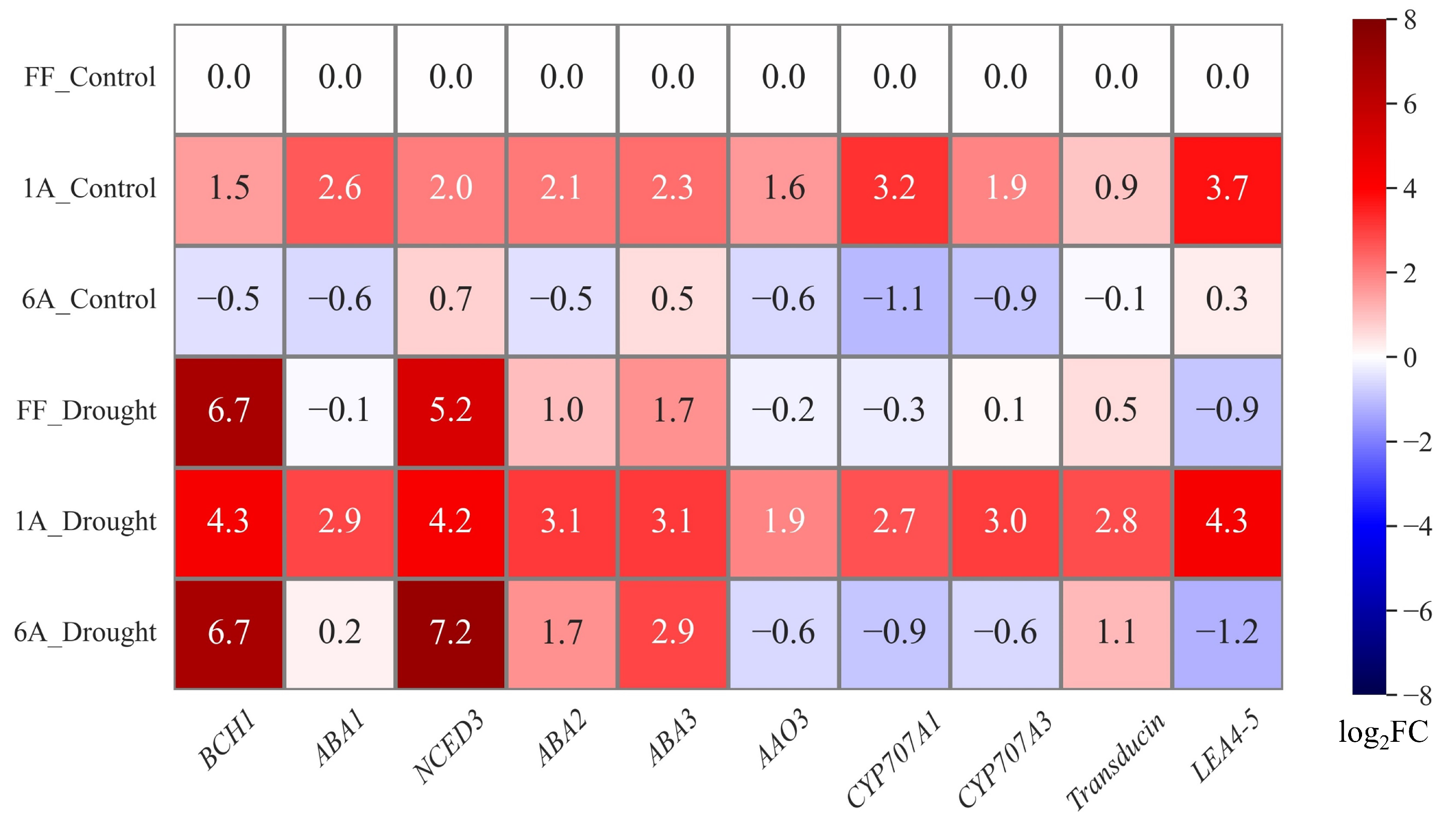
3.2. Expression Analysis of ABA-Related Genes in the Monosomic Addition Lines and A. fistulosum under Drought Conditions
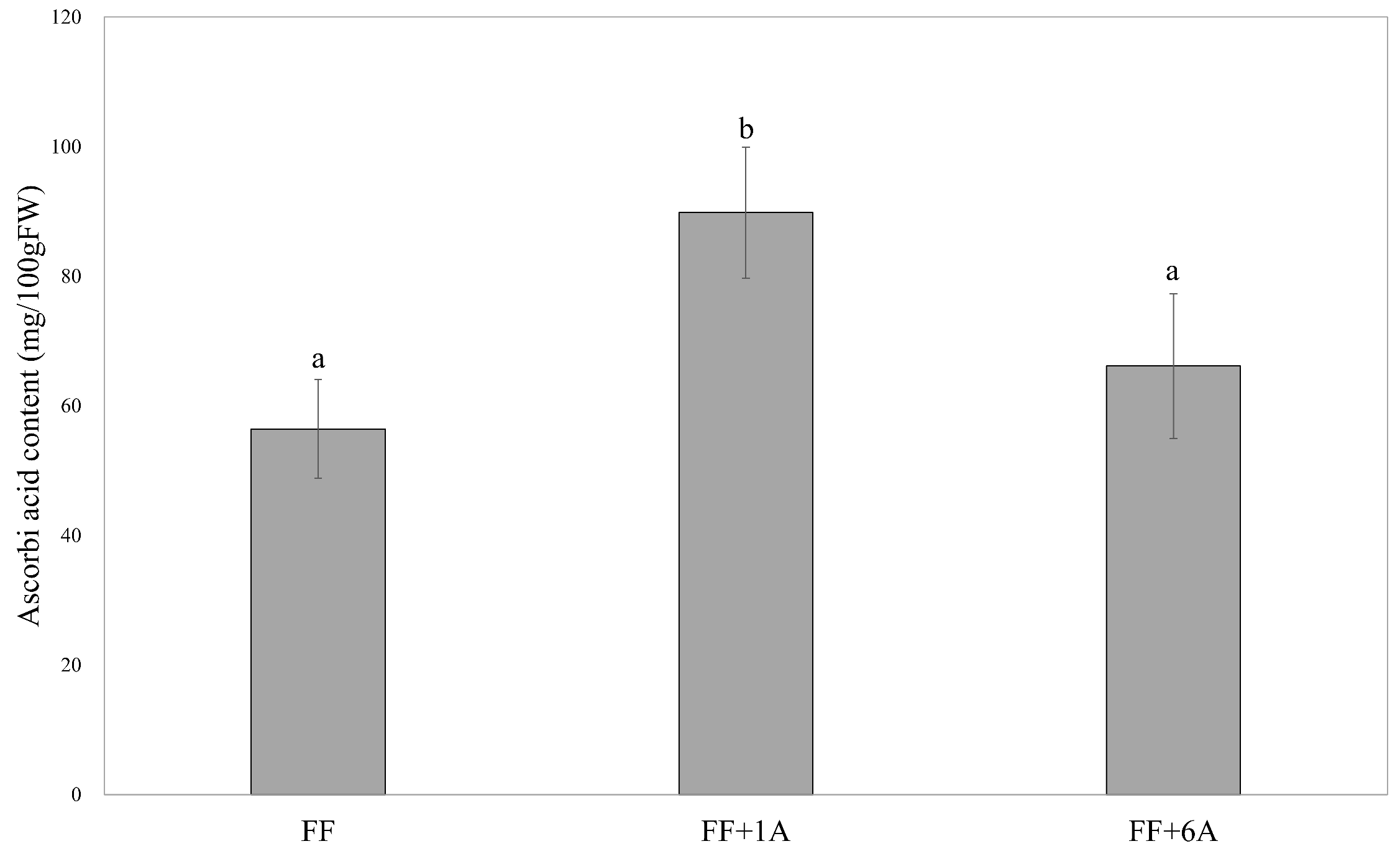
3.3. Determination of Ascorbic Acid Content in FF, FF + 1A, and FF + 6A
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumazawa, S.; Katsumata, H. Chapter 31 Negi. In Sosai-Engei Kakuron; Yokendo Press: Tokyo, Japan, 1965; pp. 280–289. [Google Scholar]
- Ford-Lloyd, B.V.; Armstrong, S.J. Welsh onion Allium fistulosum L. In Genetic Improvement of Vegetable Crops; Pergamon Press: London, UK, 1993; pp. 51–58. [Google Scholar]
- Rabinowitch, H.D.; Thomas, B. Edible Alliums; CABI: Wallingford, UK, 2023; p. 111. [Google Scholar]
- Yiu, J.-C.; Liu, C.-W.; Kuo, C.-T.; Tseng, M.-J.; Lai, Y.-S.; Lai, W.-J. Changes in antioxidant properties and their relationship to paclobutrazol- induced flooding tolerance in Welsh onion. J. Sci. Food Agric. 2008, 88, 1222–1230. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Alterations in leaf photosynthetic electron transport in Welsh onion (Allium fistulosum L.) under different light intensity and soil water conditions. Plant Biol. 2020, 23, 83–90. [Google Scholar] [CrossRef]
- Rai, G.K.; Khanday, D.M.; Choudhary, S.M.; Kumar, P.; Kumari, S.; Martínez-Andújar, C.; Martínez-Melgarejo, P.A.; Rai, P.K.; Pérez-Alfocea, F. Unlocking nature’s stress buster: Abscisic acid’s crucial role in defending plants against abiotic stress. Plant Stress 2024, 11, 100359. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, M.; Hu, J.; Zhang, X.; Wang, K.; Ashraf, M. Modulation Role of abscisic acid (ABA) on growth, water relations and glycinebetaine metabolism in two maize (Zea mays L.) cultivars under drought stress. Int. J. Mol. Sci. 2012, 13, 3189–3202. [Google Scholar] [CrossRef]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, T.; Kang, G. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci. 2015, 6, 458. [Google Scholar] [CrossRef]
- Awan, S.A.; Khan, I.; Rizwan, M.; Zhang, X.; Brestic, M.; Khan, A.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S.; Huang, L. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol. Plant. 2021, 172, 809–819. [Google Scholar] [CrossRef]
- Huan, L.; Jin-Qiang, W.; Qing, L. Photosynthesis product allocation and yield in sweet potato with spraying exogenous hormones under drought stress. J. Plant Physiol. 2020, 253, 153265. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Sirko, A.; Wawrzyńska, A.; Brzywczy, J.; Sieńko, M. Control of ABA Signaling and Crosstalk with Other Hormones by the Selective Degradation of Pathway Components. Int. J. Mol. Sci. 2021, 22, 4638. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Nishiyama, R.; Tran, C.D.; Kusano, M.; Nakabayashi, R.; Okazaki, Y.; Matsuda, F.; Chávez Montes, R.A.; Mostofa, M.G.; Li, W.; et al. Defective cytokinin signaling reprograms lipid and flavonoid gene-to-metabolite networks to mitigate high salinity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2105021118. [Google Scholar] [CrossRef]
- Şimşek, Ö.; Isak, M.A.; Dönmez, D.; Dalda Şekerci, A.; İzgü, T.; Kaçar, Y.A. Advanced Biotechnological Interventions in Mitigating Drought Stress in Plants. Plants 2024, 13, 717. [Google Scholar] [CrossRef]
- Rabinowitch, H.D.; Currah, L. (Eds.) Onions in the tropics: Cultivars and country reports. In Allium Crop Science: Recent Advances; CABI Publishing: Wallingford, UK, 2002; pp. 379–407. [Google Scholar]
- Abdelrahman, M.; Sawada, Y.; Nakabayashi, R.; Shusei, S.; Hideki, H.; El-Sayed, M.A.; Masami, Y.H.; Kazuki, S.; Naoki, Y.; Masayoshi, S. Integrating transcriptome and target metabolome variability in doubled haploids of Allium cepa Abiotic Stress Prot. Mol. Breed. 2015, 35, 195. [Google Scholar] [CrossRef]
- Multani, D.S.; Khush, G.S.; delos Reyes, B.G.; Brar, D.S. Alien genes introgression and development of monosomic alien addition lines from Oryza latifolia Desv. to rice, Oryza Sativa L. Theor. Appl. Genet. 2003, 107, 395–405. [Google Scholar] [CrossRef]
- Reamon-Ramos, S.M.; Wricke, G. A full set of monosomic addition lines in Beta Vulgaris Beta Webbiana: Morphol. Isozyme Markers. Theor. Appl. Genet. 1992, 84, 411–418. [Google Scholar] [CrossRef]
- Chevre, A.M.; Eber, F.; This, P.; Barret, P.; Tanguy, X.; Brun, H.; Delseny, M.; Renard, M. Characterization of Brassica Nigra Chromosom. Blackleg Resist. B. Napus–B. Nigra Addit. Lines. Plant Breed. 1996, 115, 113–118. [Google Scholar]
- Fu, S.; Sun, C.; Yang, M.; Fei, Y.; Tan, F.; Yan, B.; Ren, Z.; Tang, Z. Genetic and Epigenetic Variations Induced by Wheat-Rye 2R and 5R Monosomic Addition Lines. PLoS ONE 2013, 8, e54057. [Google Scholar] [CrossRef]
- Shigyo, M.; Tashiro, Y.; Isshiki, S.; Miyazaki, S. Establishment of a series of alien monosomic addition lines of Japanese bunching onion (Allium fistulosum L.) with extra chromosomes from shallot (A. cepa L. aggregatum group). Genes Genet Syst. 1996, 71, 363–371. [Google Scholar] [CrossRef]
- Wako, T.; Yamashita, K.; Tsukazaki, H.; Ohara, T.; Kojima, A.; Yaguchi, S.; Shimazaki, S.; Midorikawa, N.; Sakai. T.; Yamauchi, N.; Shigyo, M. Screening and incorporation of rust resistance from Allium cepa into bunching onion (Allium fistulosum) via alien chromosome addition. Genome 2015, 58, 135–142. [Google Scholar] [CrossRef]
- Vu, H.Q.; El-Sayed, M.A.; Ito, S.; Yamauchi, N.; Shigyo, M. Discovery of a new source of resistance to Fusarium oxysporum, cause of Fusarium wilt in Allium fistulosum, located on chromosome 2 of Allium cepa Aggregatum group. Genome 2012, 55, 797–807. [Google Scholar] [CrossRef]
- Endang, S.; Tashiro, Y.; Shigyo, M.; Isshiki, S. Morphological and cytological characteristics of haploid shallot (A. cepa L. Aggregatum group). Bull. Fac. Agric. Saga Univ. 1997, 82, 7–15. [Google Scholar]
- Yaguchi, S.; Atarashi, M.; Iwai, M.; Masuzaki, S.; Yamauchi, N.; Shigyo, M. Production of Alien Addition Lines in Polyploid Bunching Onion (Allium fistulosum) Carrying 1A Chromosome(s) of Shallot (Allium cepa) and Their Application to Breeding for a New Vitamin C-Rich Vegetable. J. Am. Soc. Hort. Sci. 2008, 133, 363–373. [Google Scholar] [CrossRef]
- Kushiro, T.; Okamoto, M.; Nakabayashi, K.; Yamagishi, K.; Kitamura, S.; Asami, T.; Hirai, N.; Koshiba, T.; Kamiya, Y.; Nambara, E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8’-hydroxylases: Key enzymes in ABA catabolism. EMBO J. 2004, 23, 1647–1656. [Google Scholar] [CrossRef]
- Liao, N.; Hu, Z.; Miao, J.; Hu, X.; Lyu, X.; Fang, H.; Zhou, Y.M.; Mahmoud, A.; Deng, G.; Meng, Y.Q.; et al. Chromosome-level genome assembly of bunching onion illuminates genome evolution and flavor formation in Allium crops. Nat. Commun. 2022, 13, 6690. [Google Scholar] [CrossRef]
- Hao, F.; Liu, X.; Zhou, B.; Tian, Z.; Zhou, L.; Zong, H.; Qi, J.; He, J.; Zhang, Y.; Zeng, P.; et al. Chromosome-level genomes of three key Allium Crop. Their Trait Evolution. Nat. Genet. 2023, 55, 1976–1986. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Y.; Wang, Y.; Wang, L.; Chen, S.; Li, H. Identification of a sugar beet BvM14-MADS box gene through differential gene expression analysis of monosomic addition line M14. Plant Physiol. 2011, 168, 1980–1986. [Google Scholar] [CrossRef]
- Hu, T.; Zhu, S.; Tan, L.; Qi, W.; He, S.; Wang, G. Overexpression of OsLEA4 enhances drought, high salt and heavy metal stress tolerance in transgenic rice (Oryza sativa L.). Environ. Exp. Bot. 2016, 123, 68–77. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Shi, Y.; Wu, X.; Wang, Y.; Liu, J.; Fang, Z.; Li, C.; Dong, K. Overexpression of Medicago sativa LEA4-4 can improve the salt, drought, and oxidation resistance of transgenic Arabidopsis. PLoS ONE 2020, 15, e0234085. [Google Scholar] [CrossRef]
- Audran, C.; Borel, C.; Frey, A.; Sotta, B.; Meyer, C.; Simonneau, T.; Marion-Poll, A. Expression studies of the zeaxanthin epoxidase gene in nicotiana plumbaginifolia. Plant Physiol. 1998, 118, 1021–1028. [Google Scholar] [CrossRef]
- Thompson, A.J.; Jackson, A.C.; Parker, R.A.; Morpeth, D.R.; Burbidge, A.; Taylor, I.B. Abscisic acid biosynthesis in tomato: Regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Mol Biol. 2000, 42, 833–845. [Google Scholar] [CrossRef]
- Schwarz, N.; Armbruster, U.; Iven, T.; Brückle, L.; Melzer, M.; Feussner, I.; Jahns, P. Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple functions of the enzyme in plastids. Plant Cell Physiol. 2015, 56, 346–357. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.-L.; Havaux, M. Chemical Quenching of Singlet Oxygen by Carotenoids in Plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef]
- Pospíšil, P.; Prasad, A. Formation of singlet oxygen and protection against its oxidative damage in Photosystem II under abiotic stress. J. Photochem. Photobiol. B Biol. 2014, 137, 39–48. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Modarres Sanavy, S.A.M.; Asilan, K.S. Effect of Ascorbic Acid Foliar Application on Yield, Yield Component and several Morphological Traits of Grain Corn under Water Deficit Stress Conditions. Not. Sci. Biol. 2010, 2, 45–50. [Google Scholar] [CrossRef]
- Gaafar, A.A.; Ali, S.I.; El-Shawadfy, M.A.; Salama, Z.A.; Sękara, A.; Ulrichs, C.; Abdelhamid, M.T. Ascorbic Acid Induces the Increase of Secondary Metabolites, Antioxidant Activity, Growth, and Productivity of the Common Bean under Water Stress Conditions. Plants 2020, 9, 627. [Google Scholar] [CrossRef]
- Sh, S.M. Role of ascorbic acid and α tocopherol in alleviating salinity stress on flax plant (Linum usitatissimum L.). J. Stress Physiol. Biochem. 2014, 10, 93–111. [Google Scholar]
- Hafez, E.; Gharib, H. Effect of exogenous application of ascorbic acid on physiological and biochemical characteristics of wheat under water stress. Int. J. Plant Prod. 2016, 10, 579–596. [Google Scholar]

| Arabidopsis Genome Initiative Code | Gene Name | Homologs | Primer Sequence Based on A. fistulosum Unigenes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A. cepa Unigene(bp) | Identity to Arabidopsis Gene (%) | Chromosomal Location | A. fistulosum Unigene(bp) | Identity to A. cepa UniGene (%) | Chromosomal Location | Forward (5′ to 3′) | Reverse (5′ to 3′) | ||
| AT4G25700.1 | BCH1 | CL831.Contig1_ DHA_Bulb (1169) | 73.4 | 8A | Unigene22611_ FFStem (339) | 97.9 | 1F, 7F | AGGCAAAAACG AAGCAGCAG | TCGCGGCAACC AAATAAGTG |
| AT5G67030.2 | ABA1 | Unigene26642_ DHA_Bulb (687) | 82.9 | 4A, 5A | CL2970.Contig3_ FFStem (1321) | 100.0 | 4F, 5F | TCCTCTTTCTG CAGCAGGTG | AAGAGGTCATG AGTGCTGGC |
| AT3G14440.1 | NCED3 | Unigene2060_ DHA_Bulb (237) | 59.2 | 7A | Unigene35308_ FF_5AStem (510) | 86.8 | 1F | GTTGGTTCACC GGTGCAAAG | ACATATTCCAA CAAGCTGCAGC |
| AT1G52340.1 | ABA2 | CL2419.Contig1_ DHA_Bulb (799) | 62.9 | 4A | CL736.Contig4_ FFStem (960) | 97.7 | 6F | TGGTGCTCCTG AGACAACAAG | GAGCGTTGATG ACCTCAATAGC |
| AT2G27150.2 | AAO | CL1978.Contig1_ DHA_Bulb (423) | 70.1 | 3A, 5A, 8A | Unigene26780_ FFStem (865) | 89.5 | 2F, 3F, 5F, 7F, 8F | TCCACCAAAAC CTCCTCCAAC | CATGCCAAGCC CCTCAATTTAC |
| AT1G16540.1 | ABA3 | Unigene33397_ DHA_Bulb (272) | 48.9 | 4A, 5A, 8A | Unigene34756_ FFStem (260) | 97.7 | 6F | TGGTGCTCCTG AGACAACAAG | GAGCGTTGATG ACCTCAATAGC |
| AT4G19230.1 | CYP707A1 | CL2918.Contig1_ DHA_Bulb (1782) | 72.0 | 5A, 8A | CL5541.Contig1_ FFStem (249) | 99.2 | 7F | TTGTGGTCAGG TGATGAAGC | GCACCAAAGCC AAACACTTTC |
| AT5G45340.2 | CYP707A3 | Unigene28686_ DHA_Bulb (437) | 76.6 | 4A | Unigene30555_ FFStem (440) | 99.3 | 4F | ACCTTTACCTCC AGGCTCTATG | GGCAACCCAAGA TGTGAGTTTTG |
| AT1G49450.1 | Transducin | Unigene7829_ DHA_Bulb (423) | 45.5 | 1A | Unigene31313_ FFStem (590) | 97.8 | 1F | TGTGTCCACGG CGATTTTAC | AATTGCTCGGTC TGTTCACC |
| AT1G01470.1 | LEA4-5 | CL838.Contig2_ DHA_Bulb (610) | 53.7 | 3A, 4A, 6A | Unigene30105_ FFStem (491) | 95.7 | 2F, 5F, 6F, 7F, 8F | TTTCTTGGTCA CTGGAAGCG | TGGTGCCAATG AAAGTGGAC |
| AT3G53750.1 | -actin | CL575.Contig2_ DHA_Bulb (454) | 99.3 | - | CL1482.Contig7_ FFStem (351) | 98.0 | - | GTTGGTATGGG GCAAAAAGA | AGCCTTTGGAT TGAGTGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, T.; Yaguchi, S.; Hirata, S.; Abdelrahman, M.; Wada, T.; Mega, R.; Shigyo, M. Effects of Drought Stress on Abscisic Acid Content and Its Related Transcripts in Allium fistulosum—A. cepa Monosomic Addition Lines. Genes 2024, 15, 754. https://doi.org/10.3390/genes15060754
Nakajima T, Yaguchi S, Hirata S, Abdelrahman M, Wada T, Mega R, Shigyo M. Effects of Drought Stress on Abscisic Acid Content and Its Related Transcripts in Allium fistulosum—A. cepa Monosomic Addition Lines. Genes. 2024; 15(6):754. https://doi.org/10.3390/genes15060754
Chicago/Turabian StyleNakajima, Tetsuya, Shigenori Yaguchi, Sho Hirata, Mostafa Abdelrahman, Tomomi Wada, Ryosuke Mega, and Masayoshi Shigyo. 2024. "Effects of Drought Stress on Abscisic Acid Content and Its Related Transcripts in Allium fistulosum—A. cepa Monosomic Addition Lines" Genes 15, no. 6: 754. https://doi.org/10.3390/genes15060754








